m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03638
|
[1], [2] | |||
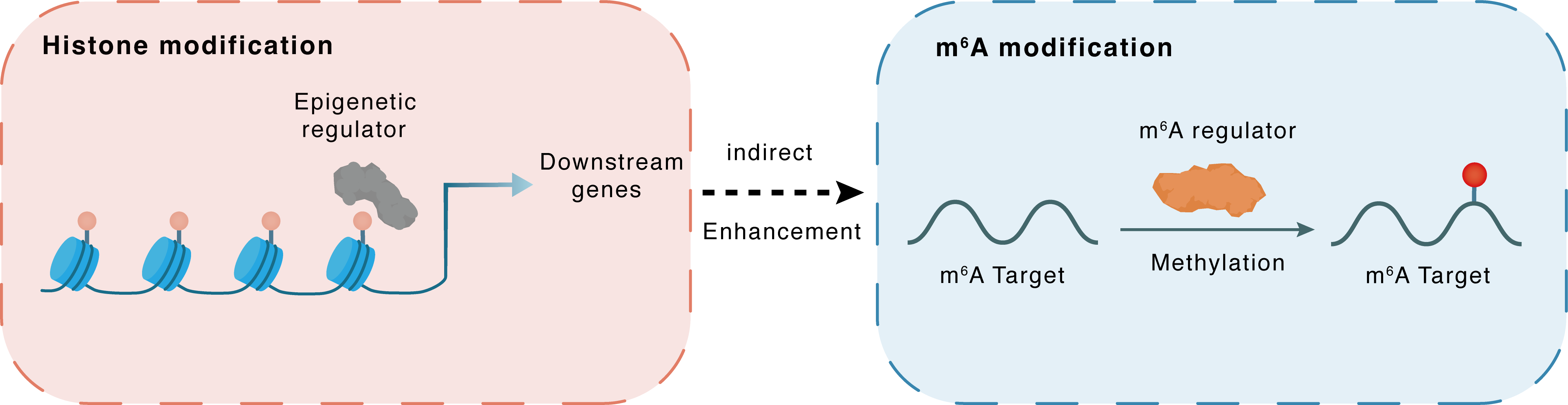 Histone modification
H3K27me3
EZH2
miR-338-5p
Indirect
Enhancement
m6A modification
RPS6KB1
RPS6KB1
METTL3
Methylation
Histone modification
H3K27me3
EZH2
miR-338-5p
Indirect
Enhancement
m6A modification
RPS6KB1
RPS6KB1
METTL3
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Ribosomal protein S6 kinase beta-1 (RPS6KB1/p70S6K) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone-lysine N-methyltransferase EZH2 (EZH2) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 27 trimethylation (H3K27me3) | View Details | |||
| Downstream Gene | miR-338-5p | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | Collectively, EZH2 downregulated hsa-miR-338-5p through Histone H3 lysine 27 trimethylation (H3K27me3), which in turn impaired miR-338-5p-dependent METTL3 inhibition and enhanced CDCP1 translation, therefore contributing to the development of GC. Down-regulation of METTL3 inhibits the proliferation and mobility of human gastric cancer cells and leads to inactivation of the AKT signaling pathway, suggesting that METTL3 is a potential target for the treatment of human gastric cancer. METTL3 knockdown decreased Bcl2 and increased Bax and active Caspase-3 in gastric cancer cells, which suggested the apoptotic pathway was activated. METTL3 led to inactivation of the AKT signaling pathway in human gastric cancer cells, including decreased phosphorylation levels of AKT and expression of down-stream effectors Ribosomal protein S6 kinase beta-1 (RPS6KB1/p70S6K) and Cyclin D1. | ||||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | |||
| Pathway Response | Apoptosis | hsa04210 | |||
| PI3K-Akt signaling pathway | hsa04151 | ||||
| Cell Process | Cell proliferation | ||||
| Cell migration | |||||
| Cell invasion | |||||
In-vitro Model |
AGS | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | |
| MKN45 | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Histone-lysine N-methyltransferase EZH2 (EZH2) | 74 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Tazemetostat | Approved | [3] | ||
| Synonyms |
EPZ-6438; 1403254-99-8; EPZ6438; UNII-Q40W93WPE1; N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(morpholinomethyl)-[1,1'-biphenyl]-3-carboxamide; Q40W93WPE1; EPZ 6438; E7438; (1,1'-Biphenyl)-3-carboxamide, N-((1,2-dihydro-4,6-dimethyl-2-oxo-3-pyridinyl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(4-morpholinylmethyl)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 2.5 nM | |||
| External Link | ||||
| DS-3201b | Phase 2 | [4] | ||
| Synonyms |
Valemetostat; 1809336-39-7; UNII-60RD0234VE; 60RD0234VE; 1809336-39-7 (free base); DS-3201; (2R)-7-chloro-2-[trans-4-(dimethylamino)cyclohexyl]-N-[(4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl]-2,4-dimethyl-1,3-benzodioxole-5-carboxamide; Valemetostat 2HCl; Valemetostat [INN]; Valemetostat (DS-3201); CHEMBL4597193; EZH1/2 inhibitor DS-3201; SCHEMBL18393626; SCHEMBL18393627; SCHEMBL18639210; EX-A3423; DS3201; NSC813381; s8926; NSC-813381; HY-109108; CS-0039740; D11551
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CPI-1205 | Phase 1/2 | [5] | ||
| Synonyms |
HPODOLXTMDHLLC-QGZVFWFLSA-N; 1621862-70-1; UNII-455J2479FY; CPI1205; CPI 1205; 455J2479FY; (R)-N-((4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-methyl-1-(1-(1-(2,2,2-trifluoroethyl)piperidin-4-yl)ethyl)-1H-indole-3-carboxamide; GTPL9115; SCHEMBL17329268; MolPort-044-560-382; KS-000006BA; EX-A1068; s8353; AKOS030628484; ZINC220982768; CS-7648; compound 13 [PMID: 27739677]; HY-100021; J3.556.402K; N-[(4-methoxy-6-methyl-2-oxo-1H-pyridin-3-yl)methyl]-2-methyl-1-[(1R)-1-[1-(2,2,2-trifluoroethyl)piperidin-4-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SHR2554 | Phase 1/2 | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| CPI-0209 | Phase 1/2 | [7] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| GSK2816126 | Phase 1 | [8] | ||
| Synonyms |
GSK 126; GSK-126
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | Ki = 0.5 nM | |||
| External Link | ||||
| PF-06821497 | Phase 1 | [9] | ||
| Synonyms |
UNII-S4L4MM20B6; S4L4MM20B6; CHEMBL4080228; PF06821497; 1844849-10-0; 5,8-dichloro-2-[(4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl]-7-[(R)-methoxy(oxetan-3-yl)methyl]-3,4-dihydroisoquinolin-1(2H)-one; SCHEMBL17330426; GTPL10516; BDBM50246967; NSC800019; DB14799; HY-101571A; NSC-800019; compound 23a [PMID: 29211475]; CS-0092626; Q29209799; 1(2H)-Isoquinolinone, 5,8-dichloro-2-((1,2-dihydro-4-methoxy-6-methyl-2-oxo-3-pyridinyl)methyl)-3,4-dihydro-7-((R)-methoxy-3-oxetanylmethyl)-; 5,8-dichloro-2-[(4-methoxy-6-methyl-2-oxo-1H-pyridin-3-yl)methyl]-7-[(R)-methoxy(oxetan-3-yl)methyl]-3,4-dihydroisoquinolin-1-one; CJD
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| DS-3201 | Phase 1 | [5] | ||
| Synonyms |
QTGYNKYZRZATJB-UHFFFAOYSA-N; 701899-63-0; 2-(2-(2-Methyl-1H-imidazol-5-yl)ethyl)isoindoline-1,3-dione; 2-(2-(2-Methyl-1H-imidazol-5-yl)-ethyl)isoindoline-1,3-dione; SCHEMBL7743461; MolPort-035-945-474; MolPort-035-690-353; ZINC98086069; AKOS024459002; AKOS024262663; MCULE-2708350770; FCH4077443; AK158834; AX8292467; ST2403812; 2-[2-(2-methyl-1H-imidazol-5-yl)ethyl]-2,3-dihydro-1H-isoindole-1,3-dione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| HH2853 | Phase 1 | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-33 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID28394193-Compound-Figure3bI | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-35 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| PMID28394193-Compound-54 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-24 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-27 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-25 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-50 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-47 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2 nM; Ki < 1 nM | |||
| External Link | ||||
| PMID28394193-Compound-21 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-41 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-53 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 80 nM | |||
| External Link | ||||
| PMID28394193-Compound-Figure5aVIII | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-38 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-51 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-31 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 20 nM | |||
| External Link | ||||
| PMID28394193-Compound-42 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-15 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-52 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-32 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| PMID28394193-Compound-23 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-29 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID28394193-Compound-30 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 16 nM | |||
| External Link | ||||
| PMID28394193-Compound-39 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-49 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-43 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-40 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-Figure3bIII | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-36 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 316 nM | |||
| External Link | ||||
| PMID28394193-Compound-28 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 32 nM | |||
| External Link | ||||
| PMID28394193-Compound-22 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-18 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-16 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-44 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-20 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-19 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-37 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-Figure3bII | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-26 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-17 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-34 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID28394193-Compound-56 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.93 nM | |||
| External Link | ||||
| PMID28394193-Compound-46 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| PMID28394193-Compound-57 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6.45 nM | |||
| External Link | ||||
| PMID28394193-Compound-14 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 21900 nM | |||
| External Link | ||||
| PMID28394193-Compound-12 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 10 nM | |||
| External Link | ||||
| PMID26882240-Compound-1 | Patented | [12] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-55 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 8.13 nM | |||
| External Link | ||||
| PMID28394193-Compound-45 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 168000 nM | |||
| External Link | ||||
| PMID28394193-Compound-13 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1470 nM | |||
| External Link | ||||
| EPZ005687 | Investigative | [13] | ||
| Synonyms |
EPZ-005687; EPZ 005687
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| EI1 | Investigative | [14] | ||
| Synonyms |
KB-145943
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| UNC1999 | Investigative | [15] | ||
| Synonyms |
UNC 1999; UNC-1999
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MS1943 | Investigative | [6] | ||
| Synonyms |
2225938-17-8; SCHEMBL21271666; EX-A3962; s8918; HY-133129; CS-0112146; 6-(6-(4-(2-(2-((3r,5r,7r)-adamantan-1-yl)acetamido)ethyl)piperazin-1-yl)pyridin-3-yl)-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-1-isopropyl-1H-indazole-4-carboxamide; 6-(6-(4-(2-(2-(Adamantan-1-yl)acetamido)ethyl)piperazin-1-yl)pyridin-3-yl)-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-1-isopropyl-1H-indazole-4-carboxamide
Click to Show/Hide
|
|||
| MOA | Degrader | |||
| External Link | ||||
| GSK343 | Investigative | [16] | ||
| Synonyms |
compound 6 [PMID 24900432]; GSK 343
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 174 nM | |||
| External Link | ||||
| PMID28394193-Compound-11 | Patented | [17] | ||
| External Link | ||||
| PMID28394193-Compound-10 | Patented | [17] | ||
| External Link | ||||
| PMID28394193-Compound-14 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 21900 nM | |||
| External Link | ||||
| PMID28394193-Compound-12 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 10 nM | |||
| External Link | ||||
| PMID28394193-Compound-13 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1470 nM | |||
| External Link | ||||
| PMID28394193-Compound-56 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.93 nM | |||
| External Link | ||||
| PMID28394193-Compound-46 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| PMID28394193-Compound-57 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6.45 nM | |||
| External Link | ||||
| PMID28394193-Compound-55 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 8.13 nM | |||
| External Link | ||||
| PMID28394193-Compound-45 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 168000 nM | |||
| External Link | ||||
| Ribosomal protein S6 kinase beta-1 (RPS6KB1/p70S6K) | 17 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| NNI-362 | Phase 1 | [18] | ||
| MOA | Stimulator | |||
| External Link | ||||
| M2698 | Phase 1 | [5] | ||
| Synonyms |
HXAUJHZZPCBFPN-QGZVFWFLSA-N; 1379545-95-5; SCHEMBL15262358; EX-A1187; AKOS030627134; M2698(MSC-2363318A)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PF-4708671 | Clinical trial | [19] | ||
| Synonyms |
PF4708671; PF 4708671
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27410995-Compound-Figure3c | Patented | [20] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Pyrimido[4,5-d] pyrimidines and pyrido[4,3-d] pyrimidine derivative 1 | Patented | [20] | ||
| Synonyms |
PMID27410995-Compound-Figure3d
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SB-415286 | Investigative | [21] | ||
| Synonyms |
SB 415286; 264218-23-7; SB415286; 3-(3-chloro-4-hydroxyphenylamino)-4-(2-nitrophenyl)-1H-pyrrole-2,5-dione; 3-(3-chloro-4-hydroxyphenylamino)-4-(4-nitrophenyl)-1H-pyrrole-2,5-dione; CHEMBL322970; 3-(3-chloro-4-hydroxyanilino)-4-(2-nitrophenyl)pyrrole-2,5-dione; 3-[(3-chloro-4-hydroxyphenyl)amino]-4-(2-nitrophenyl)-1H-pyrrole-2,5-dione; 1H-Pyrrol-2,5-dione, 3-((3-chloro-4-hydroxyphenyl)amino)-4-(2-nitrophenyl)-; SMR000568415; SR-01000075855
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SB-747651A | Investigative | [22] | ||
| Synonyms |
CHEMBL188434; compound 26; SCHEMBL4719834; GTPL8130; BDBM24996; oxadiazole-containing compound, 9; MBCJUIJWPYUEBX-UHFFFAOYSA-N; ZINC13998530; NCGC00273984-05; NCGC00273984-03; SB-747651; 4-{1-ethyl-7-[(piperidin-4-ylamino)methyl]-1H-imidazo[4,5-c]pyridin-2-yl}-1,2,5-oxadiazol-3-amine; [2-(4-Amino-furazan-3-yl)-1-ethyl-1H-imidazo[4,5-c]pyridin-7-ylmethyl]-piperidin-4-yl-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 270 nM | |||
| External Link | ||||
| ACTB-1003 | Investigative | [23] | ||
| Synonyms |
Multi-mode kinase inhibitor (oral, cancer), ACT Biotech; Multi-mode kinase inhibitor (oral, cancer), Bayer
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID20005102C1 | Investigative | [24] | ||
| Synonyms |
GTPL8181; BDBM50305878; B99
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7 nM | |||
| External Link | ||||
| KT-5720 | Investigative | [25] | ||
| Synonyms |
KT 5720; KT5720; 108068-98-0; GTPL337; ZINC3873013; KT 5720, > hexyl (15R,16R,18S)-16-hydroxy-15-methyl-3-oxo-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1^{15,18}.0^{2,6}.0^{7,27}.0^{8,13}.0^{19,26}.0^{20,25}]octacosa-1(26),2(6),7(27),8,10,12,20,22,24-nonaene-16-carboxylate; (9S,10S,12R)-2,3,9,10,11,12-Hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3 inverted exclamation marka,2 inverted exclamation marka,1 inverted exclamation marka-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-c
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| STAUROSPORINONE | Investigative | [25] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Ro31-8220 | Investigative | [25] | ||
| Synonyms |
Bisindolylmaleimide IX; ro 31-8220; 125314-64-9; Ro 31 8220; Ro 318220; UNII-W9A0B5E78O; Ro-318220; Ro-31-8220; CHEMBL6291; W9A0B5E78O; CHEBI:38912; 3-{3-[4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-3-yl]-1H-indol-1-yl}propyl carbamimidothioate; 3-{3-[4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-3-yl]-1H-indol-1-yl}propyl imidothiocarbamate; CHEMBL1591531; Carbamimidothioic acid, 3-(3-(2,5-dihydro-4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-1H-pyrrol-3-yl)-1H-indol-1-yl)propyl; bisindolymaleimide IX
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 15 nM | |||
| External Link | ||||
| KN-62 | Investigative | [25] | ||
| Synonyms |
KN-62 (non-isomeric); GTPL6001; HMS3229A04; CCG-206863
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CI-1040 | Investigative | [25] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 4,5,6,7-tetrabromo-1H-benzo[d][1,2,3]triazole | Investigative | [26] | ||
| Synonyms |
4,5,6,7-tetrabromobenzotriazole
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-[(3,5-diamino-1H-pyrazol-4-yl)diazenyl]phenol | Investigative | [27] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| RO-316233 | Investigative | [25] | ||
| Synonyms |
119139-23-0; bisindolylmaleimide iv; 3,4-di(1H-indol-3-yl)-1H-pyrrole-2,5-dione; Arcyriarubin A; 3,4-Bis(3-indolyl)maleimide; 3,4-Di-1H-indol-3-yl-1H-pyrrole-2,5-dione; UNII-MBK3OO5K8T; BIM IV; 3,4-bis(1H-indol-3-yl)pyrrole-2,5-dione; MBK3OO5K8T; CHEMBL266487; 3,4-bis(1H-indol-3-yl)-2,5-dihydro-1H-pyrrole-2,5-dione; DQYBRTASHMYDJG-UHFFFAOYSA-N; 2,3-bis(1H-Indol-3-yl)maleimide; 1H-Pyrrole-2,5-dione, 3,4-di-1H-indol-3-yl-; Ro-31-6233; AK-15401; 3,4-bis(3-indolyl)-1H-pyrrole-2,5-dione; Bisindoylmaleimide; Bisindolyl deriv. 3
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2B72: Gastric cancer | 81 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Leniolisib | Approved | [28] | ||
| Synonyms |
1354690-24-6; Leniolisib free base; UNII-L22772Z9CP; (S)-1-(3-((6-(6-methoxy-5-(trifluoromethyl)pyridin-3-yl)-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4-yl)amino)pyrrolidin-1-yl)propan-1-one; L22772Z9CP; 1354690-24-6 (free base); leniolisib(CDZ 173); CDZ173; CDZ-173; 1-[(3S)-3-[[6-[6-methoxy-5-(trifluoromethyl)pyridin-3-yl]-7,8-dihydro-5H-pyrido[4,3-d]pyrimidin-4-yl]amino]pyrrolidin-1-yl]propan-1-one; Leniolisib [INN]; Leniolisib (CDZ173); Leniolisib (USAN/INN); CDZ173-NX; SCHEMBL323054; GTPL9424; CHEMBL3643413; BDBM118299; EX-A2854; MFCD30470232; s8752; ZB1510; CS-7524; DC22326; SB18839; Example 67 [WO2012004299]; 1-{(S)-3-[6-(6-methoxy-5-trifluoromethyl-pyridin-3-yl)-5,6,7,8-tetrahydro-pyrido[4,3-d]pyrimidin-4-y; 1-{(S)-3-[6-(6-methoxy-5-trifluoromethyl-pyridin-3-yl)-5,6,7,8-tetrahydro-pyrido[4,3-d]pyrimidin-4-ylamino]-pyrrolidin-1-yl}-propan-1-one; AS-56217; HY-17635; A16796; D11158; US8653092, 67; Q27282602; 1-Propanone, 1-((3S)-3-((5,6,7,8-tetrahydro-6-(6-methoxy-5-(trifluoromethyl)-3-pyridinyl)pyrido(4,3-d)pyrimidin-4-yl)amino)-1-pyrrolidinyl)-; 9NQ
Click to Show/Hide
|
|||
| External Link | ||||
| Atezolizumab | Approved | [5] | ||
| External Link | ||||
| Bavencio | Approved | [5] | ||
| External Link | ||||
| Tebentafusp | Approved | [29] | ||
| External Link | ||||
| Merimepodib | Approved | [30] | ||
| Synonyms |
Merimebodib; Merimepodib [USAN:INN]; Tyverb/Tykerb; MMPD; 198821-22-6; 2ZL2BA06FU; C23H24N4O6; CHEMBL304087; MERIMEPODIB, VI-21497, VX-497; UNII-2ZL2BA06FU; VI-21497; VX-497; VX497; Vx 497; carbamic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Taxol | Approved | [31] | ||
| Synonyms |
C47H51NO14; weekly paclitaxel; Micellar Paclitaxel; Paclitaxel [USAN:INN:BAN]; SCHEMBL15000506; Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, (2aR-(2aalpha,4beta,4abeta,6beta,9alpha(alphaR*,betaS*),11alpha,12alpha,12aalpha,12balpha))-
Click to Show/Hide
|
|||
| External Link | ||||
| Ramucirumab | Approved | [32] | ||
| Synonyms |
LY3009806
Click to Show/Hide
|
|||
| External Link | ||||
| Tucatinib | Approved | [33] | ||
| Synonyms |
Irbinitinib; 937263-43-9; ONT-380; UNII-234248D0HH; 234248D0HH; N6-(4,4-Dimethyl-4,5-dihydrooxazol-2-yl)-N4-(3-methyl-4-((1,2,4)triazolo(1,5-a)pyridin-7-yloxy)phenyl)quinazoline-4,6-diamine; 4,6-Quinazolinediamine, N6-(4,5-dihydro-4,4-dimethyl-2-oxazolyl)-N4-(3-methyl-4-((1,2,4)triazolo(1,5-a)pyridin-7-yloxy)phenyl)-; ONT 380; 4,6-QuinazolinediaMine, N6-(4,5-dihydro-4,4-diMethyl-2-oxazolyl)-N4-[3-Methyl-4-([1,2,4]triazolo[1,5-a]pyridin-7-yloxy)phenyl]-; Tucatinib [USAN:INN]; 6-DIAMINE
Click to Show/Hide
|
|||
| External Link | ||||
| Antacids | Approved | [34] | ||
| External Link | ||||
| Trastuzumab | Approved | [5] | ||
| Synonyms |
Herceptin; Herceptin (TN); Trastuzumab (INN); Trastuzumab (genetical recombination); Trastuzumab (genetical recombination) (JAN); Trastuzumab (ERBB2 mAb inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| Carbamazepine | Phase 3 | [35] | ||
| Synonyms |
Carbamazepine (iv, epilepsy); Carbamazepine (iv, epilepsy), Lundbeck; Carbamazepine (iv, epilepsy), Ovation Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| Margetuximab | Approved | [5] | ||
| External Link | ||||
| Nivolumab | Approved | [5] | ||
| External Link | ||||
| GRANITE | Phase 3 | [36] | ||
| Synonyms |
Penoxsulam; 219714-96-2; 2-(2,2-difluoroethoxy)-N-(5,8-dimethoxy-[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; UNII-784ELC1SCZ; 784ELC1SCZ; CHEBI:81776; 2-(2,2-difluoroethoxy)-n-(5,8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; Penoxsulam [ISO]; PXD; DSSTox_CID_14803; DSSTox_RID_79204; DSSTox_GSID_34803; SCHEMBL116968; CHEMBL1895913; DTXSID0034803; HSDB 7887; AMY12535; BCP18718; EBD18529; Tox21_301010; MFCD07363876; ZINC13827750; AKOS025401685; NCGC00163715-01; NCGC00163715-02; NCGC00163715-03; NCGC00254912-01; AC-24494; Penoxsulam 100 microg/mL in Acetonitrile; CAS-219714-96-2; FT-0696708; Penoxsulam, PESTANAL(R), analytical standard; C18481; Q22808507; 2-(2,2-Difluoroethoxy)-6-trifluoromethyl-N-(5, 8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)benzenesulfonamide; 2-(2,2-Difluoroethoxy)-N-(5,8-dimethoxy[1,2,4]-triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; 2-(2,2-difluoroethoxy)-N-{5,8-dimethoxy-[1,2,4]triazolo[1,5-c]pyrimidin-2-yl}-6-(trifluoromethyl)benzene-1-sulfonamide; 2-(2,2-difluoroethyl)-N-(5,8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; Benzenesulfonamide, 2-(2,2-difluoroethoxy)-N-(5,8-dimethoxy(1,2,4)triazolo(1,5-c)pyrimidin-2-yl)-6-(trifluoromethyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| Zolbetuximab | Phase 3 | [37] | ||
| Synonyms |
IMAB362
Click to Show/Hide
|
|||
| External Link | ||||
| Tusamitamab ravtansine | Phase 3 | [38] | ||
| Synonyms |
SAR408701
Click to Show/Hide
|
|||
| External Link | ||||
| Andecaliximab | Phase 3 | [39] | ||
| External Link | ||||
| ABP 980 | Phase 3 | [40] | ||
| External Link | ||||
| GS-5745 | Phase 3 | [31] | ||
| External Link | ||||
| S-1 | Phase 3 | [41] | ||
| Synonyms |
Ciprofibrate-coa; Ciprofibrate-coenzyme A; Coenzyme A, ciprofibrate-; AC1L4TRG; AC1Q3T4H; 111900-25-5; s-{1-[(2r,3s,4r,5r)-5-(6-amino-9h-purin-9-yl)-4-hydroxy-3-(phosphonooxy)tetrahydrofuran-2-yl]-3,5,9-trihydroxy-8,8-dimethyl-3,5-dioxido-10,14-dioxo-2,4,6-trioxa-11,15-diaza-3; E5,5; E5-diphosphaheptadecan-17-yl} 2-[4-(2,2-dichlorocyclopropyl)phenoxy]-2-methylpropanethioate(non-preferred name); Coenzyme A, S-(2-(4-(2,2-dichlorocyclopropyl)phenoxy)-2-methylpropanoate)
Click to Show/Hide
|
|||
| External Link | ||||
| Lonsurf | Phase 3 | [5] | ||
| External Link | ||||
| GDC-0068 | Phase 3 | [31] | ||
| Synonyms |
RG7440
Click to Show/Hide
|
|||
| External Link | ||||
| Edotecarin | Phase 3 | [42] | ||
| Synonyms |
ED-749; Edotecarin < Prop INN; J-107088; PF-804950; 12-(beta-D-Glucopyranosyl)-2,10-dihydroxy-6-[2-hydroxy-1-(hydroxymethyl)ethylamino]-6,7,12,13-tetrahydro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-5,7-dione
Click to Show/Hide
|
|||
| External Link | ||||
| RG3638 | Phase 3 | [43] | ||
| Synonyms |
Onartuzumab
Click to Show/Hide
|
|||
| External Link | ||||
| G17DT | Phase 3 | [44] | ||
| Synonyms |
Gastrimmune; Insegia
Click to Show/Hide
|
|||
| External Link | ||||
| DE-766 | Phase 3 | [45] | ||
| External Link | ||||
| Tesetaxel | Phase 2 | [46] | ||
| Synonyms |
DJ-927; 333754-36-2; UNII-UG97LO5M8Y; UG97LO5M8Y; Tesetaxel [INN]; DJ927; DJ 927; CHEMBL2107787; SCHEMBL12060837; DB12019; Z-3104; (2AS,2BR,3S,4S,6S,8AR,10R,11AS,11BR,13AR)-2A-ACETOXY-6-(((2R,3S)-3-((TERT-BUTOXYCARBONYL)AMINO)-3-(3-FLUOROPYRIDIN-2-YL)-2-HYDROXYPROPANOYL)OXY)-10-((DIMETHYLAMINO)METHYL)-4-HYDROXY-7,11B,14,14-TETRAMETHYL-2A,2B,3,4,5,6,8A,11A,11B,12,13,13A-DODECAHYDRO-2H-4,8-METHANOOXETO[3'',2'':3',4']BENZO[1',2':3,4]CYCLODECA[1,2-D][1,3]DIOXOL-3-YL BENZOATE
Click to Show/Hide
|
|||
| External Link | ||||
| Nelipepimut S | Phase 3 | [47] | ||
| Synonyms |
E75
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-986205 | Phase 3 | [5] | ||
| Synonyms |
KRTIYQIPSAGSBP-KLAILNCOSA-N; 1923833-60-6; BMS986205; UNII-0A7729F42K; 0A7729F42K; GTPL9707; SCHEMBL18826792; SCHEMBL17740982; SCHEMBL19105151; EX-A2606; AKOS032954040; HY-101560; CS-0021719; Q29213697; (R)-N-(4-chlorophenyl)-2-((1s,4S)-4-(6-fluoroquinolin-4-yl)cyclohexyl)propanamide; (2R)-N-(4-chlorophenyl)-2-[4-(6-fluoroquinolin-4-yl)cyclohexyl]propanamide; (2R)-N-(4-Chlorophenyl)-2-(4-(6-fluoro-4-quinolyl)cyclohexyl)propanamide, cis; Cyclohexaneacetamide, N-(4-chlorophenyl)-4-(6-fluoro-4-quinolinyl)-alpha-methyl-, cis-(alphaR)-
Click to Show/Hide
|
|||
| External Link | ||||
| Rivoceranib | Phase 3 | [5] | ||
| External Link | ||||
| Claudiximab | Phase 3 | [5] | ||
| Synonyms |
IMAB-362; Anti-GC182 mAbs (cancer), Ganymed; Anti-GC182 monoclonal antibodies (cancer), Ganymed; Anti-CLDN18-2 mAbs (cancer), Ganymed; Anti-CLDN18-2 monoclonal antibodies (cancer), Ganymed
Click to Show/Hide
|
|||
| External Link | ||||
| OS-440 | Phase 3 | [48] | ||
| Synonyms |
CNS modulator (spasticity), Osmotica
Click to Show/Hide
|
|||
| External Link | ||||
| Oraxol | Phase 3 | [5] | ||
| External Link | ||||
| ICI 118,551 | Phase 3 | [31] | ||
| Synonyms |
Ici 118551; (2R,3S)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-3-(propan-2-ylamino)butan-2-ol; CHEMBL198059; CHEBI:73289; ICI-118551; ICI118551; erythro-DL-1-(7-Methylindan-4-yloxy)-3-isopropylaminobutan-2-ol; (2R,3S)-3-(isopropylamino)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]butan-2-ol; 2-Butanol, 1-((2,3-dihydro-7-methyl-1H-inden-4-yl)oxy)-3-((1-methylethyl)amino)-, (2R,3S)-rel-; 2-Butanol, 1-((2,3-dihydro-7-methyl-1H-inden-4-yl)oxy)-3-((1-methylethyl)amino)-, (R*,S*)-(+-)-; ICI-118,551; Ici 111,581; AC1NUNSO
Click to Show/Hide
|
|||
| External Link | ||||
| Evorpacept | Phase 2/3 | [49] | ||
| Synonyms |
ALX148
Click to Show/Hide
|
|||
| External Link | ||||
| BNT141 | Phase 2 | [50] | ||
| External Link | ||||
| Anti-LAG3 | Phase 2 | [40] | ||
| External Link | ||||
| GSK1292263 | Phase 2 | [51] | ||
| External Link | ||||
| MM-111 | Phase 2 | [52] | ||
| External Link | ||||
| Plevitrexed | Phase 2 | [53] | ||
| Synonyms |
ZD 9331; ZD9331; 153537-73-6; Plevitrexed [INN]; ZD-9331; NSC 696259; UNII-L9P2881C3H; CHEMBL126648; (2s)-2-[(2-fluoro-4-{[(4-hydroxy-2,7-dimethylquinazolin-6-yl)methyl](prop-2-yn-1-yl)amino}benzoyl)amino]-4-(2h-tetrazol-5-yl)butanoic acid; L9P2881C3H; Plevitrexed (INN); 172521-94-7; (2S)-2-[[4-[(2,7-dimethyl-4-oxo-1H-quinazolin-6-yl)methyl-prop-2-ynylamino]-2-fluorobenzoyl]amino]-4-(2H-tetrazol-5-yl)butanoic acid; 1H-Tetrazole-5-butanoic acid,
Click to Show/Hide
|
|||
| External Link | ||||
| DS-8201 | Phase 1 | [40] | ||
| Synonyms |
9-Aminofluorene; 9H-Fluoren-9-amine; 525-03-1; FLUOREN-9-AMINE; Fluoren-9-ylamine; UNII-4NHO2K4K5B; CCRIS 7000; BRN 2209545; 4NHO2K4K5B; OUGMRQJTULXVDC-UHFFFAOYSA-N; fluorene-9-ylamine; 9-Amino-fluoren; 9-amino-fluorene; 9H-9-fluorenamine; 9H-fluoren-9-yl-amine; AC1L1VP5; 4-12-00-03390 (Beilstein Handbook Reference); SCHEMBL353865; AC1Q53A2; AC1Q53A1; KS-00000JGC; CTK1H0380; DTXSID90200496; MolPort-001-794-448; HMS1780P20; 9H-fluoren-9-ylamine hydrochloride; ZINC1724407; ALBB-023296; CA-733; SBB005783; AKOS000264388; MCULE-8757055914; DS-
Click to Show/Hide
|
|||
| External Link | ||||
| XL880 | Phase 2 | [54] | ||
| Synonyms |
GSK 089; GSK 1363089; GSK1363089; XL 880; GSK1363089, GSK089, foretinib, EXEL-2880, XL880; 88Z; MET inhibitors
Click to Show/Hide
|
|||
| External Link | ||||
| Matuzumab | Phase 2 | [55] | ||
| Synonyms |
EMD-62000; EMD-72000; Anti-EGF receptor humanized antibody (iv, cancer), Merck KGaA/ Takeda Pharmaceuticals; Anti-EGFR humanized mAb (iv, cancer), Merck KGaA/ Takeda Pharmaceuticals; Anti-epidermal growth factor receptor humanized antibody (iv, cancer), Merck KGaA/ Takeda Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| BAY-57-9352 | Phase 2 | [31] | ||
| Synonyms |
Telatinib; Bay 57-9352
Click to Show/Hide
|
|||
| External Link | ||||
| Bemarituzumab | Phase 2 | [56] | ||
| External Link | ||||
| PEGPH20 | Phase 2 | [5] | ||
| External Link | ||||
| Plevitrexed (R)-isomer | Phase 2 | [57] | ||
| Synonyms |
YW3548
Click to Show/Hide
|
|||
| External Link | ||||
| APR-246 | Phase 2 | [58] | ||
| Synonyms |
Eprenetapopt
Click to Show/Hide
|
|||
| External Link | ||||
| CRS-207 | Phase 2 | [47] | ||
| External Link | ||||
| Opdivo + Yervoy | Phase 3 | [5] | ||
| External Link | ||||
| CT-041 | Phase 1/2 | [59] | ||
| External Link | ||||
| BPX-601 | Phase 1/2 | [60] | ||
| External Link | ||||
| Anti-MUC1 CAR-T cells | Phase 1/2 | [61] | ||
| External Link | ||||
| Anti-Mesothelin CAR-T cells | Phase 1/2 | [62] | ||
| External Link | ||||
| Anti-HER2 CAR-T | Phase 1/2 | [63] | ||
| External Link | ||||
| CAR-T Cells targeting EpCAM | Phase 1/2 | [64] | ||
| External Link | ||||
| PAT-SC1 | Phase 1/2 | [65] | ||
| Synonyms |
SC-1; Adjuvant therapy (gastric cancer), University of Wurzburg; SC-1 (gastric cancer), CAT; SC-1 (gastric cancer), Debiopharm; SC-1 (gastric cancer), Patrys; SC-1 (stomach cancer), OncoMab
Click to Show/Hide
|
|||
| External Link | ||||
| ASP2138 | Phase 1 | [66] | ||
| External Link | ||||
| SAR443216 | Phase 1 | [67] | ||
| External Link | ||||
| AMG 199 | Phase 1 | [68] | ||
| External Link | ||||
| AMG 910 | Phase 1 | [69] | ||
| External Link | ||||
| Alofanib | Phase 1 | [70] | ||
| Synonyms |
1612888-66-0; 3-(N-(4-methyl-2-nitro-5-(pyridin-3-yl)phenyl)sulfamoyl)benzoic acid; RPT-835(alofanib); UNII-LQX7RFK8MZ; RPT-835; RPT835; LQX7RFK8MZ; ES000835; Alofanib [INN]; Alofanib(RPT835); Syn007154; CHEMBL4594436; SCHEMBL18660613; AMY16650; BCP31905; EX-A2731; MFCD30533418; NSC790182; s8754; Benzoic acid, 3-(((4-methyl-2-nitro-5-(3-pyridinyl)phenyl)amino)sulfonyl)-; NSC-790182; SB19665; AC-31695; AK668992; AS-56846; HY-17601; CS-0014684; RPT 835; Q27283135; 3-{[4-methyl-2-nitro-5-(pyridin-3-yl)phenyl]sulfamoyl}benzoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| HER2-specific CAR T cell | Phase 1 | [71] | ||
| External Link | ||||
| Anti-CEA-CAR T | Phase 1 | [72] | ||
| External Link | ||||
| XR-5944 | Phase 1 | [73] | ||
| Synonyms |
MLN-944; XR-11576 analogs; XR-5000 analogs; XR-5942
Click to Show/Hide
|
|||
| External Link | ||||
| A168 | Phase 1 | [74] | ||
| External Link | ||||
| EGFR806-specific CAR T cell | Phase 1 | [75] | ||
| External Link | ||||
| AbGn-107 | Phase 1 | [5] | ||
| External Link | ||||
| FPA144 | Phase 1 | [40] | ||
| External Link | ||||
| Minnelide 001 | Phase 1 | [31] | ||
| External Link | ||||
| CAR-T cells targeting EpCAM | Phase 1 | [76] | ||
| External Link | ||||
| Anti-CEA CAR-T cells | Phase 1 | [77] | ||
| External Link | ||||
| EPCAM-targeted CAR-T cells | Clinical trial | [78] | ||
| External Link | ||||
| PMID28460551-Compound-1 | Patented | [79] | ||
| External Link | ||||
| Conjugated 3-(indolyl)-and 3-(azaindolyl)-4-arylmaleimide compound 1 | Patented | [80] | ||
| Synonyms |
PMID28621580-Compound-WO2012084683c62
Click to Show/Hide
|
|||
| External Link | ||||
| TOPIXANTRONE HYDROCHLORIDE | Discontinued in Phase 2 | [81] | ||
| Synonyms |
SCHEMBL1418986; Topixantrone hydrochloride < Prop INNM; BBR-3409 (dimaleate); 5-[2-(Dimethylamino)ethylamino]-2-[2-(2-hydroxyethylamino)ethyl]indazolo[4,3-gh]isoquinolin-6(2H)-one dihydrochloride
Click to Show/Hide
|
|||
| External Link | ||||
| MDL 101,731 | Discontinued in Phase 2 | [82] | ||
| Synonyms |
Tezacitabine; Fmdc cpd; 130306-02-4; UNII-7607Y95N9S; Mdl 101731; (E)-2'-Deoxy-2'-(fluoromethylene) cytidine; MDL-101731; 2'-Deoxy-2'-(fluoromethylene)cytidine; 7607Y95N9S; Cytidine, 2'-deoxy-2'-(fluoromethylene)-, (2E)-; (E)-2'-Deoxy-2'-(fluoromethylene)cytidine; Tezacitabine [INN]; tezaciabine; Tezacitabine, anhydrous; AC1O5KIG; SCHEMBL18724; SCHEMBL18725; Tezacitabine, anhydrous [INN]; CHEMBL2105467; C10H12FN3O4; DTXSID10156446; GFFXZLZWLOBBLO-ASKVSEFXSA-N; ZINC3777826; KW-2331
Click to Show/Hide
|
|||
| External Link | ||||
| BBR-3438 | Discontinued in Phase 2 | [83] | ||
| Synonyms |
Nortopixantrone; UNII-PH2639TAB4; PH2639TAB4; Nortopixantrone [INN:BAN]; AC1MI4ZO; CHEMBL150303; SCHEMBL7804438
Click to Show/Hide
|
|||
| External Link | ||||
| IPI-493 | Discontinued in Phase 1 | [84] | ||
| Synonyms |
[(3R,5R,6S,7R,8E,10R,11R,12Z,14E)-21-amino-6-hydroxy-5,11-dimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-17-azabicyclo[16.3.1]docosa-1(21),8,12,14,18-pentaen-10-yl] carbamate; AC1NS08X; SCHEMBL16226496; SCHEMBL16225851
Click to Show/Hide
|
|||
| External Link | ||||
| Kanjinti | Application submitted | [5] | ||
| External Link | ||||
| Anti-CD9 mab | Investigative | [85] | ||
| Synonyms |
ALB-6; Anti-CD9 mAb (gastric cancer); Anti-CD9 mAb (gastric cancer), Osaka University
Click to Show/Hide
|
|||
| External Link | ||||
References