m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03608
|
[1], [2] | |||
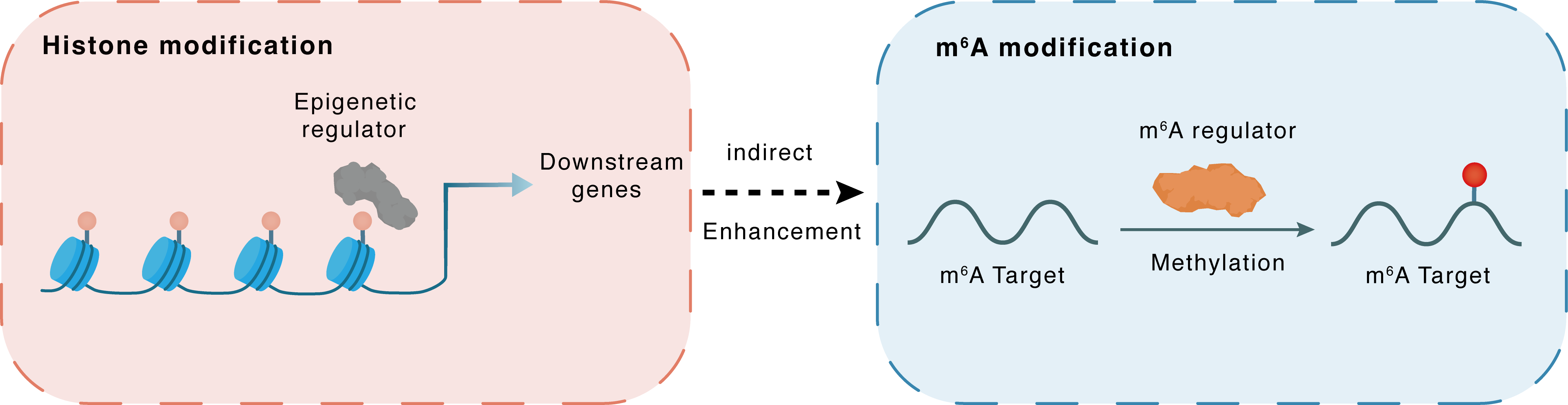 Histone modification
H3K4me3
SMYD2
LINC01605
Indirect
Enhancement
m6A modification
LDHA
LDHA
METTL3
Methylation
Histone modification
H3K4me3
SMYD2
LINC01605
Indirect
Enhancement
m6A modification
LDHA
LDHA
METTL3
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Lactate dehydrogenase A (LDHA) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | N-lysine methyltransferase SMYD2 (SMYD2) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 4 trimethylation (H3K4me3) | View Details | |||
| Downstream Gene | LINC01605 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | LINC01605 was regulated by SMYD2-EP300-mediated modifications of histone Histone H3 lysine 4 trimethylation (H3K4me3) as well as H3K27ac. LINC01605 was found to bind to METTL3 and promote the m6A modification of SPTBN2 mRNA, thereby facilitating the translation of SPTBN2. METTL3 enhances the expression of Lactate dehydrogenase A (LDHA), which catalyzes the conversion of pyruvate to lactate, to trigger glycolysis and 5-FU resistance. METTL3/LDHA axis-induced glucose metabolism is a potential therapy target to overcome 5-FU resistance in CRC cells. | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Pathway Response | HIF-1 signaling pathway | hsa04066 | |||
| Glycolysis / Gluconeogenesis | hsa00010 | ||||
| Cell Process | Glycolysis | ||||
In-vitro Model |
HCT 116 | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW480 | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| In-vivo Model | For subcutaneous transplanted model, sh-control and sh-METTL3 HCT-116/5-FU cells (5 × 106 per mouse) were diluted in 100ul PBS + 100 ul Matrigel (BD Biosciences, San Jose, CA, USA) and injected subcutaneously in the rear flank fat pad of the nude mice. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Lactate dehydrogenase A (LDHA) | 1 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Nedosiran | Approved | [3] | ||
| Synonyms |
nedosiran
Click to Show/Hide
|
|||
| External Link | ||||
| N-lysine methyltransferase SMYD2 (SMYD2) | 6 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| AZ505 | Preclinical | [4] | ||
| Synonyms |
AZ 505; AZ-505
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 120 nM | |||
| External Link | ||||
| LLY-507 | Preclinical | [5] | ||
| Synonyms |
1793053-37-8; LLY507; CHEMBL3414623; 5-Cyano-2'-{4-[2-(3-Methyl-1h-Indol-1-Yl)ethyl]piperazin-1-Yl}-N-[3-(Pyrrolidin-1-Yl)propyl]biphenyl-3-Carboxamide; 3-cyano-5-(2-{4-[2-(3-methyl-1H-indol-1-yl)ethyl]piperazin-1-yl}phenyl)-N-[3-(pyrrolidin-1-yl)propyl]benzamide; 3-Cyano-5-[2-[4-[2-(3-methyl-1H-indol-1-yl)ethyl]piperazin-1-yl]phenyl]-N-[3-(pyrrolidin-1-yl)propyl]benzamide; GTPL8239; SCHEMBL19760400; EX-A899; LLY 507; MolPort-042-624-530; BCP17114; s7575; BDBM50075102; ZINC231558920; AKOS027470175; CS-5126
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 15 nM | |||
| External Link | ||||
| EPZ032597 | Preclinical | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| EPZ033294 | Preclinical | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| A-893 | Preclinical | [7] | ||
| Synonyms |
1868232-32-9; (R)-N-cyclohexyl-3-((3,4-dichlorophenethyl)amino)-N-(2-((2-hydroxy-2-(3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazin-8-yl)ethyl)amino)ethyl)propanamide; CHEMBL3590526; N-Cyclohexyl-N~3~-[2-(3,4-Dichlorophenyl)ethyl]-N-(2-{[(2r)-2-Hydroxy-2-(3-Oxo-3,4-Dihydro-2h-1,4-Benzoxazin-8-Yl)ethyl]amino}ethyl)-Beta-Alaninamide; SCHEMBL17476248; EX-A2769; BDBM50095537; AKOS030235552; ZINC230499113; ACN-037539; AC-29886; HY-19563; CS-0015655; Q27454706; 4GQ; N-Cyclohexyl-3-[2-(3,4-dichlorophenyl)ethylamino]-N-[2-[[(2R)-2-hydroxy-2-(3-oxo-4H-1,4-benzoxazin-8-yl)ethyl]amino]ethyl]propanamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BAY 598 | Preclinical | [8] | ||
| Synonyms |
BAY-598; 1906919-67-2; CHEMBL3818617; 1906919-67-2 (S-isomer); (S,E)-N-(1-(N'-cyano-N-(3-(difluoromethoxy)phenyl)carbamimidoyl)-3-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-4-yl)-N-ethyl-2-hydroxyacetamide; BAY 598 - Bio-X; BAY598; GTPL8953; EX-A1835; BDBM50180955; ZINC504786915; AC-31567; BS-16389; HY-19546; CS-0015642; J3.601.000B; Q27074893; (S)-N-(1-(N'-Cyano-N-(3-(difluoromethoxy)phenyl)carbamimidoyl)-3-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-4-yl)-N-ethyl-2-hydroxyacetamide; (S,E)-N-(1-(N-cyano-N'-(3-(difluoromethoxy)phenyl)carbamimidoyl)-3-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-4-yl)-N-ethyl-2-hydroxyacetamide; (S,Z)-N-(1-(N-cyano-N'-(3-(difluoromethoxy)phenyl)carbamimidoyl)-3-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-4-yl)-N-ethyl-2-hydroxyacetamide; N-[(4S)-1-[(Cyanoamino)[[3-(difluoromethoxy)phenyl]imino]methyl]-3-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-4-yl]-N-ethyl-2-hydroxyacetamide; N-[(4S)-1-[(Z)-N'-cyano-N-[3-(difluoromethoxy)phenyl]carbamimidoyl]-3-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-4-yl]-N-ethyl-2-hydroxyacetamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2B91: Colorectal cancer | 25 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Retifanlimab | Approved | [9] | ||
| Synonyms |
INCMGA0012; Retifanlimab
Click to Show/Hide
|
|||
| External Link | ||||
| Aflibercept | Approved | [10] | ||
| Synonyms |
Ziv-Aflibercept; Zaltrap (TN); VEGF Trap; VEGF Trap-Eye
Click to Show/Hide
|
|||
| External Link | ||||
| Regorafenib | Approved | [11] | ||
| Synonyms |
755037-03-7; BAY 73-4506; Regorafenibum; Stivarga; 4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide; BAY73-4506; Regorafenib (BAY 73-4506); UNII-24T2A1DOYB; 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide; BAY-73-4506; 24T2A1DOYB; CHEMBL1946170; CHEBI:68647; Stivarga (TN); BAY73-4506 hydrochloride; Regorafenib [USAN:INN]
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [12] | ||
| Synonyms |
Bevacizumab (ophthalmic slow-release tissue tablet)
Click to Show/Hide
|
|||
| External Link | ||||
| SYM-004 | Phase 3 | [12] | ||
| Synonyms |
Chimeric IgG1 antibody 1024 (cancer), Symphogen; Chimeric IgG1 antibody 992 (cancer), Symphogen; Chimeric IgG1 antibodies992 + 1024 (cancer), Symphogen
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab + Erlotinib | Phase 3 | [13] | ||
| External Link | ||||
| CPI-613 | Phase 3 | [12] | ||
| Synonyms |
95809-78-2; 6,8-bis(benzylthio)octanoic acid; CPI 613; MLS006010202; SCHEMBL1062218; 6,8-Bis(benzylsulfanyl)octanoic acid; Octanoic acid, 6,8-bis((phenylmethyl)thio)-; Octanoic acid, 6,8-bis[(phenylMethyl)thio]-; 6,8-Bis[(phenylmethyl)thio]octanoic acid; CPI613; CHEMBL3186849; QCR-193; AOB1058; MolPort-023-219-128; HMS3656L06; C22H28O2S2; BCP04663; EX-A2043; s2776; AKOS025142095; BCP9000552; DB12109; RL06062; CS-0961; NCGC00344764-01; SMR004701300; AS-16613; BC261916; AK174899; HY-15453; BCP0726000030; KB-293127; AB0035874
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [10] | ||
| External Link | ||||
| AlloStim | Phase 2/3 | [14] | ||
| Synonyms |
AlloStim (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Sibrotuzumab | Phase 2 | [15] | ||
| External Link | ||||
| CV301 | Phase 2 | [16] | ||
| External Link | ||||
| Efatutazone | Phase 2 | [17] | ||
| Synonyms |
Inolitazone; 223132-37-4; 5-[[4-[[6-(4-amino-3,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-yl]methoxy]phenyl]methyl]-2,4-Thiazolidinedione; Efatutazone [INN]; RS5444; CS-7017; SCHEMBL3246054; CHEMBL3545280; JCYNMRJCUYVDBC-UHFFFAOYSA-N; Efatutazone;CS-7017;RS5444; BCP07478; AKOS030526729; DB11894; CS-0778; KB-77905; DA-07988; HY-14792; QC-10456; 4CA-1384; FT-0737589; 5-[4-[6-(4-amino-3 ,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-ylmethoxy]benzyl]thiazolidine-2,4-dione
Click to Show/Hide
|
|||
| External Link | ||||
| LOR-2040 | Phase 2 | [18] | ||
| External Link | ||||
| RG7221 | Phase 2 | [19] | ||
| External Link | ||||
| PEG-SN38 | Phase 2 | [20] | ||
| Synonyms |
EZN-2208
Click to Show/Hide
|
|||
| External Link | ||||
| MEGF0444A | Phase 2 | [21] | ||
| External Link | ||||
| Encapsulated cell therapy | Phase 1/2 | [22] | ||
| External Link | ||||
| AB928 | Phase 1/2 | [23] | ||
| External Link | ||||
| MGD007 | Phase 1 | [19] | ||
| External Link | ||||
| BNC-101 | Phase 1 | [24] | ||
| External Link | ||||
| Navicixizumab | Phase 1 | [12] | ||
| External Link | ||||
| RG7160 | Discontinued in Phase 2 | [25] | ||
| External Link | ||||
| Nimesulide | Terminated | [26] | ||
| Synonyms |
51803-78-2; N-(4-Nitro-2-phenoxyphenyl)methanesulfonamide; Mesulid; Flogovital; Sulidene; Nimed; R-805; 4-NITRO-2-PHENOXYMETHANESULFONANILIDE; Nisulid; Nimesulidum [INN-Latin]; Nimesulida [INN-Spanish]; R 805; UNII-V4TKW1454M; 4-Nitro-2-phenoxy-methanesulfonanilide; 4'-Nitro-2'-phenoxymethanesulfonanilide; Methanesulfonamide, N-(4-nitro-2-phenoxyphenyl)-; EINECS 257-431-4; 4'-Nitro-2'-phenoxymethansulfonanilid; BRN 2421175; CHEMBL56367; MLS000069680; V4TKW1454M; Methanesulfonanilide, 4'-nitro-2'-phenoxy-; CHEBI:44445; Dulanermin
Click to Show/Hide
|
|||
| External Link | ||||
| Saracatinib | Phase 2 | [27] | ||
| External Link | ||||
| G3139 + Irinotecan | Investigative | [28] | ||
| External Link | ||||
References