m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03656
|
[1], [2] | |||
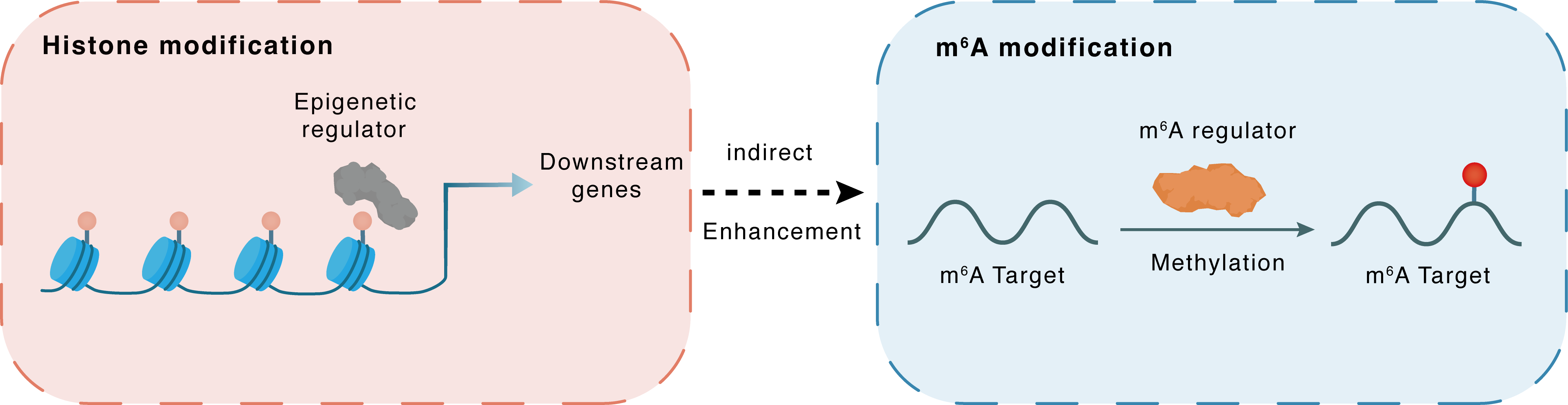 Histone modification
H3K27me3
EZH2
miR-338-5p
Indirect
Enhancement
m6A modification
PARP1
PARP1
METTL3
Methylation
Histone modification
H3K27me3
EZH2
miR-338-5p
Indirect
Enhancement
m6A modification
PARP1
PARP1
METTL3
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Poly [ADP-ribose] polymerase 1 (PARP1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone-lysine N-methyltransferase EZH2 (EZH2) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 27 trimethylation (H3K27me3) | View Details | |||
| Downstream Gene | miR-338-5p | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | Collectively, EZH2 downregulated hsa-miR-338-5p through Histone H3 lysine 27 trimethylation (H3K27me3), which in turn impaired miR-338-5p-dependent METTL3 inhibition and enhanced CDCP1 translation, therefore contributing to the development of GC. m6A methyltransferase METTL3 facilitates oxaliplatin resistance in CD133+ gastric cancer stem cells by Promoting Poly [ADP-ribose] polymerase 1 (PARP1) mRNA stability which increases base excision repair pathway activity. METTTL3 enhances the stability of PARP1 by recruiting YTHDF1 to target the 3'-untranslated Region (3'-UTR) of PARP1 mRNA. | ||||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | |||
| Responsed Drug | Oxaliplatin | ||||
| Pathway Response | Nucleotide excision repair | hsa03420 | |||
| Signaling pathways regulating pluripotency of stem cells | hsa04550 | ||||
| Cell Process | RNA stability | ||||
| Excision repair | |||||
In-vitro Model |
MKN74 | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_2791 | |
| HEK293T | Normal | Homo sapiens | CVCL_0063 | ||
| AGS | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| SNU-719 | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_5086 | ||
| In-vivo Model | 100,000 pLKO and PARP1-sh1 (PT1 and PT2) cells were mixed with matrix gel and inoculate into BALB/C nude mice, respectively. After 25 days, 6 organoid transplanted tumor mice were treated with oxaliplatin (Sellekchem, s1224) twice a week for 4 weeks at a dose of 5 mg/kg. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Histone-lysine N-methyltransferase EZH2 (EZH2) | 74 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Tazemetostat | Approved | [3] | ||
| Synonyms |
EPZ-6438; 1403254-99-8; EPZ6438; UNII-Q40W93WPE1; N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(morpholinomethyl)-[1,1'-biphenyl]-3-carboxamide; Q40W93WPE1; EPZ 6438; E7438; (1,1'-Biphenyl)-3-carboxamide, N-((1,2-dihydro-4,6-dimethyl-2-oxo-3-pyridinyl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(4-morpholinylmethyl)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 2.5 nM | |||
| External Link | ||||
| DS-3201b | Phase 2 | [4] | ||
| Synonyms |
Valemetostat; 1809336-39-7; UNII-60RD0234VE; 60RD0234VE; 1809336-39-7 (free base); DS-3201; (2R)-7-chloro-2-[trans-4-(dimethylamino)cyclohexyl]-N-[(4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl]-2,4-dimethyl-1,3-benzodioxole-5-carboxamide; Valemetostat 2HCl; Valemetostat [INN]; Valemetostat (DS-3201); CHEMBL4597193; EZH1/2 inhibitor DS-3201; SCHEMBL18393626; SCHEMBL18393627; SCHEMBL18639210; EX-A3423; DS3201; NSC813381; s8926; NSC-813381; HY-109108; CS-0039740; D11551
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CPI-1205 | Phase 1/2 | [5] | ||
| Synonyms |
HPODOLXTMDHLLC-QGZVFWFLSA-N; 1621862-70-1; UNII-455J2479FY; CPI1205; CPI 1205; 455J2479FY; (R)-N-((4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-methyl-1-(1-(1-(2,2,2-trifluoroethyl)piperidin-4-yl)ethyl)-1H-indole-3-carboxamide; GTPL9115; SCHEMBL17329268; MolPort-044-560-382; KS-000006BA; EX-A1068; s8353; AKOS030628484; ZINC220982768; CS-7648; compound 13 [PMID: 27739677]; HY-100021; J3.556.402K; N-[(4-methoxy-6-methyl-2-oxo-1H-pyridin-3-yl)methyl]-2-methyl-1-[(1R)-1-[1-(2,2,2-trifluoroethyl)piperidin-4-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SHR2554 | Phase 1/2 | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| CPI-0209 | Phase 1/2 | [7] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| GSK2816126 | Phase 1 | [8] | ||
| Synonyms |
GSK 126; GSK-126
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | Ki = 0.5 nM | |||
| External Link | ||||
| PF-06821497 | Phase 1 | [9] | ||
| Synonyms |
UNII-S4L4MM20B6; S4L4MM20B6; CHEMBL4080228; PF06821497; 1844849-10-0; 5,8-dichloro-2-[(4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl]-7-[(R)-methoxy(oxetan-3-yl)methyl]-3,4-dihydroisoquinolin-1(2H)-one; SCHEMBL17330426; GTPL10516; BDBM50246967; NSC800019; DB14799; HY-101571A; NSC-800019; compound 23a [PMID: 29211475]; CS-0092626; Q29209799; 1(2H)-Isoquinolinone, 5,8-dichloro-2-((1,2-dihydro-4-methoxy-6-methyl-2-oxo-3-pyridinyl)methyl)-3,4-dihydro-7-((R)-methoxy-3-oxetanylmethyl)-; 5,8-dichloro-2-[(4-methoxy-6-methyl-2-oxo-1H-pyridin-3-yl)methyl]-7-[(R)-methoxy(oxetan-3-yl)methyl]-3,4-dihydroisoquinolin-1-one; CJD
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| DS-3201 | Phase 1 | [5] | ||
| Synonyms |
QTGYNKYZRZATJB-UHFFFAOYSA-N; 701899-63-0; 2-(2-(2-Methyl-1H-imidazol-5-yl)ethyl)isoindoline-1,3-dione; 2-(2-(2-Methyl-1H-imidazol-5-yl)-ethyl)isoindoline-1,3-dione; SCHEMBL7743461; MolPort-035-945-474; MolPort-035-690-353; ZINC98086069; AKOS024459002; AKOS024262663; MCULE-2708350770; FCH4077443; AK158834; AX8292467; ST2403812; 2-[2-(2-methyl-1H-imidazol-5-yl)ethyl]-2,3-dihydro-1H-isoindole-1,3-dione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| HH2853 | Phase 1 | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-33 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID28394193-Compound-Figure3bI | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-35 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| PMID28394193-Compound-54 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-24 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-27 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-25 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-50 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-47 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2 nM; Ki < 1 nM | |||
| External Link | ||||
| PMID28394193-Compound-21 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-41 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-53 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 80 nM | |||
| External Link | ||||
| PMID28394193-Compound-Figure5aVIII | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-38 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-51 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-31 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 20 nM | |||
| External Link | ||||
| PMID28394193-Compound-42 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-15 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-52 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-32 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| PMID28394193-Compound-23 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-29 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID28394193-Compound-30 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 16 nM | |||
| External Link | ||||
| PMID28394193-Compound-39 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-49 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-43 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-40 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-Figure3bIII | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-36 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 316 nM | |||
| External Link | ||||
| PMID28394193-Compound-28 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 32 nM | |||
| External Link | ||||
| PMID28394193-Compound-22 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-18 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-16 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-44 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-20 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-19 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-37 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-Figure3bII | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-26 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-17 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-34 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID28394193-Compound-56 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.93 nM | |||
| External Link | ||||
| PMID28394193-Compound-46 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| PMID28394193-Compound-57 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6.45 nM | |||
| External Link | ||||
| PMID28394193-Compound-14 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 21900 nM | |||
| External Link | ||||
| PMID28394193-Compound-12 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 10 nM | |||
| External Link | ||||
| PMID26882240-Compound-1 | Patented | [12] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-55 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 8.13 nM | |||
| External Link | ||||
| PMID28394193-Compound-45 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 168000 nM | |||
| External Link | ||||
| PMID28394193-Compound-13 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1470 nM | |||
| External Link | ||||
| EPZ005687 | Investigative | [13] | ||
| Synonyms |
EPZ-005687; EPZ 005687
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| EI1 | Investigative | [14] | ||
| Synonyms |
KB-145943
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| UNC1999 | Investigative | [15] | ||
| Synonyms |
UNC 1999; UNC-1999
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MS1943 | Investigative | [6] | ||
| Synonyms |
2225938-17-8; SCHEMBL21271666; EX-A3962; s8918; HY-133129; CS-0112146; 6-(6-(4-(2-(2-((3r,5r,7r)-adamantan-1-yl)acetamido)ethyl)piperazin-1-yl)pyridin-3-yl)-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-1-isopropyl-1H-indazole-4-carboxamide; 6-(6-(4-(2-(2-(Adamantan-1-yl)acetamido)ethyl)piperazin-1-yl)pyridin-3-yl)-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-1-isopropyl-1H-indazole-4-carboxamide
Click to Show/Hide
|
|||
| MOA | Degrader | |||
| External Link | ||||
| GSK343 | Investigative | [16] | ||
| Synonyms |
compound 6 [PMID 24900432]; GSK 343
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 174 nM | |||
| External Link | ||||
| PMID28394193-Compound-11 | Patented | [17] | ||
| External Link | ||||
| PMID28394193-Compound-10 | Patented | [17] | ||
| External Link | ||||
| PMID28394193-Compound-14 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 21900 nM | |||
| External Link | ||||
| PMID28394193-Compound-12 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 10 nM | |||
| External Link | ||||
| PMID28394193-Compound-13 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1470 nM | |||
| External Link | ||||
| PMID28394193-Compound-56 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.93 nM | |||
| External Link | ||||
| PMID28394193-Compound-46 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| PMID28394193-Compound-57 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6.45 nM | |||
| External Link | ||||
| PMID28394193-Compound-55 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 8.13 nM | |||
| External Link | ||||
| PMID28394193-Compound-45 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 168000 nM | |||
| External Link | ||||
| Poly [ADP-ribose] polymerase 1 (PARP1) | 131 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Nicotinamide | Approved | [18] | ||
| Synonyms |
Aminicotin; Amixicotyn; Amnicotin; Benicot; Dipegyl; Endobion; Enduramide; Hansamid; Mediatric; Niacevit; Niacinamide; Niacotinamide; Niamide; Nicamina; Nicamindon; Nicasir; Nicobion; Nicofort; Nicogen; Nicomidol; Nicosylamide; Nicota; Nicotamide; Nicotilamide; Nicotililamido; Nicotinamid; Nicotinamida; Nicotinamidum; Nicotinsaeureamid; Nicotinsaureamid; Nicotol; Nicotylamide; Nicotylamidum; Nicovit; Nicovitina; Nicovitol; Nicozymin; Nikasan; Nikazan; Nikotinamid; Nikotinsaeureamid; Niocinamide; Niozymin; Papulex; Pelmin; Pelmine; Savacotyl; Amid kyseliny nikotinove; Amid kyseliny nikotinove [Czech]; Amide PP; Astra Brand of Niacinamide; Austrovit PP; Delonin amide; Factor pp; Inovitan PP; Jenapharm Brand of Niacinamide; Merck Brand of Niacinamide; Niacinamide Astra Brand; Niacinamide Jenapharm Brand; Niacinamide Merck Brand; Niacinamide Pharmagenix Brand; Niacinamide [USAN]; Niavit PP; Nicotine acid amide; Nicotine amide; Nicotinic acid amide; Nicotinic amide; Nicotinsaureamid Jenapharm; Nicotinsaureamid [German]; Nikotinsaeureamid [German]; Pelonin amide; Pharmagenix Brand of Niacinamide; Vitamin B; Vitamin PP; Witamina PP; Nicosan 2; Vitamin H1; B 3, Vitamin; B3, Vitamin; Beta-Pyridinecarboxamide; Jenapharm, Nicotinsaureamid; Nandervit-N; Niacin-Vitamin B3; Niacinamide (USP); Nicotinamida [INN-Spanish]; Nicotinamide (Niacinamide); Nicotinamidum [INN-Latin]; Niko-tamin; PP-Faktor; Vi-Nicotyl; Vitamin B (VAN); M-(Aminocarbonyl)pyridine; Niacinamide, Nicotinic acid amide, Nicotinamide; Nicotinamide (JP15/INN); Nicotinamide, niacin, vitamin B3; Nicotinamide-carbonyl-14C; Pyridine-3-carboxamide; Pyridine-3-carboxylic acid amide; 3 Pyridinecarboxamide; 3-Carbamoylpyridine; 3-Pyridinecarboxamide; 3-Pyridinecarboxylic acid amide
Click to Show/Hide
|
|||
| MOA | Binder | |||
| Activity | IC50 = 210000 nM | |||
| External Link | ||||
| KU-0058948 | Approved | [19] | ||
| Synonyms |
CHEMBL380648; 4-[3-(1,4-diazepan-1-ylcarbonyl)-4-fluorobenzyl]phthalazin-1(2H)-one; 4-(3-(1,4-diazepane-1-carbonyl)-4-fluorobenzyl)phthalazin-1(2H)-one; Homopiperazine analogue, 14; SCHEMBL864319; BDBM27533; HGEPGGJUGUMFHT-UHFFFAOYSA-N; ZINC3821234; DB08058; NCGC00386677-01; KU-58948; FT-0670691; TL80090044; 4-[3-([1,4]diazepane-1-carbonyl)-4 -fluorobenzyl]-2H-phthalazin-1-one; 4-[3-([1,4]diazepane-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7 nM | |||
| External Link | ||||
| Niraparib Tosylate | Approved | [20] | ||
| Synonyms |
1038915-73-9; MK-4827 (tosylate); MK-4827 tosylate; UNII-75KE12AY9U; MK-4827(Niraparib) tosylate; 75KE12AY9U; MK-4827-tosylate; MK 4827 tosylate; Niraparib(MK-4827) tosylate; KS-00000TSH; MolPort-044-556-849; s7625; HY-10619B; AKOS030632785; CS-2283; AC-30383; KB-335358; AX8326059
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Nicaraven | Phase 3 | [21] | ||
| Synonyms |
AVS; Antevan; Antevas
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| CC-486 | Phase 3 | [22] | ||
| Synonyms |
AG-14361; AG14361; 328543-09-5; UNII-48N0U0K50I; AG 14361; CHEMBL65892; 48N0U0K50I; Imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one, 2-[4-[(dimethylamino)methyl]phenyl]-5,6-dihydro-; AG-014361; 1-(4-((dimethylamino)methyl)phenyl)-8,9-dihydro-2,7,9a-triazabenzo[cd]azulen-6(7H)-one; Imidazo(4,5,1-jk)(1,4)benzodiazepin-7(4H)-one, 2-(4-((dimethylamino)methyl)phenyl)-5,6-dihydro-; 2-[4-[(Dimethylamino)methyl]phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one; SMR000486393; MLS006011157; MLS001065917; Nucleoside analogue
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 5.8 nM | |||
| External Link | ||||
| AG140699 | Phase 2 | [23] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| AZD5305 | Phase 2 | [24] | ||
| Synonyms |
16MZ1V3RBT; 2589531-76-8; 2-Pyridinecarboxamide, 5-(4-((7-ethyl-5,6-dihydro-6-oxo-1,5-naphthyridin-3-yl)methyl)-1-piperazinyl)-N-methyl-; 2-Pyridinecarboxamide, 5-[4-[(7-ethyl-5,6-dihydro-6-oxo-1,5-naphthyridin-3-yl)methyl]-1-piperazinyl]-N-methyl-; 5-(4-((7-Ethyl-6-oxo-5,6-dihydro-1,5-naphthyridin-3-yl)methyl)piperazin-1-yl)-N-methylpicolinamide; 5-[4-[(7-Ethyl-6-oxo-5,6-dihydro-1,5-naphthyridin-3-yl)methyl]-1-piperazinyl]-N-methylpicolinamide; 5-[4-[(7-ethyl-6-oxo-5H-1,5-naphthyridin-3-yl)methyl]piperazin-1-yl]-N-methylpyridine-2-carboxamide; 5-{4-[(7-ethyl-5,6-dihydro-6-oxo-1,5-naphthyridin-3- yl)methyl]piperazin-1-yl}-N-methylpyridine-2- carboxamide; AC-37130; Azd 5305; AZD 5305 [WHO-DD]; AZD5305; AZD-5305; AZD-5305 [WHO-DD]; CHEMBL5095220; CS-0163534; E80364; EX-A5234; example 4 [WO2021013735]; GTPL11526; HY-132167; MS-26971; NSC834196; NSC-834196; Saruparib; saruparib [INN]; SCHEMBL22912701; SY295016; UNII-16MZ1V3RBT
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27841036-Compound-37 | Phase 2 | [25] | ||
| Synonyms |
2X-121
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2 nM | |||
| External Link | ||||
| Stenoparib | Phase 2 | [26] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| AMXI 5001 | Phase 1/2 | [27] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| NMS-03305293 | Phase 1 | [28] | ||
| Synonyms |
NMS-P293
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-oxo-2,3-dihydro-1H-indazole-4-carboxamide derivative 1 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-6
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 230 nM | |||
| External Link | ||||
| Benzimidazole carboxamide derivative 1 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-I
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 1 nM | |||
| External Link | ||||
| PMID27841036-Compound-33 | Patented | [25] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| 3-oxo-2,3-dihydro-1H-indazole-4-carboxamide derivative 4 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-9
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 44 nM | |||
| External Link | ||||
| Phthalazine ketone derivative 1 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-16
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 6000 nM | |||
| External Link | ||||
| Quinazolinedione derivative 3 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-13
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 140 nM | |||
| External Link | ||||
| 4-Carboxamido-isoindolinone derivative 2 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-3
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 11 nM | |||
| External Link | ||||
| 3-oxo-2,3-dihydro-1H-indazole-4-carboxamide derivative 3 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-8
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 15 nM; Kd < 10 nM | |||
| External Link | ||||
| 7-azaindole derivative 8 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-10
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100 nM | |||
| External Link | ||||
| 3-oxo-2,3-dihydro-1H-indazole-4-carboxamide derivative 5 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-IV
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Phthalazine ketone derivative 3 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-18
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 9000 nM | |||
| External Link | ||||
| Dihydropyrido phthalazinone derivative 1 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-21
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 800 nM | |||
| External Link | ||||
| Phthalazine ketone derivative 2 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-17
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 8000 nM | |||
| External Link | ||||
| Tricyclic indole compound 13 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-XVI
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 to 270 nM | |||
| External Link | ||||
| 3-phenyl isoquinolin-1(2H) derivative 2 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-VII
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Carboxamido-isoindolinone derivative 5 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-III
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Quinazolinedione derivative 2 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-12
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.37 nM | |||
| External Link | ||||
| Quinazolinedione derivative 1 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-11
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.21 nM | |||
| External Link | ||||
| Dihydropyrido phthalazinone derivative 2 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-22
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 800 nM | |||
| External Link | ||||
| 3-oxo-2,3-dihydro-1H-indazole-4-carboxamide derivative 2 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-7
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 26 nM; Kd < 10 nM | |||
| External Link | ||||
| Phthalazine derivative 3 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-19
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| Tetra-hydro-quinoline derivative 1 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-VIII
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1 to 500 nM | |||
| External Link | ||||
| 4-Carboxamido-isoindolinone derivative 1 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-2
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 20 nM | |||
| External Link | ||||
| 4-Carboxamido-isoindolinone derivative 3 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-4
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| 4-Carboxamido-isoindolinone derivative 4 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-5
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 20 nM | |||
| External Link | ||||
| Dihydrodiazepinocarbazolone derivative 1 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-26
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2.9 nM | |||
| External Link | ||||
| Tetra-cyclic pyridophthalazinone derivative 1 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-25
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5.1 nM | |||
| External Link | ||||
| 3-phenyl isoquinolin-1(2H) derivative 1 | Patented | [25] | ||
| Synonyms |
PMID27841036-Compound-20
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 10 nM | |||
| External Link | ||||
| CPH-102 | Preclinical | [29] | ||
| Synonyms |
IABP; INH2BP, Crimson Pharmaceutical; INH2BP, Octamer; PARP inhibitors, Crimson Pharmaceutical; PARP inhibitors, Octamer; CPH-101, Crimson Pharma
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PJ34 | Preclinical | [30] | ||
| Synonyms |
344458-19-1; pj-34; N~2~,N~2~-DIMETHYL-N~1~-(6-OXO-5,6-DIHYDROPHENANTHRIDIN-2-YL)GLYCINAMIDE; CHEMBL372303; P34; 2-(dimethylamino)-N-(6-oxo-5,6-dihydrophenanthridin-2-yl)acetamide; Acetamide, N-(5,6-dihydro-6-oxo-2-phenanthridinyl)-2-(dimethylamino)-; UYJZZVDLGDDTCL-UHFFFAOYSA-N; PJ34(free base); 1xk9; SCHEMBL422317; ZINC8960; AC1L1J45; BDBM27497; CTK1B7701; MolPort-035-395-737; Ibrutinib (PCI32765 pound(c); HMS3651B06; BCP07990; HY-13688A; 2662AH; AKOS030229047; SB19292; DB08348; CS-1463; NCGC00370866-10; DA-42692; BC600341
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10.9 nM | |||
| External Link | ||||
| NU1025 | Terminated | [31] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 48 nM | |||
| External Link | ||||
| 3-Ethynylquinoline-8-carboxamide | Investigative | [32] | ||
| Synonyms |
CHEMBL501330; 8-Quinolinecarboxamide, 3-ethynyl-; BDBM50255268
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2300 nM | |||
| External Link | ||||
| 2-(4-Amino-phenyl)-8-hydroxy-3H-quinazolin-4-one | Investigative | [33] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 289 nM | |||
| External Link | ||||
| N-(4-Phenylthiazol-2-yl)isonicotinamide | Investigative | [34] | ||
| Synonyms |
N-(4-phenyl-1,3-thiazol-2-yl)pyridine-4-carboxamide; CHEMBL482012; 5245-66-9; BAS 03572091; AC1Q5OBS; AC1LG7OX; CBMicro_015073; n-(4-phenyl-1,3-thiazol-2-yl)isonicotinamide; Cambridge id 5245669; Oprea1_303023; Oprea1_553111; SCHEMBL17107144; CTK4J5937; DTXSID30355050; MolPort-001-992-732; ZINC290573; STK483947; BDBM50255300; AKOS000569765; MCULE-9247268363; BIM-0015234.P001; N-(4-Phenyl-thiazol-2-yl)-isonicotinamide; ST50017829; N~4~-(4-phenyl-1,3-thiazol-2-yl)isonicotinamide; Z27772062
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 753 nM | |||
| External Link | ||||
| 5-Chloro-2-methyl-3H-quinazolin-4-one | Investigative | [35] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1200 nM | |||
| External Link | ||||
| BZ3 | Investigative | [36] | ||
| Synonyms |
5-METHOXYINDOLE; 1006-94-6; 5-Methoxy-1H-indole; 1H-Indole, 5-methoxy-; Femedol; 5-Methoxy indole; Indole, 5-methoxy-; Methoxy-5 indole; Indol-5-yl methyl ether; UNII-DQM3AS43PQ; Methoxy-5 indole [French]; 5-Methoxyindole, 99%; EINECS 213-745-3; DQM3AS43PQ; NSC 521752; CHEMBL280311; DWAQDRSOVMLGRQ-UHFFFAOYSA-N; MFCD00005674; 916979-77-6; 5Methoxyindole; 5-methoxyindol; 5-methoxy-indole; 3img; 3imc; PubChem7432; 1,3-dihydro-5-methyl-2H-Indol-2-one; ACMC-1BMAQ; 5-(methyloxy)-1H-indole; AC1Q4F1F; AC1L22NW; SCHEMBL74720; KSC177G3N; WLN: T
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(3'-Methoxyphenyl) Benzimidazole-4-Carboxamide | Investigative | [31] | ||
| Synonyms |
2-(3-methoxyphenyl)-1H-benzimidazole-4-carboxamide; CHEMBL134022; BZC; 1efy; AC1L1BMO; SCHEMBL4321727; CTK7A9229; NVVWVYYHTKCIAE-UHFFFAOYSA-N; ZINC11565446; BDBM50093373; DB04010; 2-(3'-Methoxyphenyl)-1-H-benzimidazole-4-carboxamide; 2-(3-methoxyphenyl)-1H-1,3-benzodiazole-4-carboxamide; 2-(3''-METHOXYPHENYL) BENZIMIDAZOLE-4-CARBOXAMIDE; 2-(3-Methoxy-phenyl)-1H-benzoimidazole-4-carboxylic acid amide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 6 nM | |||
| External Link | ||||
| CEP-6800 | Investigative | [37] | ||
| Synonyms |
UNII-0X7U7SRK9H; 0X7U7SRK9H; CHEMBL247374; 609848-02-4; SCHEMBL12256417; BDBM50197585; 1H-Cyclopenta(a)pyrrolo(3,4-C)carbazole-1,3(2H)-dione, 10-(aminomethyl)-4,5,6,7-tetrahydro-; 8-aminomethyl-1,2,3,11-tetrahydro-5,11-diaza-benzo[a]trindene-4,6-dione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 18 nM | |||
| External Link | ||||
| ANG-2864 | Investigative | [29] | ||
| Synonyms |
PARP inhibitor (ischemia/cancer), Angion Biomedica
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-Methoxybenzamide | Investigative | [38] | ||
| Synonyms |
5813-86-5; m-Methoxybenzamide; m-Anisamide; 3-Methoxy-benzamide; Benzamide, 3-methoxy-; UNII-M8502TLK98; EINECS 227-379-7; NSC 28589; NSC 209527; BRN 2206857; CHEMBL123978; VKPLPDIMEREJJF-UHFFFAOYSA-N; M8502TLK98; 3MB; 3pax; 5-methoxybenzamide; 3-methoxy-benzamid; ACMC-1ASRE; AC1Q5DMC; M-METHOXY BENZAMIDE; bmse000775; 3-Methoxybenzamide, 97%; Oprea1_695428; MLS001066418; 4-10-00-00326 (Beilstein Handbook Reference); cid_98487; SCHEMBL283787; AC1L407F; KS-00000VVU; CTK1H2082; VKPLPDIMEREJJF-UHFFFAOYSA-; DTXSID00206848
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 17000 nM | |||
| External Link | ||||
| 2-Benzyl-2H-indazole-7-carboxamide | Investigative | [39] | ||
| Synonyms |
CHEMBL1094951; SCHEMBL2268172
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 130 nM | |||
| External Link | ||||
| 5-methylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione | Investigative | [40] | ||
| Synonyms |
CHEMBL201723
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5000 nM | |||
| External Link | ||||
| 4-amino-1,8-naphthalimide | Investigative | [31] | ||
| Synonyms |
1742-95-6; 4-Aminonaphthalimide; 6-AMINO-BENZO[DE]ISOQUINOLINE-1,3-DIONE; 6-Amino-1H-benzo[de]isoquinoline-1,3(2H)-dione; 4-Aminonaphthalene-1,8-dicarboximide; Naphthalimide, 4-amino-; DFP 1; 1H-Benz[de]isoquinoline-1,3(2H)-dione, 6-amino-; EINECS 217-110-1; BRN 0177185; 6-aminobenzo[de]isoquinoline-1,3-dione; PARP Inhibitor V, 4-ANI; CHEMBL338790; CHEBI:40071; SSMIFVHARFVINF-UHFFFAOYSA-N; 6-Amino-1H-benz(de)isoquinoline-1,3(2H)-dione; 4-AMINO-1,8 NAPHTHALIMIDE
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 26 nM | |||
| External Link | ||||
| Pyrrolo[3,4-e]indole-1,3(2H,6H)-dione | Investigative | [40] | ||
| Synonyms |
CHEMBL373066; pyrroloisoindoledione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 750 nM | |||
| External Link | ||||
| 8-Amino-6H,11H-indeno[1,2-c]isoquinolin-5-one | Investigative | [41] | ||
| Synonyms |
SCHEMBL4662780; CHEMBL363363; BDBM27514; LQEYAIKMIJUZNT-UHFFFAOYSA-N; indeno[1,2-c]isoquinolinone, 2a; ZINC13652898; 5,6-Dihydro-5-oxo-8-amino-indeno[1,2-c]isoquinoline
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 300 nM | |||
| External Link | ||||
| EB-47 | Investigative | [23] | ||
| Synonyms |
EB 47; 366454-36-6; 5'-Deoxy-5'-[4-[2-[(2,3-dihydro-1-oxo-1H-isoindol-4-yl)amino]-2-oxoethyl]-1-piperazinyl]-5'-oxoadenosine Dihydrochloride; DTXSID40692822; ZINC98052573; NCGC00370771-01; KB-76747; FT-0667818; 4-[1-(6-Amino-9H-purin-9-yl)-1-deoxy-; A-D-ribofuranuronoyl]-N-(2,3-dihydro-1-oxo-1H-isoindol-4-yl)-1-piperazineacetamide Dihydrochloride; 2-{4-[(2R,3R,4S,5S)-5-(6-Amino-9H-purin-9-yl)-3,4-dihydroxyoxolane-2-carbonyl]piperazin-1-yl}-N-(1-oxo-2,3-dihydro-1H-isoindol-4-yl)acetamide (non-preferred name)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BZ6 | Investigative | [36] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(4-methoxyphenyl)quinoline-8-carboxamide | Investigative | [32] | ||
| Synonyms |
CHEMBL481591; 655222-47-2; CTK1J6622; DTXSID70649086; BDBM50255383; AKOS030560276; 8-Quinolinecarboxamide, 2-(4-methoxyphenyl)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1100 nM | |||
| External Link | ||||
| 9-Amino-6H,11H-indeno[1,2-c]isoquinolin-5-one | Investigative | [41] | ||
| Synonyms |
CHEMBL194155; SCHEMBL4078284; BDBM27515; BIBLEFNXUYTZIB-UHFFFAOYSA-N; indeno[1,2-c]isoquinolinone, 2b; ZINC13652899; 5,6-Dihydro-5-oxo-9-amino-indeno[1,2-c]isoquinoline
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 95 nM | |||
| External Link | ||||
| (E)-N-(4-Phenylthiazol-2-yl) cinnamamide | Investigative | [34] | ||
| Synonyms |
CHEMBL452100; 2-Cinnamamido-4-phenylthiazole; (2E)-3-phenyl-N-(4-phenyl-1,3-thiazol-2-yl)prop-2-enamide; 1107621-03-3; AC1LEPJR; ZINC60264; WOJRHCOBUKJCAJ-VAWYXSNFSA-N; MolPort-019-760-060; MolPort-001-931-977; HMS1397P15; STK173781; BDBM50255301; N-(4-phenylthiazol-2-yl)cinnamamide; AKOS000523355; MCULE-8934603681; BAS 00417267; ST4016450; 3-Phenyl-N-(4-phenyl-thiazol-2-yl)-acrylamide; AG-690/11629440; 3-phenyl-N-(4-phenyl-1,3-thiazol-2-yl)acrylamide; F0298-0058; A0793/0037152
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 224 nM | |||
| External Link | ||||
| ANG-2684 | Investigative | [29] | ||
| Synonyms |
ANG-3038; PARP-1 inhibitors (acute pancreatitis/stroke); PARP-1 inhibitors (acute pancreatitis/stroke), Angion Biomedica
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-methylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione | Investigative | [40] | ||
| Synonyms |
CHEMBL380940; SCHEMBL5828673
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2000 nM | |||
| External Link | ||||
| 2,3-dihydro-1H-benzo[de]isoquinolin-1-one | Investigative | [42] | ||
| Synonyms |
CHEMBL594596; 2,3-dihydro-benzo[de]isoquinolin-1-one; SCHEMBL832168; ZINC24216; BDBM50306285; FCH1866210
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 540 nM | |||
| External Link | ||||
| 5-amino-3,4-dihydroisoquinolin-1(2H)-one | Investigative | [42] | ||
| Synonyms |
129075-53-2; CHEMBL594759; SCHEMBL7581623; RTPKPVYTPRJRBY-UHFFFAOYSA-N; ZINC45353622; BDBM50306284; AKOS023598631; AB53700; FCH1123505; KS-9128; CM10348; AJ-110485; 5-amino-3,4-dihydro-1(2H)-isoquinolinone; EN300-254419
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 410 nM | |||
| External Link | ||||
| KR-33889 | Investigative | [29] | ||
| Synonyms |
KR-34285; PARP-1 inhibitors (myocardial infarction), KRICT
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-(5-Morpholin-4-yl-pentyl)-2H-phthalazin-1-one | Investigative | [43] | ||
| Synonyms |
CHEMBL194684
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 119 nM | |||
| External Link | ||||
| AG-014376 | Investigative | [44] | ||
| Synonyms |
CHEMBL361489; SCHEMBL7159231; BDBM50154730; 6-(4-Dimethylaminomethyl-phenyl)-3,4-dihydro-2H-[1,4]diazepino[6,7,1-hi]indol-1-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 6.4 nM | |||
| External Link | ||||
| 3-Hydroxy-benzamide | Investigative | [33] | ||
| Synonyms |
3-hydroxybenzamide; 618-49-5; benzamide, 3-hydroxy-; CHEMBL419424; 3-Hydroxy benzamide; NSC379289; 3-hydroxybenzenecarboxamide; AC1Q4ZB3; AC1L7W2U; ACMC-1B71B; Oprea1_435073; SCHEMBL161861; 3-Hydroxybenzamide, AldrichCPR; CTK2F7291; DTXSID90321635; NGMMGKYJUWYIIG-UHFFFAOYSA-N; MolPort-001-791-593; ZINC1590754; KM0548; BDBM50068769; 9282AB; ANW-33964; 3-HYDROXY-BENZOIC ACID,AMIDE; SBB079277; AKOS000207073; VZ26952; NSC-379289; MB00281; MCULE-9599926365; NCGC00323509-01; KB-32185; CJ-25437; AJ-27681; CJ-05592; SC-47787
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 9100 nM | |||
| External Link | ||||
| 8-Methoxy-2-phenyl-3H-quinazolin-4-one | Investigative | [33] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 10000 nM | |||
| External Link | ||||
| Quinoline-8-carboxamide | Investigative | [32] | ||
| Synonyms |
8-Quinolinecarboxamide; 55706-61-1; CCRIS 6967; CHEMBL502330; HPQRQAOVNXWEEQ-UHFFFAOYSA-N; 8-Carbamoylquinoline; 8-Quinolinecarboxamide #; AC1L44SC; SCHEMBL460456; CTK5A4047; quinoline-8-carboxylic acid amide; DTXSID00204243; MolPort-005-722-305; ZINC6095019; BDBM50255266; AKOS008969900; NE38855; MCULE-7978956907; LS-188644; KB-259682
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1900 nM | |||
| External Link | ||||
| 9-Fluoro-6H,11H-indeno[1,2-c]isoquinolin-5-one | Investigative | [41] | ||
| Synonyms |
CHEMBL190895; BDBM27513; indeno[1,2-c]isoquinolinone, 1e; 9-Fluoro-5,6-dihydro-11H-indeno[1,2-c]isoquinoline-5-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 180 nM | |||
| External Link | ||||
| 8-Methyl-2-(4-nitro-phenyl)-3H-quinazolin-4-one | Investigative | [33] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 104 nM | |||
| External Link | ||||
| 2H-Isoquinolin-1-one | Investigative | [33] | ||
| Synonyms |
1-Hydroxyisoquinoline; 491-30-5; Isoquinolin-1(2H)-one; isoquinolin-1-ol; 1-Isoquinolinol; Isocarbostyril; 1(2H)-ISOQUINOLINONE; Isoquinolin-1-one; 489453-23-8; 1(2H)-Isoquinolone; 1,2-dihydroisoquinolin-1-one; 1-hydroxyisoquinolin; isoquinolinol; 87602-67-3; 1(2H)-ISOQUINILONE; Isocarbostyril, 98%; UNII-95EG3HGG1P; 95EG3HGG1P; Isoquinolinone; CHEMBL339695; CHEBI:18350; VDBNYAPERZTOOF-UHFFFAOYSA-N; 1-isoquinolone; oxidoisoquinolinium; EINECS 207-732-1; Isocarbostyril(1-hydroxyisoquinoline); NSC 27273
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 9 nM | |||
| External Link | ||||
| KU-58684 | Investigative | [34] | ||
| Synonyms |
SCHEMBL863338; 623578-11-0
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2,8-Dimethyl-3H-quinazolin-4-one | Investigative | [33] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 490 nM | |||
| External Link | ||||
| 5-aminoisoquinolin-1(2H)-one | Investigative | [32] | ||
| Synonyms |
93117-08-9; 5-amino-2H-isoquinolin-1-one; 5-AMINOISOQUINOLIN-1-OL; 5-amino-1,2-dihydroisoquinolin-1-one; 5-AIQ; CHEMBL446240; 5-Amino-2H-isoquinoin-1-one; 5-AMINO-1(2H)-ISOQUINOLINONE; 5-aminoisoquinolinone; 4pnq; 32X; AC1Q6DVG; AC1L1CUR; 5-Amino-1-isoquinolinol; SCHEMBL215327; 5-Amino-1-hydroxyisoquinoline; BDBM27503; 5-amino-isoquinolin-1(2h)-one; 5-amino isoquinolin-1(2h)-one; CTK5H2122; DTXSID90274354; 1(2H)-Isoquinolinone,5-amino-; MolPort-008-423-043; MolPort-004-803-197
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 940 nM | |||
| External Link | ||||
| INO-1002 | Investigative | [29] | ||
| Synonyms |
PARP inhibitors, Inotek; PARP inhibitor (erectile dysfunction), Inotek; PARP inhibitor (prostate nerve damage), Inotek; Poly (ADP ribose) polymerase inhibitor (erectile dysfunction), Inotek; Poly (ADP ribose) polymerase inhibitor (prostate nerve damage), Inotek
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-(4-aminophenyl)quinoxaline-5-carboxamide | Investigative | [34] | ||
| Synonyms |
CHEMBL443077; quinoxaline analogue, 3f; SCHEMBL7112515
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 87 nM | |||
| External Link | ||||
| 2-phenylpyrazolo-[1,5-a]pyridine-7-carboxamide | Investigative | [39] | ||
| Synonyms |
2-phenylpyrazolo[1,5-a]pyridine-7-carboxamide; 1196713-16-2; ZINC64337832; DA-47424
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-benzylphthalazin-1(2H)-one | Investigative | [34] | ||
| Synonyms |
4-Benzyl-1(2H)-phthalazinone; 32003-14-8; 4-Benzyl-2H-phthalazin-1-one; 4-benzyl-1,2-dihydrophthalazin-1-one; CHEMBL66761; JUCCMEHWBGPJKS-UHFFFAOYSA-N; benzylphthalazinone; phthalazinone, 1; 4-benzyl-phthalazone; AC1LDDNC; SMR000135223; AC1Q6GZZ; ChemDiv2_000142; Cambridge id 5241846; Oprea1_151142; Oprea1_623913; CBDivE_015258; MLS000530246; SCHEMBL863462; CTK4G8063; BDBM27660; DTXSID30346948; MolPort-001-796-654; HMS2379K10; HMS1369G10; 4-benzyl-2-hydrophthalazin-1-one; 4-Benzyl-1(2H)-phthalazinone #
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 770 nM | |||
| External Link | ||||
| 3-Methylquinoline-8-carboxamide | Investigative | [32] | ||
| Synonyms |
CHEMBL504998; 8-Quinolinecarboxamide, 3-methyl-; BDBM50255267
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3400 nM | |||
| External Link | ||||
| 2-(4-Chlorophenyl)-5-Quinoxalinecarboxamide | Investigative | [38] | ||
| Synonyms |
3-(4-CHLOROPHENYL)QUINOXALINE-5-CARBOXAMIDE; 4tju; 1wok; AC1LCVX7; quinoxaline analogue, 3b; SCHEMBL424209; 3-(4-chloro-phenyl)-quinoxaline-5-carboxylic acid amide; CTK8F4675; BDBM27720; ZINC1489510; DB03509; 489457-67-2
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(4-Methoxy-phenyl)-8-methyl-3H-quinazolin-4-one | Investigative | [33] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 891 nM | |||
| External Link | ||||
| HYDAMTIQ | Investigative | [29] | ||
| Synonyms |
PARP-1 inhibitor (brain ischemia), University of Perugia
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-Nitro-6H,11H-indeno[1,2-c]isoquinolin-5-one | Investigative | [41] | ||
| Synonyms |
SCHEMBL4661629; CHEMBL370673; BDBM27510; SWBDUXDIPMGDNO-UHFFFAOYSA-N; indeno[1,2-c]isoquinolinone, 1b; ZINC13652894; 5,6-dihydro-5-oxo-8-nitro-indeno[1,2-c]isoquinoline; 8-Nitro-5,6-dihydro-11H-indeno[1,2-c]isoquinoline-5-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| PD-128763 | Investigative | [38] | ||
| Synonyms |
5-Methyl-3,4-dihydroisoquinolin-1(2H)-one; 129075-56-5; 3,4-Dihydro-5-methyl-1(2H)-isoquinolinone; 3,4-DIHYDRO-5-METHYL-ISOQUINOLINONE; 1(2H)-Isoquinolinone, 3,4-dihydro-5-methyl-; CHEBI:41928; PD128763; CHEMBL125200; 5-methyl-3,4-dihydro-2H-isoquinolin-1-one; 3,4-dihydro-5-methylisoquinolinone; 1(2H)-Isoquinolinone,3,4-dihydro-5-methyl-; 5-methyl-1,2,3,4-tetrahydroisoquinolin-1-one; DHQ; PD 128763; dihydroisoquinolinone, 1; AC1L3WIO; ACMC-1C7L4; SCHEMBL831538; AMBZ0075; KS-00000QFP; CTK4B6146; BDBM27682
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 70 nM | |||
| External Link | ||||
| 8-Methyl-2-phenyl-3H-quinazolin-4-one | Investigative | [33] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 795 nM | |||
| External Link | ||||
| TI3 | Investigative | [36] | ||
| Synonyms |
RB106; CHEMBL419499; [(2S)-2-SULFANYL-3-PHENYLPROPANOYL]-GLY-(5-PHENYLPROLINE); 1qf2; AC1L9LL2; BDBM50051785; DB02669; N-[(S)-2-Mercapto-3-phenylpropionyl]-Gly-[(5R)-5-phenyl-L-Pro-]-OH; N-[(2S)-3-phenyl-2-sulfanylpropanoyl]glycyl-(5R)-5-phenyl-L-proline; (2S,5R)-5-phenyl-1-[2-[[(2S)-3-phenyl-2-sulfanylpropanoyl]amino]acetyl]pyrrolidine-2-carboxylic acid; (2S,5R)-1-[2-((S)-2-Mercapto-3-phenyl-propionylamino)-acetyl]-5-phenyl-pyrrolidine-2-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-aminobenzo[c][1,5]naphthyridin-6(5H)-one | Investigative | [34] | ||
| Synonyms |
CHEMBL106154; Benzo[c]-1,5-naphthyridin-6(5H)-one, 3-amino-; SCHEMBL12750402; BDBM50130580; 433726-73-9
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 180 nM | |||
| External Link | ||||
| S-111 | Investigative | [29] | ||
| Synonyms |
PARP1 inhibitor (cancer), Sentinel Oncology
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Thieno-phenanthridin-6-one | Investigative | [23] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-Morpholin-4-ylmethyl-5H-phenanthridin-6-one | Investigative | [43] | ||
| Synonyms |
CHEMBL194535
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 23 nM | |||
| External Link | ||||
| 1,2,3,4,4a,5-hexahydrophenanthridin-6(10bH)-one | Investigative | [34] | ||
| Synonyms |
CHEMBL84044; 7403-93-2; NSC403412; AC1L83AN; SCHEMBL6645911; DTXSID70323238; BDBM50131013; NSC-403412; 2,3,4,4a,5,10b-hexahydro-1H-phenanthridin-6-one; 1,3,4,4a,5,10b-Hexahydro-2H-phenanthridin-6-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7500 nM | |||
| External Link | ||||
| 8-Hydroxy-2-(4-nitro-phenyl)-3H-quinazolin-4-one | Investigative | [33] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 105 nM | |||
| External Link | ||||
| 2-(4-Amino-phenyl)-8-methyl-3H-quinazolin-4-one | Investigative | [33] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 504 nM | |||
| External Link | ||||
| 4-(4-Morpholin-4-yl-butyl)-2H-phthalazin-1-one | Investigative | [43] | ||
| Synonyms |
CHEMBL373210
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 950 nM | |||
| External Link | ||||
| 2-phenyl-2H-benzo[d][1,2,3]triazole-4-carboxamide | Investigative | [39] | ||
| Synonyms |
CHEMBL1096560; SCHEMBL2265205
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 71 nM | |||
| External Link | ||||
| 2-phenyl-2H-indazole-7-carboxamide | Investigative | [39] | ||
| Synonyms |
CHEMBL594298; SCHEMBL1422404; BDBM50306166
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 24 nM | |||
| External Link | ||||
| Carba-Nicotinamide-Adenine-Dinucleotide | Investigative | [38] | ||
| Synonyms |
Carba-NAD; Carbanicotinamide adenine dinucleotide; 112345-60-5; AC1L4TS7; AC1Q5J0L; SCHEMBL16445201; 5'-o-{[({[(1r,2r,3s,4r)-4-(3-carbamoylpyridinium-1-yl)-2,3-dihydroxycyclopentyl]methoxy}phosphinato)oxy](hydroxy)phosphoryl}adenosine; [[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [(1R,2R,3S,4R)-4-(3-carbamoylpyridin-1-ium-1-yl)-2,3-dihydroxycyclopentyl]methyl phosphate; Adenosine 5'-(trihydrogen diphosphate), 5'-((4-(3-(aminocarbonyl)pyridinio)-2,3-dihydroxycyclopentyl)m
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-aminobenzamide | Investigative | [30] | ||
| Synonyms |
3544-24-9; m-Aminobenzamide; Benzamide, 3-amino-; 3-Amino-benzamide; Benzamide, m-amino-; meta-aminobenzamide; 3-ABA; 3-Aminobenzimide; 3-amino benzamide; aniline-3-carboxamide; 3-aminobenzoic acid amide; UNII-8J365YF1YH; CCRIS 3925; EINECS 222-586-9; NSC 36962; BRN 2802373; PARP Inhibitor I, 3-ABA; 3-H2NC6H4CONH2; CHEMBL81977; 3-AB; 8J365YF1YH; CHEBI:64042; GSCPDZHWVNUUFI-UHFFFAOYSA-N; MFCD00007989; 3-Aminobenzamide, 98%; SR-01000075657; HSDB 7581; 3-azanylbenzamide; m-amino benzamide; 4pml; 3-Aminobenzaminde
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 3100 nM | |||
| External Link | ||||
| 3-Ethenylquinoline-8-carboxamide | Investigative | [32] | ||
| Synonyms |
CHEMBL481793; 8-Quinolinecarboxamide, 3-ethenyl-; BDBM50255270
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5800 nM | |||
| External Link | ||||
| 3-Phenylquinoline-8-carboxamide | Investigative | [32] | ||
| Synonyms |
CHEMBL450259; BDBM50255264
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 15000 nM | |||
| External Link | ||||
| 3-(4-methoxyphenyl)quinoxaline-5-carboxamide | Investigative | [34] | ||
| Synonyms |
CHEMBL519443; quinoxaline analogue, 3e; SCHEMBL7045177
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 71 nM | |||
| External Link | ||||
| TI4 | Investigative | [36] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Benzo[c][1,5]naphthyridin-6(5H)-one | Investigative | [34] | ||
| Synonyms |
CHEMBL320031; Benzo[c]-1,5-naphthyridin-6(5H)-one; SCHEMBL12750384; BDBM50130585; 5H-Benzo[c][1,5]naphthyridin-6-one; Benzo[c][1,5]naphthyridine-6(5H)-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 116 nM | |||
| External Link | ||||
| 3-(4-cyanophenyl)quinoxaline-5-carboxamide | Investigative | [34] | ||
| Synonyms |
CHEMBL481603; quinoxaline analogue, 3c; SCHEMBL7108289
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 101 nM | |||
| External Link | ||||
| 1,7,8,9-tetrahydro-1,5-diaza-trindene-4,6-dione | Investigative | [40] | ||
| Synonyms |
CHEMBL201907; SCHEMBL2086780; ZINC28569089
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 40 nM | |||
| External Link | ||||
| 8-Fluoro-6H,11H-indeno[1,2-c]isoquinolin-5-one | Investigative | [41] | ||
| Synonyms |
CHEMBL370045; BDBM27512; indeno[1,2-c]isoquinolinone, 1d; ZINC13652896; 8-Fluoro-5,6-dihydro-11H-indeno[1,2-c]isoquinoline-5-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 225 nM | |||
| External Link | ||||
| 8-Hydroxy-2-phenyl-3H-quinazolin-4-one | Investigative | [33] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1034 nM | |||
| External Link | ||||
| 8-Methoxy-2-(4-nitro-phenyl)-3H-quinazolin-4-one | Investigative | [33] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 10000 nM | |||
| External Link | ||||
| 2-(4-Chlorophenyl)-2H-indazole-7-carboxamide | Investigative | [39] | ||
| Synonyms |
CHEMBL1099295; SCHEMBL2264064
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 24 nM | |||
| External Link | ||||
| BPI-704001 | Investigative | [29] | ||
| Synonyms |
BPI-705001; BPI-715001; PARP-1 inhibitors (cancer); PARP-1 inhibitors (cancer), Beta Pharma
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione | Investigative | [40] | ||
| Synonyms |
CHEMBL370869; SCHEMBL5381581
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10000 nM | |||
| External Link | ||||
| 2-Methylquinoline-8-carboxamide | Investigative | [32] | ||
| Synonyms |
CHEMBL471966; 8-Quinolinecarboxamide, 2-methyl-; SCHEMBL422282; BDBM50255329; AKOS022882220
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| 8-Methoxy-2-methyl-3H-quinazolin-4-one | Investigative | [33] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-Prop-1-ynylquinoline-8-carboxamide | Investigative | [32] | ||
| Synonyms |
CHEMBL504903; ZINC40829471
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2200 nM | |||
| External Link | ||||
| 2-(2-Chlorophenyl)-2H-indazole-7-carboxamide | Investigative | [39] | ||
| Synonyms |
CHEMBL1094952; SCHEMBL2265265
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| 2-(3-Piperidin-1-yl-propyl)-3H-quinazolin-4-one | Investigative | [35] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1100 nM | |||
| External Link | ||||
| 3-Ethylquinoline-8-carboxamide | Investigative | [32] | ||
| Synonyms |
CHEMBL453989; 8-Quinolinecarboxamide, 3-ethyl-; BDBM50255269
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3700 nM | |||
| External Link | ||||
| A-620223 | Investigative | [29] | ||
| Synonyms |
A-866111; A-966492; ABT-472; ABT-999; PARP inhibitor, Abbott; PARP inhibitors, Abbott; Poly (ADP)ribose polymer inhibitor, Abbott; Poly (ADP)ribose inhibitors (cancer), Abbott
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 3 nM | |||
| External Link | ||||
| DR2313 | Investigative | [23] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(4-Azido-phenyl)-8-methoxy-3H-quinazolin-4-one | Investigative | [33] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(3-Chlorophenyl)-2H-indazole-7-carboxamide | Investigative | [39] | ||
| Synonyms |
CHEMBL1094953; SCHEMBL2265628
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 14 nM | |||
| External Link | ||||
| 2-(4-Hydroxy-phenyl)-8-methyl-3H-quinazolin-4-one | Investigative | [33] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1200 nM | |||
| External Link | ||||
| 2-ethylquinoline-8-carboxamide | Investigative | [32] | ||
| Synonyms |
CHEMBL526128; 8-Quinolinecarboxamide, 2-ethyl-; BDBM50255330
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 800 nM | |||
| External Link | ||||
| 2-phenylquinoline-8-carboxamide | Investigative | [32] | ||
| Synonyms |
CHEMBL480429; SCHEMBL6442515; ZINC3939668
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 900 nM | |||
| External Link | ||||
| BZ5 | Investigative | [36] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| ME0328 | Investigative | [45] | ||
| Synonyms |
ME-0328; compound 5b [PMID 24188023]
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 6300 nM | |||
| External Link | ||||
| [2(R,S)-2-Sulfanylheptanoyl]-Phe-Ala | Investigative | [36] | ||
| Synonyms |
TI1; AC1NRDCJ; 1qf1; DB02597; N-[(2S)-2-sulfanylheptanoyl]-L-phenylalanyl-L-alanine; N-[N-[(S)-2-Mercaptoheptanoyl]-L-phenylalanyl]-L-alanine; (2S)-2-[[(2S)-3-phenyl-2-[[(2S)-2-sulfanylheptanoyl]amino]propanoyl]amino]propanoic acid; (2S)-2-[(2S)-3-phenyl-2-[(2S)-2-sulfanylheptanamido]propanamido]propanoic
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| S-070 | Investigative | [29] | ||
| Synonyms |
S-158; Targeted synergy program (solid tumor), Sentinel Oncology; Chk1/PARP-1 inhibitors (cancer); Chk1/PARP-1 inhibitors (cancer), Sentinel Oncology
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2B72: Gastric cancer | 81 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Leniolisib | Approved | [46] | ||
| Synonyms |
1354690-24-6; Leniolisib free base; UNII-L22772Z9CP; (S)-1-(3-((6-(6-methoxy-5-(trifluoromethyl)pyridin-3-yl)-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4-yl)amino)pyrrolidin-1-yl)propan-1-one; L22772Z9CP; 1354690-24-6 (free base); leniolisib(CDZ 173); CDZ173; CDZ-173; 1-[(3S)-3-[[6-[6-methoxy-5-(trifluoromethyl)pyridin-3-yl]-7,8-dihydro-5H-pyrido[4,3-d]pyrimidin-4-yl]amino]pyrrolidin-1-yl]propan-1-one; Leniolisib [INN]; Leniolisib (CDZ173); Leniolisib (USAN/INN); CDZ173-NX; SCHEMBL323054; GTPL9424; CHEMBL3643413; BDBM118299; EX-A2854; MFCD30470232; s8752; ZB1510; CS-7524; DC22326; SB18839; Example 67 [WO2012004299]; 1-{(S)-3-[6-(6-methoxy-5-trifluoromethyl-pyridin-3-yl)-5,6,7,8-tetrahydro-pyrido[4,3-d]pyrimidin-4-y; 1-{(S)-3-[6-(6-methoxy-5-trifluoromethyl-pyridin-3-yl)-5,6,7,8-tetrahydro-pyrido[4,3-d]pyrimidin-4-ylamino]-pyrrolidin-1-yl}-propan-1-one; AS-56217; HY-17635; A16796; D11158; US8653092, 67; Q27282602; 1-Propanone, 1-((3S)-3-((5,6,7,8-tetrahydro-6-(6-methoxy-5-(trifluoromethyl)-3-pyridinyl)pyrido(4,3-d)pyrimidin-4-yl)amino)-1-pyrrolidinyl)-; 9NQ
Click to Show/Hide
|
|||
| External Link | ||||
| Atezolizumab | Approved | [5] | ||
| External Link | ||||
| Bavencio | Approved | [5] | ||
| External Link | ||||
| Tebentafusp | Approved | [47] | ||
| External Link | ||||
| Merimepodib | Approved | [48] | ||
| Synonyms |
Merimebodib; Merimepodib [USAN:INN]; Tyverb/Tykerb; MMPD; 198821-22-6; 2ZL2BA06FU; C23H24N4O6; CHEMBL304087; MERIMEPODIB, VI-21497, VX-497; UNII-2ZL2BA06FU; VI-21497; VX-497; VX497; Vx 497; carbamic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Taxol | Approved | [49] | ||
| Synonyms |
C47H51NO14; weekly paclitaxel; Micellar Paclitaxel; Paclitaxel [USAN:INN:BAN]; SCHEMBL15000506; Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, (2aR-(2aalpha,4beta,4abeta,6beta,9alpha(alphaR*,betaS*),11alpha,12alpha,12aalpha,12balpha))-
Click to Show/Hide
|
|||
| External Link | ||||
| Ramucirumab | Approved | [50] | ||
| Synonyms |
LY3009806
Click to Show/Hide
|
|||
| External Link | ||||
| Tucatinib | Approved | [51] | ||
| Synonyms |
Irbinitinib; 937263-43-9; ONT-380; UNII-234248D0HH; 234248D0HH; N6-(4,4-Dimethyl-4,5-dihydrooxazol-2-yl)-N4-(3-methyl-4-((1,2,4)triazolo(1,5-a)pyridin-7-yloxy)phenyl)quinazoline-4,6-diamine; 4,6-Quinazolinediamine, N6-(4,5-dihydro-4,4-dimethyl-2-oxazolyl)-N4-(3-methyl-4-((1,2,4)triazolo(1,5-a)pyridin-7-yloxy)phenyl)-; ONT 380; 4,6-QuinazolinediaMine, N6-(4,5-dihydro-4,4-diMethyl-2-oxazolyl)-N4-[3-Methyl-4-([1,2,4]triazolo[1,5-a]pyridin-7-yloxy)phenyl]-; Tucatinib [USAN:INN]; 6-DIAMINE
Click to Show/Hide
|
|||
| External Link | ||||
| Antacids | Approved | [52] | ||
| External Link | ||||
| Trastuzumab | Approved | [5] | ||
| Synonyms |
Herceptin; Herceptin (TN); Trastuzumab (INN); Trastuzumab (genetical recombination); Trastuzumab (genetical recombination) (JAN); Trastuzumab (ERBB2 mAb inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| Carbamazepine | Phase 3 | [53] | ||
| Synonyms |
Carbamazepine (iv, epilepsy); Carbamazepine (iv, epilepsy), Lundbeck; Carbamazepine (iv, epilepsy), Ovation Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| Margetuximab | Approved | [5] | ||
| External Link | ||||
| Nivolumab | Approved | [5] | ||
| External Link | ||||
| GRANITE | Phase 3 | [54] | ||
| Synonyms |
Penoxsulam; 219714-96-2; 2-(2,2-difluoroethoxy)-N-(5,8-dimethoxy-[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; UNII-784ELC1SCZ; 784ELC1SCZ; CHEBI:81776; 2-(2,2-difluoroethoxy)-n-(5,8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; Penoxsulam [ISO]; PXD; DSSTox_CID_14803; DSSTox_RID_79204; DSSTox_GSID_34803; SCHEMBL116968; CHEMBL1895913; DTXSID0034803; HSDB 7887; AMY12535; BCP18718; EBD18529; Tox21_301010; MFCD07363876; ZINC13827750; AKOS025401685; NCGC00163715-01; NCGC00163715-02; NCGC00163715-03; NCGC00254912-01; AC-24494; Penoxsulam 100 microg/mL in Acetonitrile; CAS-219714-96-2; FT-0696708; Penoxsulam, PESTANAL(R), analytical standard; C18481; Q22808507; 2-(2,2-Difluoroethoxy)-6-trifluoromethyl-N-(5, 8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)benzenesulfonamide; 2-(2,2-Difluoroethoxy)-N-(5,8-dimethoxy[1,2,4]-triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; 2-(2,2-difluoroethoxy)-N-{5,8-dimethoxy-[1,2,4]triazolo[1,5-c]pyrimidin-2-yl}-6-(trifluoromethyl)benzene-1-sulfonamide; 2-(2,2-difluoroethyl)-N-(5,8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; Benzenesulfonamide, 2-(2,2-difluoroethoxy)-N-(5,8-dimethoxy(1,2,4)triazolo(1,5-c)pyrimidin-2-yl)-6-(trifluoromethyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| Zolbetuximab | Phase 3 | [55] | ||
| Synonyms |
IMAB362
Click to Show/Hide
|
|||
| External Link | ||||
| Tusamitamab ravtansine | Phase 3 | [56] | ||
| Synonyms |
SAR408701
Click to Show/Hide
|
|||
| External Link | ||||
| Andecaliximab | Phase 3 | [57] | ||
| External Link | ||||
| ABP 980 | Phase 3 | [58] | ||
| External Link | ||||
| GS-5745 | Phase 3 | [49] | ||
| External Link | ||||
| S-1 | Phase 3 | [59] | ||
| Synonyms |
Ciprofibrate-coa; Ciprofibrate-coenzyme A; Coenzyme A, ciprofibrate-; AC1L4TRG; AC1Q3T4H; 111900-25-5; s-{1-[(2r,3s,4r,5r)-5-(6-amino-9h-purin-9-yl)-4-hydroxy-3-(phosphonooxy)tetrahydrofuran-2-yl]-3,5,9-trihydroxy-8,8-dimethyl-3,5-dioxido-10,14-dioxo-2,4,6-trioxa-11,15-diaza-3; E5,5; E5-diphosphaheptadecan-17-yl} 2-[4-(2,2-dichlorocyclopropyl)phenoxy]-2-methylpropanethioate(non-preferred name); Coenzyme A, S-(2-(4-(2,2-dichlorocyclopropyl)phenoxy)-2-methylpropanoate)
Click to Show/Hide
|
|||
| External Link | ||||
| Lonsurf | Phase 3 | [5] | ||
| External Link | ||||
| GDC-0068 | Phase 3 | [49] | ||
| Synonyms |
RG7440
Click to Show/Hide
|
|||
| External Link | ||||
| Edotecarin | Phase 3 | [60] | ||
| Synonyms |
ED-749; Edotecarin < Prop INN; J-107088; PF-804950; 12-(beta-D-Glucopyranosyl)-2,10-dihydroxy-6-[2-hydroxy-1-(hydroxymethyl)ethylamino]-6,7,12,13-tetrahydro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-5,7-dione
Click to Show/Hide
|
|||
| External Link | ||||
| RG3638 | Phase 3 | [61] | ||
| Synonyms |
Onartuzumab
Click to Show/Hide
|
|||
| External Link | ||||
| G17DT | Phase 3 | [62] | ||
| Synonyms |
Gastrimmune; Insegia
Click to Show/Hide
|
|||
| External Link | ||||
| DE-766 | Phase 3 | [63] | ||
| External Link | ||||
| Tesetaxel | Phase 2 | [64] | ||
| Synonyms |
DJ-927; 333754-36-2; UNII-UG97LO5M8Y; UG97LO5M8Y; Tesetaxel [INN]; DJ927; DJ 927; CHEMBL2107787; SCHEMBL12060837; DB12019; Z-3104; (2AS,2BR,3S,4S,6S,8AR,10R,11AS,11BR,13AR)-2A-ACETOXY-6-(((2R,3S)-3-((TERT-BUTOXYCARBONYL)AMINO)-3-(3-FLUOROPYRIDIN-2-YL)-2-HYDROXYPROPANOYL)OXY)-10-((DIMETHYLAMINO)METHYL)-4-HYDROXY-7,11B,14,14-TETRAMETHYL-2A,2B,3,4,5,6,8A,11A,11B,12,13,13A-DODECAHYDRO-2H-4,8-METHANOOXETO[3'',2'':3',4']BENZO[1',2':3,4]CYCLODECA[1,2-D][1,3]DIOXOL-3-YL BENZOATE
Click to Show/Hide
|
|||
| External Link | ||||
| Nelipepimut S | Phase 3 | [65] | ||
| Synonyms |
E75
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-986205 | Phase 3 | [5] | ||
| Synonyms |
KRTIYQIPSAGSBP-KLAILNCOSA-N; 1923833-60-6; BMS986205; UNII-0A7729F42K; 0A7729F42K; GTPL9707; SCHEMBL18826792; SCHEMBL17740982; SCHEMBL19105151; EX-A2606; AKOS032954040; HY-101560; CS-0021719; Q29213697; (R)-N-(4-chlorophenyl)-2-((1s,4S)-4-(6-fluoroquinolin-4-yl)cyclohexyl)propanamide; (2R)-N-(4-chlorophenyl)-2-[4-(6-fluoroquinolin-4-yl)cyclohexyl]propanamide; (2R)-N-(4-Chlorophenyl)-2-(4-(6-fluoro-4-quinolyl)cyclohexyl)propanamide, cis; Cyclohexaneacetamide, N-(4-chlorophenyl)-4-(6-fluoro-4-quinolinyl)-alpha-methyl-, cis-(alphaR)-
Click to Show/Hide
|
|||
| External Link | ||||
| Rivoceranib | Phase 3 | [5] | ||
| External Link | ||||
| Claudiximab | Phase 3 | [5] | ||
| Synonyms |
IMAB-362; Anti-GC182 mAbs (cancer), Ganymed; Anti-GC182 monoclonal antibodies (cancer), Ganymed; Anti-CLDN18-2 mAbs (cancer), Ganymed; Anti-CLDN18-2 monoclonal antibodies (cancer), Ganymed
Click to Show/Hide
|
|||
| External Link | ||||
| OS-440 | Phase 3 | [66] | ||
| Synonyms |
CNS modulator (spasticity), Osmotica
Click to Show/Hide
|
|||
| External Link | ||||
| Oraxol | Phase 3 | [5] | ||
| External Link | ||||
| ICI 118,551 | Phase 3 | [49] | ||
| Synonyms |
Ici 118551; (2R,3S)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-3-(propan-2-ylamino)butan-2-ol; CHEMBL198059; CHEBI:73289; ICI-118551; ICI118551; erythro-DL-1-(7-Methylindan-4-yloxy)-3-isopropylaminobutan-2-ol; (2R,3S)-3-(isopropylamino)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]butan-2-ol; 2-Butanol, 1-((2,3-dihydro-7-methyl-1H-inden-4-yl)oxy)-3-((1-methylethyl)amino)-, (2R,3S)-rel-; 2-Butanol, 1-((2,3-dihydro-7-methyl-1H-inden-4-yl)oxy)-3-((1-methylethyl)amino)-, (R*,S*)-(+-)-; ICI-118,551; Ici 111,581; AC1NUNSO
Click to Show/Hide
|
|||
| External Link | ||||
| Evorpacept | Phase 2/3 | [67] | ||
| Synonyms |
ALX148
Click to Show/Hide
|
|||
| External Link | ||||
| BNT141 | Phase 2 | [68] | ||
| External Link | ||||
| Anti-LAG3 | Phase 2 | [58] | ||
| External Link | ||||
| GSK1292263 | Phase 2 | [69] | ||
| External Link | ||||
| MM-111 | Phase 2 | [70] | ||
| External Link | ||||
| Plevitrexed | Phase 2 | [71] | ||
| Synonyms |
ZD 9331; ZD9331; 153537-73-6; Plevitrexed [INN]; ZD-9331; NSC 696259; UNII-L9P2881C3H; CHEMBL126648; (2s)-2-[(2-fluoro-4-{[(4-hydroxy-2,7-dimethylquinazolin-6-yl)methyl](prop-2-yn-1-yl)amino}benzoyl)amino]-4-(2h-tetrazol-5-yl)butanoic acid; L9P2881C3H; Plevitrexed (INN); 172521-94-7; (2S)-2-[[4-[(2,7-dimethyl-4-oxo-1H-quinazolin-6-yl)methyl-prop-2-ynylamino]-2-fluorobenzoyl]amino]-4-(2H-tetrazol-5-yl)butanoic acid; 1H-Tetrazole-5-butanoic acid,
Click to Show/Hide
|
|||
| External Link | ||||
| DS-8201 | Phase 1 | [58] | ||
| Synonyms |
9-Aminofluorene; 9H-Fluoren-9-amine; 525-03-1; FLUOREN-9-AMINE; Fluoren-9-ylamine; UNII-4NHO2K4K5B; CCRIS 7000; BRN 2209545; 4NHO2K4K5B; OUGMRQJTULXVDC-UHFFFAOYSA-N; fluorene-9-ylamine; 9-Amino-fluoren; 9-amino-fluorene; 9H-9-fluorenamine; 9H-fluoren-9-yl-amine; AC1L1VP5; 4-12-00-03390 (Beilstein Handbook Reference); SCHEMBL353865; AC1Q53A2; AC1Q53A1; KS-00000JGC; CTK1H0380; DTXSID90200496; MolPort-001-794-448; HMS1780P20; 9H-fluoren-9-ylamine hydrochloride; ZINC1724407; ALBB-023296; CA-733; SBB005783; AKOS000264388; MCULE-8757055914; DS-
Click to Show/Hide
|
|||
| External Link | ||||
| XL880 | Phase 2 | [72] | ||
| Synonyms |
GSK 089; GSK 1363089; GSK1363089; XL 880; GSK1363089, GSK089, foretinib, EXEL-2880, XL880; 88Z; MET inhibitors
Click to Show/Hide
|
|||
| External Link | ||||
| Matuzumab | Phase 2 | [73] | ||
| Synonyms |
EMD-62000; EMD-72000; Anti-EGF receptor humanized antibody (iv, cancer), Merck KGaA/ Takeda Pharmaceuticals; Anti-EGFR humanized mAb (iv, cancer), Merck KGaA/ Takeda Pharmaceuticals; Anti-epidermal growth factor receptor humanized antibody (iv, cancer), Merck KGaA/ Takeda Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| BAY-57-9352 | Phase 2 | [49] | ||
| Synonyms |
Telatinib; Bay 57-9352
Click to Show/Hide
|
|||
| External Link | ||||
| Bemarituzumab | Phase 2 | [74] | ||
| External Link | ||||
| PEGPH20 | Phase 2 | [5] | ||
| External Link | ||||
| Plevitrexed (R)-isomer | Phase 2 | [75] | ||
| Synonyms |
YW3548
Click to Show/Hide
|
|||
| External Link | ||||
| APR-246 | Phase 2 | [76] | ||
| Synonyms |
Eprenetapopt
Click to Show/Hide
|
|||
| External Link | ||||
| CRS-207 | Phase 2 | [65] | ||
| External Link | ||||
| Opdivo + Yervoy | Phase 3 | [5] | ||
| External Link | ||||
| CT-041 | Phase 1/2 | [77] | ||
| External Link | ||||
| BPX-601 | Phase 1/2 | [78] | ||
| External Link | ||||
| Anti-MUC1 CAR-T cells | Phase 1/2 | [79] | ||
| External Link | ||||
| Anti-Mesothelin CAR-T cells | Phase 1/2 | [80] | ||
| External Link | ||||
| Anti-HER2 CAR-T | Phase 1/2 | [81] | ||
| External Link | ||||
| CAR-T Cells targeting EpCAM | Phase 1/2 | [82] | ||
| External Link | ||||
| PAT-SC1 | Phase 1/2 | [83] | ||
| Synonyms |
SC-1; Adjuvant therapy (gastric cancer), University of Wurzburg; SC-1 (gastric cancer), CAT; SC-1 (gastric cancer), Debiopharm; SC-1 (gastric cancer), Patrys; SC-1 (stomach cancer), OncoMab
Click to Show/Hide
|
|||
| External Link | ||||
| ASP2138 | Phase 1 | [84] | ||
| External Link | ||||
| SAR443216 | Phase 1 | [85] | ||
| External Link | ||||
| AMG 199 | Phase 1 | [86] | ||
| External Link | ||||
| AMG 910 | Phase 1 | [87] | ||
| External Link | ||||
| Alofanib | Phase 1 | [88] | ||
| Synonyms |
1612888-66-0; 3-(N-(4-methyl-2-nitro-5-(pyridin-3-yl)phenyl)sulfamoyl)benzoic acid; RPT-835(alofanib); UNII-LQX7RFK8MZ; RPT-835; RPT835; LQX7RFK8MZ; ES000835; Alofanib [INN]; Alofanib(RPT835); Syn007154; CHEMBL4594436; SCHEMBL18660613; AMY16650; BCP31905; EX-A2731; MFCD30533418; NSC790182; s8754; Benzoic acid, 3-(((4-methyl-2-nitro-5-(3-pyridinyl)phenyl)amino)sulfonyl)-; NSC-790182; SB19665; AC-31695; AK668992; AS-56846; HY-17601; CS-0014684; RPT 835; Q27283135; 3-{[4-methyl-2-nitro-5-(pyridin-3-yl)phenyl]sulfamoyl}benzoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| HER2-specific CAR T cell | Phase 1 | [89] | ||
| External Link | ||||
| Anti-CEA-CAR T | Phase 1 | [90] | ||
| External Link | ||||
| XR-5944 | Phase 1 | [91] | ||
| Synonyms |
MLN-944; XR-11576 analogs; XR-5000 analogs; XR-5942
Click to Show/Hide
|
|||
| External Link | ||||
| A168 | Phase 1 | [92] | ||
| External Link | ||||
| EGFR806-specific CAR T cell | Phase 1 | [93] | ||
| External Link | ||||
| AbGn-107 | Phase 1 | [5] | ||
| External Link | ||||
| FPA144 | Phase 1 | [58] | ||
| External Link | ||||
| Minnelide 001 | Phase 1 | [49] | ||
| External Link | ||||
| CAR-T cells targeting EpCAM | Phase 1 | [94] | ||
| External Link | ||||
| Anti-CEA CAR-T cells | Phase 1 | [95] | ||
| External Link | ||||
| EPCAM-targeted CAR-T cells | Clinical trial | [96] | ||
| External Link | ||||
| PMID28460551-Compound-1 | Patented | [97] | ||
| External Link | ||||
| Conjugated 3-(indolyl)-and 3-(azaindolyl)-4-arylmaleimide compound 1 | Patented | [98] | ||
| Synonyms |
PMID28621580-Compound-WO2012084683c62
Click to Show/Hide
|
|||
| External Link | ||||
| TOPIXANTRONE HYDROCHLORIDE | Discontinued in Phase 2 | [99] | ||
| Synonyms |
SCHEMBL1418986; Topixantrone hydrochloride < Prop INNM; BBR-3409 (dimaleate); 5-[2-(Dimethylamino)ethylamino]-2-[2-(2-hydroxyethylamino)ethyl]indazolo[4,3-gh]isoquinolin-6(2H)-one dihydrochloride
Click to Show/Hide
|
|||
| External Link | ||||
| MDL 101,731 | Discontinued in Phase 2 | [100] | ||
| Synonyms |
Tezacitabine; Fmdc cpd; 130306-02-4; UNII-7607Y95N9S; Mdl 101731; (E)-2'-Deoxy-2'-(fluoromethylene) cytidine; MDL-101731; 2'-Deoxy-2'-(fluoromethylene)cytidine; 7607Y95N9S; Cytidine, 2'-deoxy-2'-(fluoromethylene)-, (2E)-; (E)-2'-Deoxy-2'-(fluoromethylene)cytidine; Tezacitabine [INN]; tezaciabine; Tezacitabine, anhydrous; AC1O5KIG; SCHEMBL18724; SCHEMBL18725; Tezacitabine, anhydrous [INN]; CHEMBL2105467; C10H12FN3O4; DTXSID10156446; GFFXZLZWLOBBLO-ASKVSEFXSA-N; ZINC3777826; KW-2331
Click to Show/Hide
|
|||
| External Link | ||||
| BBR-3438 | Discontinued in Phase 2 | [101] | ||
| Synonyms |
Nortopixantrone; UNII-PH2639TAB4; PH2639TAB4; Nortopixantrone [INN:BAN]; AC1MI4ZO; CHEMBL150303; SCHEMBL7804438
Click to Show/Hide
|
|||
| External Link | ||||
| IPI-493 | Discontinued in Phase 1 | [102] | ||
| Synonyms |
[(3R,5R,6S,7R,8E,10R,11R,12Z,14E)-21-amino-6-hydroxy-5,11-dimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-17-azabicyclo[16.3.1]docosa-1(21),8,12,14,18-pentaen-10-yl] carbamate; AC1NS08X; SCHEMBL16226496; SCHEMBL16225851
Click to Show/Hide
|
|||
| External Link | ||||
| Kanjinti | Application submitted | [5] | ||
| External Link | ||||
| Anti-CD9 mab | Investigative | [103] | ||
| Synonyms |
ALB-6; Anti-CD9 mAb (gastric cancer); Anti-CD9 mAb (gastric cancer), Osaka University
Click to Show/Hide
|
|||
| External Link | ||||
References