m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05011
|
[1], [2] | |||
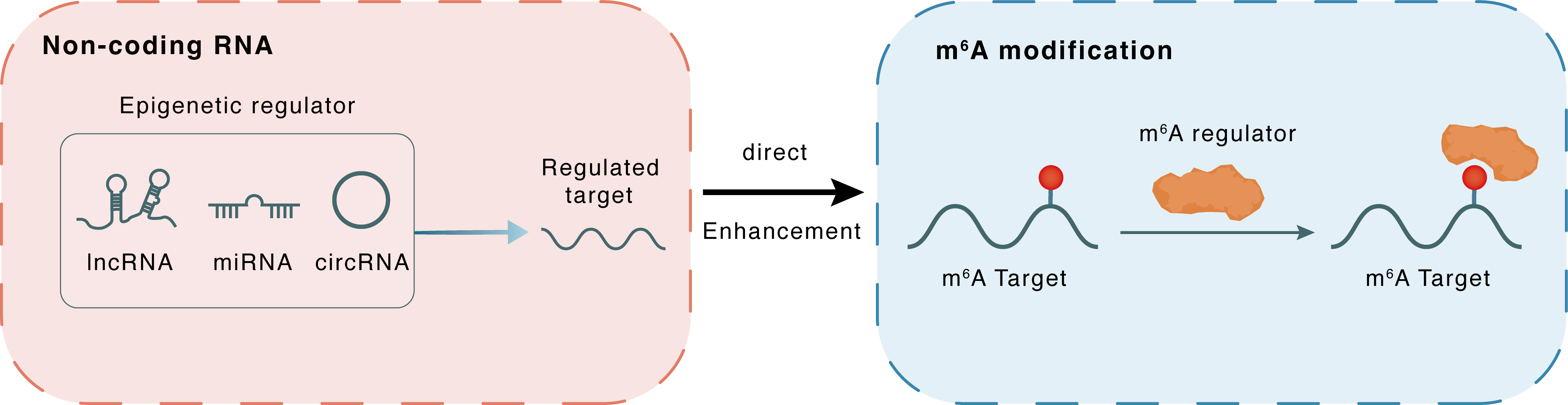 Non-coding RNA
DARS-AS1
IGF2BP3
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
TK1
TK1
IGF2BP3
Non-coding RNA
DARS-AS1
IGF2BP3
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
TK1
TK1
IGF2BP3
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3) | READER | |||
| m6A Target | Thymidine kinase, cytosolic (TK1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | DARS1 antisense RNA 1 (DARS1-AS1) | LncRNA | View Details | ||
| Regulated Target | Insulin like growth factor 2 mRNA binding protein 3 (IGF2BP3) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Enhancement | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | Downregulation of LncRNA DARS1-AS1 Inhibits the Tumorigenesis of Cervical Cancer via Inhibition of IGF2BP3, In contrast to the mRNA-decay-promoting function of YTH domain-containing family protein 2, IGF2BPs promote the stability and storage of their target mRNAs (for example, MYC) in an m6A-dependent manner under normal and stress conditions and therefore affect gene expression output. Four representative high confidence targets, including MYC, FSCN1, Thymidine kinase, cytosolic (TK1), and MARCKSL1, exhibit strong binding with IGF2BPs around their m6A motifs in control cells. Knocking down of each individual IGF2BPs in Hela (cervical cancer) and HepG2 (liver cancer) cells significantly repressed MYC expression. | ||||
| Responsed Disease | Cervical cancer | ICD-11: 2C77 | |||
| Cell Process | RNA decay | ||||
In-vitro Model |
SiHa | Cervical squamous cell carcinoma | Homo sapiens | CVCL_0032 | |
| HeLa | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| HEK293T | Normal | Homo sapiens | CVCL_0063 | ||
| HeLa | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Thymidine kinase, cytosolic (TK1) | 39 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| DEOXYCYTIDINE | Approved | [3] | ||
| Synonyms |
Cytosine deoxyribonucleoside; 2'-dC; bmse000323; ACMC-209rv6; CYTIDINE, 2'-DEOXY-; Cytosine deoxy nucleoside hydrochloride; 4-amino-1-[4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2(1H)-one; Desoxycytidine; 4-amino-1-(2-deoxypentofuranosyl)pyrimidin-2(1H)-one; 4-amino-1-[4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2-one; 3h-deoxycytidine; 4-amino-1-(4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-one; 40093-94-5; AC1L19OG; TimTec1_003892; NCIOpen2_004589; Oprea1_817993
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Penciclovir | Approved | [4] | ||
| Synonyms |
39809-25-1; Denavir; Penciclovirum; Vectavir; Penciclovirum [INN-Latin]; Pencyclovir; BRL-39123; BRL 39123; Penciclovir [USAN:INN:BAN]; Penciceovir; UNII-359HUE8FJC; CCRIS 9213; PE2
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| TK-DLI | Preregistration | [5] | ||
| Synonyms |
TBI-0301; Herpes simplex thymidine kinase suicide gene therapy, MolMed/Takara Bio; HSV thymidine kinase gene therapy (GvH, retroviral vector), Istituto Scientifico H San Raffaele; HSV-TK suicide gene therapy, MolMed/Takara Bio; TK cell therapy (haploidentical haematopoietic stem cell transplantation), MolMed/Takara Bio; HSV-TK gene therapy (haematological malignancies), MolMed/Takara Bio; TK-DLI, San Raffaele/MolMed/Takara Bio; TK gene/cell therapy (bone marrow transplantation-associated GvHD prevention), MolMed/Takara Bio; TK-expressing donor T-cells (bone marrow transplantation-associated GvHD prevention), San Raffaele/MolMed; Thymidine kinase expressing donor T-cells (bone marrow transplantation-associated GvHD prevention), San Raffaele/MolMed/Takara
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| FV-100 | Phase 3 | [6] | ||
| Synonyms |
CF-1001; CF-1094; CF-1368; CF-1369; CF-1449; CF-1452; CF-1698; CF-1712; CF-1743; CF-1821; CF-1835; CF-1837; CF-1838; CF-1851; CF-2004; CF-2160; CF-2161; CF-2200; BCNAs (antiviral), FermaVir Pharmaceuticals; Bicyclic nucleoside analogs (VZV infection), FermaVir; Bicyclic nucleoside analogs (VZV infection), Inhibitex; Antivirals (nucleoside derivatives), Welsh School of Pharmacy/Rega; BCNAs (antiviral), Rega/Welsh School of Pharmacy; Bicyclic nucleoside analogs (antiviral), Rega/Welsh School of Pharmacy
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Radiosensitizer gene therapy | Phase 3 | [7] | ||
| Synonyms |
Radiosensitizer gene therapy (prostate cancer)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RP101 | Phase 2/3 | [8] | ||
| Synonyms |
SCHEMBL15589316; CHEMBL3703295; BDBM149820; US8975415,
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 100 nM | |||
| External Link | ||||
| HQK-1004 | Phase 2 | [9] | ||
| Synonyms |
Arginine butyrate; VX-105; VX-105); Arginine butyrate (hematological malignancies), HemaQuest
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Ad-OC-hsvTK/valacyclovir | Phase 1 | [10] | ||
| Synonyms |
Ad-OC-hsvTK; Gene therapy (prostate cancer), Winship Cancer Institute; Adenovirus osteocalcin-promoter-driven HSV thymidine Kinase, Winship Cancer Institute; Ad-OC-hsvTK/valacyclovir, Winship Cancer Institute
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Thymidine kinase-expressing adenovirus and ganciclovir suicide gene therapy | Phase 1 | [11] | ||
| Synonyms |
Thymidine kinase-expressing adenovirus and ganciclovir suicide genetherapy (cancer); Ad5-SSTR/TK-RGD; Thymidine kinase-expressing adenovirus and ganciclovir suicide gene therapy (cancer), University of Alabama
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Rilapladib | Phase 1 | [12] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Sitimagene ceradenovec | Discontinued in Phase 3 | [13] | ||
| Synonyms |
Cerepro; EG-009; HSV thymidine kinase gene therapy, Ark
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| 6-Hydroxypropylthymine | Investigative | [4] | ||
| Synonyms |
6-(3-hydroxypropyl)thymine; 6-(3-hydroxypropyl)-5-methylpyrimidine-2,4(1H,3H)-dione; 2,4(1H,3H)-Pyrimidinedione, 6-(3-hydroxypropyl)-5-methyl-; 156569-47-0; AC1L1CEO; SCHEMBL4315838; CHEBI:43299; CTK8A4001; DB04139
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-(2-propyn-1-yl)thymidine | Investigative | [14] | ||
| Synonyms |
3-Propargylthymidine; SCHEMBL1619074; CHEMBL524872
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ITdU | Investigative | [12] | ||
| Synonyms |
3-Hydroxyandrost-5-en-17-one; Diandron, 17-Hormoforin; A-hydroxy-5-androsten-17-one; Spectrum_000661; SpecPlus_000094; Androst-5-en-17-one, 3-hydroxy-, (3.beta.)-; Spectrum3_000116; Spectrum5_000130; Spectrum4_001395; Spectrum2_000359; AC1L18FD; KBioSS_001141; KBioGR_001750; CHEMBL31399; DivK1c_006190; SPBio_000457; SCHEMBL9969169; KBio1_001134; KBio3_000872; KBio2_006277; KBio2_003709; KBio2_001141; CHEBI:95212; MolPort-003-891-893; ALBB-023670; CCG-38634; Androst-5-en-17-one, 3-hydroxy-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-Iodo-2'-Deoxyuridine-5'-Monophosphate | Investigative | [4] | ||
| Synonyms |
AC1L52UF; [(2R,3S)-3-hydroxy-5-(5-iodo-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methyl dihydrogen phosphate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1-[2-(2-triphenylmethoxyethoxy)ethyl]thymine | Investigative | [15] | ||
| Synonyms |
CHEMBL219905
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (South)-Methanocarba-Thymidine | Investigative | [4] | ||
| Synonyms |
south-methanocarbathymidine; 1-[(1S,3S,4R,5S)-3-hydroxy-4-(hydroxymethyl)bicyclo[3.1.0]hex-1-yl]-5-methylpyrimidine-2,4(1H,3H)-dione; SCT; 1-[(1S,3S,4R,5S)-3-hydroxy-4-(hydroxymethyl)bicyclo[3.1.0]hexan-1-yl]thymine; (1S,3S,4R,5S)-3-hydroxy-4-hydroxymethyl-1-(5-methyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl)bicyclo[3.1.0]hexane; [(S)-Methanocarba-T]; South-Methanocarba-thymine; AC1L9L1U; 2'-exo-Methanocarbathymidine; CHEBI:45586; XRMLXZVSFIBRRJ-PEFMBERDSA-N; DB02921
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-(Dihydroxy-Isobutyl)-Thymine | Investigative | [4] | ||
| Synonyms |
DHBT; 6-(1,3-dihydroxyisobutyl)thymine; 6-[3-hydroxy-2-(hydroxymethyl)propyl]-5-methylpyrimidine-2,4(1H,3H)-dione; 6-[3-hydroxy-2-(hydroxymethyl)propyl]-5-methyl-2,4(1h,3h)-pyrimidinedione; 6-[3-hydroxy-2-(hydroxymethyl)propyl]thymine; CCV; AC1L1CE0; CTK8A1595; CHEBI:41485; DB02500; 6-[3-hydroxy-2-(hydroxymethyl)propyl]-5-methyl-1H-pyrimidine-2,4-dione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-propyl-2'-deoxyuridine | Investigative | [16] | ||
| Synonyms |
27826-74-0; CHEMBL221982; 5-Propyldeoxyuridine; 2'-Deoxy-5-propyluridine; 5-Propyl-dUrd; 5-Propyl-2'-desoxyuridine; AC1L4N4W; 2'-deoxy-5-n-propyluridine; Uridine,2'-deoxy-5-propyl-; Uridine, 2'-deoxy-5-propyl-; SCHEMBL2396529; CTK4G0305; DTXSID30182141; MBERTAKFBYOAHR-IVZWLZJFSA-N; BDBM50375778; 1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-propylpyrimidine-2,4-dione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 20000 nM | |||
| External Link | ||||
| L-5-(bromovinyl)deoxyuridine | Investigative | [16] | ||
| Synonyms |
CHEMBL261850; SCHEMBL4314668; BDBM50375781
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 9-(4-Hydroxybutyl)-N2-Phenylguanine | Investigative | [4] | ||
| Synonyms |
HBPG; CHEMBL406254; BPG; 6H-Purin-6-one, 1,9-dihydro-9-(4-hydroxybutyl)-2-(phenylamino)-; 161363-19-5; 1QHI; AC1L9LM8; SCHEMBL1506775; SCHEMBL17485742; CTK0A9784; BDBM21866; DTXSID00332272; AKOS030558959; N2-Phenyl-9-(4-hydroxybutyl) guanine; DB02495; 9-(4-Hydroxybuthyl)-N2-Phenylguanine; 2-anilino-9-(4-hydroxybutyl)-1H-purin-6-one; 2-anilino-9-(4-hydroxybutyl)-3H-purin-6-one; 9-(4-hydroxybutyl)-2-(phenylamino)-1,9-dihydro-6H-purin-6-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 50 nM | |||
| External Link | ||||
| L-5-iodo-2'-deoxyuridine | Investigative | [16] | ||
| Synonyms |
CHEMBL408518; URIDINE, 2'-DEOXY-5-IODO-; AC1LAEAG; SCHEMBL51859; XQFRJNBWHJMXHO-XVMARJQXSA-N; ZINC5223557; BDBM50375780; FT-0620507; 5-Iodo-1-(2-deoxy-alpha-D-ribofuranosyl)uracil
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3'-(1,2,3-Triazol-1-yl)-3'-deoxy-beta-D-thymidine | Investigative | [17] | ||
| Synonyms |
3'-(1,2,3-Triazol)dT; 3'-Deoxy-3'-(1,2,3-triazol-1-yl)thymidine; 122370-58-5; CHEMBL1092729; Thymidine,3'-deoxy-3'-(1H-1,2,3-triazol-1-yl)- (9CI); AC1L9Q42; SCHEMBL9965127; CTK4B3087; DTXSID60153586; BDBM50314847; 3'-(1H-1,2,3-Triazol-1-yl)-3'-deoxythymidine; 3''-(1,2,3-Triazol-1-yl)-3''-deoxy-beta-D-thymidine; 1-[(2R,4S,5S)-5-(hydroxymethyl)-4-(triazol-1-yl)tetrahydrofuran-2-yl]-5-methyl-pyrimidine-2,4-dione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 >= 500000 nM | |||
| External Link | ||||
| BVDU-MP | Investigative | [18] | ||
| Synonyms |
SCHEMBL4287705
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 9-Hydroxypropyladenine, S-Isomer | Investigative | [4] | ||
| Synonyms |
(S)-1-(6-Amino-9H-purin-9-yl)propan-2-ol; 14047-27-9; (S)-9-(2-Hydroxypropyl)adenine; (2S)-1-(6-amino-9H-purin-9-yl)propan-2-ol; AC1L9HLO; Tenofovir Related Compound 9; S-9-(2-hydroxypropyl)adenine; SCHEMBL5810639; 9H-Purine-9-ethanol, 6-amino-alpha-methyl-, (alphaS)-; CTK8C2128; DTXSID60332177; MolPort-003-848-032; ZINC2046907; ANW-67863; 6827AA; DB03000; SC-43410; AK-82057; AJ-33425; KB-106920; TX-015702; (2S)-1-(6-aminopurin-9-yl)propan-2-ol; AX8236781; FT-0696938; ST24035731
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-phenylamino-9-(4-hydroxy-butyl)-6-oxopurine | Investigative | [16] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-Bromothienyldeoxyuridine | Investigative | [4] | ||
| Synonyms |
CHEMBL1231486; 5-(5-Bromo-2-thienyl)-2'-deoxyuridine; 5-(5-Bromothien-2-yl)-2'-deoxyuridine; BTD; 134333-70-3; BTDU; Uridine,5-(5-bromo-2-thienyl)-2'-deoxy-; AC1L9K0Y; SCHEMBL1636648; CHEMBL358374; CTK4B9107; BDBM50054768; DB03804; 5-(5-Bromothien-2-yl)-2'-deoxyuridine-; 5-(5-bromothiophen-2-yl)-2'-deoxyuridine; 5-(5-Bromothien-2-yl)-1- (.beta.-D-2-deoxyribofuranos-1-yl)uracil; 5-(5-Bromo-thiophen-2-yl)-1-(4-hydroxy-5-hydroxymethyl-tetrahydro-furan-2-yl)-1H-pyrimidine-2,4-dione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1-[7-(triphenylmethoxy)heptyl]thymine | Investigative | [15] | ||
| Synonyms |
CHEMBL219367; 921587-94-2; CTK3G1843; DTXSID20582671; BDBM50200997; 5-Methyl-1-[7-(triphenylmethoxy)heptyl]pyrimidine-2,4(1H,3H)-dione; 2,4(1H,3H)-Pyrimidinedione, 5-methyl-1-[7-(triphenylmethoxy)heptyl]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 9-Hydroxypropyladenine, R-Isomer | Investigative | [4] | ||
| Synonyms |
14047-28-0; (R)-9-(2-Hydroxypropyl)adenine; (R)-1-(6-Amino-9H-purin-9-yl)propan-2-ol; (R)-(+)-9-(2-HYDROXYPROPYL)ADENINE; (2R)-1-(6-aminopurin-9-yl)propan-2-ol; UNII-43H6SBP55W; (R)-9-(2-hydroxypropyl) adenine; 9H-Purine-9-ethanol, 6-amino-alpha-methyl-, (alphaR)-; 43H6SBP55W; AK-59150; (2R)-1-(6-Amino-9H-purin-9-yl)propan-2-ol; ARP; W-201193; 9-(2-Hydroxypropyl)adenine, (R)-; PubChem9984; R-9-(2-hydroxypropyl)adenine [WHO-DD]; AC1L9HLR; 9H-Purine-9-ethanol, 6-amino-alpha-methyl-, D-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Edoxudine | Investigative | [16] | ||
| Synonyms |
5-ethyl-1-[4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-1,3-dihydropyrimidine-2,4-d ione; ORF-15817; RWJ-15817; Edurid (Salt/Mix); 1-(2-deoxypentofuranosyl)-5-ethylpyrimidine-2,4(1h,3h)-dione; AC1L1CAR; .beta.-5-Ethyldeoxyuridine; TimTec1_004024; SCHEMBL65580; MLS001360450; AC1Q69H9; 5-ethyl-1-[4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidine-2,4-dione; .beta.-5-Ethyl-2'-deoxyuridine; XACKNLSZYYIACO-UHFFFAOYSA-N; HMS3056J10; HMS3369L22; HMS1545G20; EDU; AKOS024282522; 5-Ethyl-2'-deoxyuridine; MCULE-3445830855; ST056929
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N2-(3-trifluoromethylphenyl)guanine | Investigative | [16] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 2'-Deoxyuridine | Investigative | [3] | ||
| Synonyms |
951-78-0; deoxyuridine; Uracil deoxyriboside; 1-((2R,4S,5R)-4-Hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)-dione; 2-Deoxyuridine; Deoxyribose uracil; 2'-Desoxyuridine; UNII-W78I7AY22C; CCRIS 2832; dURD; EINECS 213-455-7; BRN 0024433; 1-(2-Deoxy-beta-D-ribofuranosyl)uracil; W78I7AY22C; CHEBI:16450; 2 -Deoxyuridine; 1-(2-Deoxy-beta-D-erythro-pentofuranoxyl)uracil; MFCD00006527; AK-54658; 2,4(1H,3H)-Pyrimidinedione, 1-(2-deoxy-beta-D-ribofuranosyl)-; NSC 23615
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1-[5-(triphenylmethoxy)pentyl]thymine | Investigative | [15] | ||
| Synonyms |
CHEMBL216997; 921587-92-0; CTK3G1845; DTXSID10582665; BDBM50201001; 5-Methyl-1-[5-(triphenylmethoxy)pentyl]pyrimidine-2,4(1H,3H)-dione; 2,4(1H,3H)-Pyrimidinedione, 5-methyl-1-[5-(triphenylmethoxy)pentyl]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Deoxythymidine | Investigative | [4] | ||
| Synonyms |
Thymidine; 50-89-5; 2'-Deoxythymidine; Beta-Thymidine; 5-Methyldeoxyuridine; Thymidin; DThyd; 5-Methyl-2'-deoxyuridine; Thymine-2-deoxyriboside; Thyminedeoxyriboside; Thymine-2-desoxyriboside; 5-Methyldeoxyurindine; dThd; Uridine, 2'-deoxy-5-methyl-; Thymine deoxyriboside; 1-((2R,4S,5R)-4-Hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidine-2,4(1H,3H)-dione; dT; UNII-VC2W18DGKR; Deoxyribothymidine; AI3-52267; 2'-thymidine; beta-D-Ribofuranoside, thymine-1 2-deoxy-; CCRIS 1283; CHEBI:17748
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 26 nM | |||
| External Link | ||||
| 1-[6-(triphenylmethoxy)hexyl]thymine | Investigative | [15] | ||
| Synonyms |
CHEMBL216998; 921587-93-1; CTK3G1844; DTXSID00658828; BDBM50200990; 5-Methyl-1-[6-(triphenylmethoxy)hexyl]pyrimidine-2,4(1H,3H)-dione; 2,4(1H,3H)-Pyrimidinedione, 5-methyl-1-[6-(triphenylmethoxy)hexyl]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| P1-(5'-Adenosyl)P5-(5'-Thymidyl)Pentaphosphate | Investigative | [8] | ||
| Synonyms |
CHEMBL1236157; T5A; 3tmk; 1mrn; 4TMK; AC1L9KL2; BDBM50366828; DB03280; adenosine 5'-(hexahydrogen pentaphosphate), P"" 5'-ester with thymidine; 103137-88-8; [[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [hydroxy-[hydroxy-[hydroxy-[[(2R,3S,5R)-3-hydroxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methoxy]phosphoryl]oxyphosphoryl]oxyphosphoryl] hydrogen phosphate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | Investigative | [15] | ||
| Synonyms |
CHEMBL101135; 5-methyl-1-[(Z)-4-trityloxybut-2-enyl]pyrimidine-2,4-dione; AC1O54TL; 5-Methyl-1-(4-trityloxy-but-2-enyl)-1H-pyrimidine-2,4-dione; SCHEMBL19196301; BDBM50118490; 5-Methyl-1-[(2Z)-4-(trityloxy)but-2-enyl]pyrimidine-2,4(1H,3H)-dione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 180 nM | |||
| External Link | ||||
| Thymidine-5'-Phosphate | Investigative | [4] | ||
| Synonyms |
Thymidine 5'-monophosphate; dTMP(-); thymidine 5'-phosphate(1-); CHEBI:46960; thymidine 5'-(hydrogen phosphate)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2'-deoxythymidine triphosphate | Investigative | [4] | ||
| Synonyms |
Thymidine-5'-triphosphate; dTTP; thymidine 5'-triphosphate; Deoxy-TTP; Deoxythymidine 5'-triphosphate; 5'-TTP; thymidine 5'-(tetrahydrogen triphosphate); TTP (nucleotide); thymidine triphosphate; pppdT; TTP; UNII-QOP4K539MU; 5-Methyl-dUTP; dThd5'PPP; CHEBI:18077; EINECS 206-669-7; QOP4K539MU; CHEMBL363559; 2'-Deoxythymidine 5'-triphosphate; 18423-43-3; [hydroxy-[[(2R,3S,5R)-3-hydroxy-5-(5-methyl-2,4-dioxo-pyrimidin-1-yl)tetrahydrofuran-2-yl]methoxy]phosphoryl] phosphono hydrogen phosphate; DEOXYTHYMIDINE_TRIPHOSPHATE
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
References