m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03524
|
[1], [2] | |||
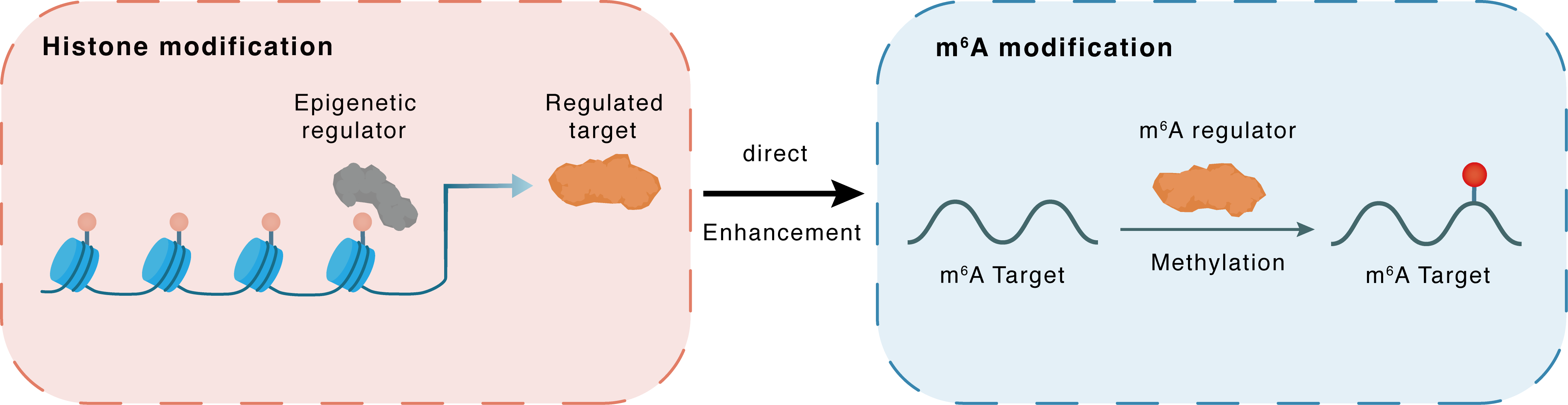 Histone modification
H3K4me3
WDR5
METTL3
Direct
Enhancement
m6A modification
CTSL
CTSL
METTL3
Methylation
Histone modification
H3K4me3
WDR5
METTL3
Direct
Enhancement
m6A modification
CTSL
CTSL
METTL3
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Procathepsin L (CTSL) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | WD repeat-containing protein 5 (WDR5) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 4 trimethylation (H3K4me3) | View Details | |||
| Downstream Gene | METTL3 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification directly impacts m6A modification through modulating the level of m6A regulator | ||||
| Crosstalk Summary | The transcription factor ETS1 recruited P300 and WDR5 which separately mediated H3K27ac and Histone H3 lysine 4 trimethylation (H3K4me3) histone modification in the promoter of METTL3 and induced METTL3 transcription activation. Furthermore, we identified TXNDC5 as a target of METTL3-mediated m6A modification through MeRIP-seq, and revealed that METTL3-mediated TXNDC5 expression relied on the m6A reader-dependent manner. METTL3 mediated the m6A modification of cathepsin L (Procathepsin L (CTSL)) mRNA at the 5'-UTR, and the m6A reader protein insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) bound to the m6A sites and enhanced CTSL mRNA stability. Our results indicated that METTL3 enhanced CTSL mRNA stability through an m6A-IGF2BP2-dependent mechanism, thereby promoting cervical cancer cell metastasis. | ||||
| Responsed Disease | Cervical cancer | ICD-11: 2C77 | |||
In-vitro Model |
Ca Ski | Cervical squamous cell carcinoma | Homo sapiens | CVCL_1100 | |
| SiHa | Cervical squamous cell carcinoma | Homo sapiens | CVCL_0032 | ||
| HEK293-FT | Normal | Homo sapiens | CVCL_6911 | ||
| In-vivo Model | Female BALB/c nude mice (4-5 weeks old) were purchased from the Center of Experimental Animals of Guangdong. To establish a tail vein metastasis model, 2 × 106 SiHa cells in 200 μ l PBS were injected into the tail vein of each mouse (n = 6 for both METTL3-overexpressing and empty vector groups). The mice were killed at approximately 8 weeks, and lung tissues were isolated and embedded in paraffin. Hematoxylin and eosin staining was then used to determine the number of lung metastasis nodules. To establish the popliteal lymph node metastasis model, 1 × 106 SiHa cells in 50 μ l PBS were injected subcutaneously into the footpad of each mouse (n = 6 for both groups). Cells from the experimental and control groups were inoculated under the right and left footpads of each mouse, respectively. After 8 weeks, the popliteal lymph nodes were excised. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Procathepsin L (CTSL) | 45 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| KGP94 | Clinical trial | [3] | ||
| Synonyms |
CHEMBL1269632; BDBM50330030
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27998201-Compound-1 | Patented | [4] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27998201-Compound-19 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 3.6 nM | |||
| External Link | ||||
| Phenylalanine derivative 1 | Patented | [4] | ||
| Synonyms |
PMID27998201-Compound-21
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27998201-Compound-7 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.6 nM | |||
| External Link | ||||
| PMID27998201-Compound-6 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 8.06 nM | |||
| External Link | ||||
| PMID27998201-Compound-17 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 56 nM | |||
| External Link | ||||
| PMID27998201-Compound-9 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 0.0031 nM | |||
| External Link | ||||
| PMID25399719-Compound-17 | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 66000 nM | |||
| External Link | ||||
| PMID27998201-Compound-5 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | Ki < 100 nM | |||
| External Link | ||||
| PMID27998201-Compound-12 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 0.25 nM | |||
| External Link | ||||
| CLIK-148 | Preclinical | [6] | ||
| Synonyms |
SCHEMBL7207304
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CLIK-181 | Preclinical | [7] | ||
| Synonyms |
J3.633.044I
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SD1002 | Preclinical | [7] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Z-Phe-Ala-diazomethylketone | Preclinical | [7] | ||
| Synonyms |
71732-53-1; CHEMBL2179950; carbobenzoxycarbonyl-phenylalanyl-alaninyldiazomethane; ZPAD; Z-FA-DMK; SCHEMBL9364460; SCHEMBL17747846; SCHEMBL17747847; ZINC4899534; BDBM50400264; MFCD00077029; (Z,3S)-1-diazonio-3-[[(2S)-3-phenyl-2-(phenylmethoxycarbonylamino)propanoyl]amino]but-1-en-2-olate; Q27278127; benzyl (S)-1-((S)-4-diazo-3-oxobutan-2-ylamino)-1-oxo-3-phenylpropan-2-ylcarbamate; benzyl N-[(2S)-1-[[(2S)-4-diazo-3-oxobutan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]carbamate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SD1003 | Preclinical | [7] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| L-006235-1 | Preclinical | [8] | ||
| Synonyms |
294623-49-7; L 006235; CHEMBL426819; N-(1-((cyanomethyl)carbamoyl)cyclohexyl)-4-(2-(4-methylpiperazin-1-yl)thiazol-4-yl)benzamide; L006235; N-[1-[[(Cyanomethyl)amino]carbonyl]cyclohexyl]-4-[2-(4-methyl-1-piperazinyl)-4-thiazolyl]benzamide; SCHEMBL6183485; CTK8E9371; BDBM19854; DTXSID90432735; MolPort-023-276-653; BCP28510; ZINC3993799; AKOS024457410; basic piperazine-containing compound, 1; NCGC00371088-01; RT-013466; CRA-013783/L-006235; J-017526; L-006235; L-006,235
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 340 nM | |||
| External Link | ||||
| [(3-Bromophenyl)-m-tolyl-ketone]thiosemicarbazone | Investigative | [9] | ||
| Synonyms |
CHEMBL602092
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 224 nM | |||
| External Link | ||||
| Bis(3-bromophenyl)(5-hydroxy)thiosemicarbazone | Investigative | [3] | ||
| Synonyms |
CHEMBL1271493
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 232.4 nM | |||
| External Link | ||||
| CAA0225 | Investigative | [10] | ||
| Synonyms |
CAA-0225; GTPL6532; (2S,3S)-2-N-[(1S)-1-(benzylcarbamoyl)-2-phenylethyl]-3-N-[2-(4-hydroxyphenyl)ethyl]oxirane-2,3-dicarboxamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BIPHENYL-4-YL-ACETALDEHYDE | Investigative | [11] | ||
| Synonyms |
2-([1,1'-biphenyl]-4-yl)acetaldehyde; 61502-90-7; 2-(4-phenylphenyl)acetaldehyde; 4-biphenylacetaldehyde; 2-{[1,1'-biphenyl]-4-yl}acetaldehyde; AC1MRDQD; (biphenyl-4-yl)ethanone; SCHEMBL850634; 2-(biphenyl-4-yl)acetaldehyde; CTK2D8632; DTXSID30392980; OIDMZCMVYZLDLI-UHFFFAOYSA-N; MolPort-020-915-677; [1,1'-Biphenyl]-4-acetaldehyde; ZINC2581188; AKOS006278290; AS-49992; BP4
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Peptide alpha-keto-beta-aldehyde-based inhibitors | Investigative | [12] | ||
| Synonyms |
CHEMBL48605; BDBM50090643; (3S)-3-[(Ac-L-Leu-L-Leu-)Amino]-6-guanidino-2-oxohexanal; (S)-2-Acetylamino-4-methyl-pentanoic acid {(S)-1-[(S)-4-guanidino-1-(2-oxo-acetyl)-butylcarbamoyl]-3-methyl-butyl}-amide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 970 nM | |||
| External Link | ||||
| (3-Bromobenzoylpyridine)thiosemicarbazone | Investigative | [3] | ||
| Synonyms |
CHEMBL1269715
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1000 nM | |||
| External Link | ||||
| 1-(1,3-diphenylpropylidene)thiosemicarbazide | Investigative | [13] | ||
| Synonyms |
CHEMBL429858; BDBM50377591
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 910 nM | |||
| External Link | ||||
| 6-(benzylamino)-9-butyl-9H-purine-2-carbonitrile | Investigative | [14] | ||
| Synonyms |
Compound 3{8,12}; CHEMBL255079
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4380 nM | |||
| External Link | ||||
| [2-Phenylacetophenone]thiosemicarbazone | Investigative | [3] | ||
| Synonyms |
CHEMBL1269810
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5410 nM | |||
| External Link | ||||
| Bis(3-Fluorophenyl)-ketone]thiosemicarbazone | Investigative | [9] | ||
| Synonyms |
CHEMBL602732
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4870 nM | |||
| External Link | ||||
| 9-benzyl-6-(benzylamino)-9H-purine-2-carbonitrile | Investigative | [14] | ||
| Synonyms |
Compound 3{8,17}; CHEMBL400454
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 12220 nM | |||
| External Link | ||||
| 1-(phenyl(p-tolyl)methylene)thiosemicarbazide | Investigative | [13] | ||
| Synonyms |
CHEMBL402465
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1020 nM | |||
| External Link | ||||
| Bis(3-bromophenyl)(4-hydroxy)thiosemicarbazone | Investigative | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 126.1 nM | |||
| External Link | ||||
| [(3-Bromophenyl)-p-tolyl-ketone]thiosemicarbazone | Investigative | [9] | ||
| Synonyms |
CHEMBL590497
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2160 nM | |||
| External Link | ||||
| N-(tert-butoxycarbonyl)-tyrosyl-glycine-nitrile | Investigative | [15] | ||
| Synonyms |
dipeptide-derived nitrile, 23; CHEMBL371893; BDBM20102; tert-butyl N-[(1S)-1-[(cyanomethyl)carbamoyl]-2-(4-hydroxyphenyl)ethyl]carbamate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (S)-tert-butyl 1-oxohexan-2-ylcarbamate | Investigative | [16] | ||
| Synonyms |
CHEMBL96875; ((S)-1-Formyl-pentyl)-carbamic acid tert-butyl ester; BML-244; Carbamic acid, [(1S)-1-formylpentyl]-, 1,1-dimethylethyl ester; SCHEMBL3285479; CTK0G6629; OBMGXPJNZKYOQY-VIFPVBQESA-N; ZINC13588585; BDBM50137790; AKOS030572335; tert-butyl(1S)-1-formylpentylcarbamate; CCG-207873; 2(S)-(tert-Butoxycarbonylamino)hexanal; tert-butyl (1S)-1-formylpentylcarbamate; (2S)-2-(tert-Butoxycarbonylamino)hexanal; (S)-2-(tert-butoxycarbonylamino)-5-methylpentanal
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 8900 nM | |||
| External Link | ||||
| N-(tert-butoxycarbonyl)-isoleucyl-glycine-nitrile | Investigative | [15] | ||
| Synonyms |
dipeptide-derived nitrile, 9; CHEMBL197113; BDBM20088; tert-butyl N-[(1S,2R)-1-[(cyanomethyl)carbamoyl]-2-methylbutyl]carbamate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(tert-butoxycarbonyl)-valyl-glycine-nitrile | Investigative | [15] | ||
| Synonyms |
N-(tert-butoxycarbonyl)-L-valyl-glycine-nitrile; 191033-03-1; dipeptide-derived nitrile, 5; CHEMBL200004; BDBM20085; tert-butyl N-[(1S)-1-[(cyanomethyl)carbamoyl]-2-methylpropyl]carbamate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(tert-butoxycarbonyl)-norleucyl-glycine-nitrile | Investigative | [15] | ||
| Synonyms |
dipeptide-derived nitrile, 13; CHEMBL383584; BDBM20092; tert-butyl N-[(1S)-1-[(cyanomethyl)carbamoyl]pentyl]carbamate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(4-phenylbenzoyl)-phenylalanyl-glycine-nitrile | Investigative | [15] | ||
| Synonyms |
phenylalanine derivative, 44; CHEMBL200235; BDBM20120
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(tert-butoxycarbonyl)-leucyl-glycine-nitrile | Investigative | [15] | ||
| Synonyms |
dipeptide-derived nitrile, 7; CHEMBL200160; SCHEMBL6257676; BDBM20087; tert-butyl N-[(1S)-1-[(cyanomethyl)carbamoyl]-3-methylbutyl]carbamate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-acetyl-phenylalanyl-glycine-nitrile | Investigative | [15] | ||
| Synonyms |
phenylalanine derivative, 42; CHEMBL197181; SCHEMBL15634553; BDBM20118; ITHLBMBCVIAAIX-LBPRGKRZSA-N; ZINC13676602; (N-acetyl-l-phenylalanyl)aminoacetonitrile; SR-03000002938; SR-03000002938-1; (S)-2-Acetamido-N-(cyanomethyl)-3-phenylpropanamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(benzyloxycarbonyl)-leucyl-glycine-nitrile | Investigative | [15] | ||
| Synonyms |
dipeptidyl nitrile, 1; Cbz-Leu-NH-CH2-CN; JMC487688 Compound 8; CHEMBL200161; SCHEMBL6183068; BDBM19768; UFXQLUZNMRVTPU-AWEZNQCLSA-N; 2-[(Z-L-Leu-)Amino]ethanenitrile; benzyl (S)-1-cyanomethylcarbamoyl-3-methylbutylcarbamate; benzyl N-[(1S)-1-[(cyanomethyl)carbamoyl]-3-methylbutyl]carbamate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 2790 nM | |||
| External Link | ||||
| N-benzoyl-phenylalanyl-glycine-nitrile | Investigative | [15] | ||
| Synonyms |
phenylalanine derivative, 43; SCHEMBL5517755; CHEMBL371466; BDBM20119
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(tert-butoxycarbonyl)-methionyl-glycine-nitrile | Investigative | [15] | ||
| Synonyms |
dipeptide-derived nitrile, 12; CHEMBL381847; BDBM20091; tert-butyl N-[(1S)-1-[(cyanomethyl)carbamoyl]-3-(methylsulfanyl)propyl]carbamate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (S)-1-benzylcyclopentyl 1-oxohexan-2-ylcarbamate | Investigative | [16] | ||
| Synonyms |
CHEMBL117658; 1-(PHENYLMETHYL)CYCLOPENTYL[(1S)-1-FORMYLPENTYL]CARBAMATE; 2auz; BDBM50148292; DB07593; 1-benzylcyclopentyl [(1S)-1-formylpentyl]carbamate; 1-benzylcyclopentyl N-[(2S)-1-oxohexan-2-yl]carbamate; ((S)-1-Formyl-pentyl)-carbamic acid 1-benzyl-cyclopentyl ester
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2000 nM | |||
| External Link | ||||
| Cysteinesulfonic Acid | Investigative | [17] | ||
| Synonyms |
Cysteic Acid; 3-Sulfoalanine; 2-amino-3-sulfopropanoic acid; DL-CYSTEIC ACID; 13100-82-8; beta-Sulfoalanine; Alanine, 3-sulfo-; Cysteinic acid; Cysteric acid; Cipteic acid; Cepteic acid; 3024-83-7; CHEBI:21260; C-9550; 2-amino-3-sulfopropanoate; cysteinsaure; Cepteate; Cysterate; Cipteate; Cysteinesulfonate; NSC 254030; (2R)-2-amino-3-sulfo-propanoic acid; L-Cysteic acid, 8; ACMC-209kii; 3-Sulfoalanine, (L)-; 2-Amino-3-sulfopropionate; AC1L19KC; SCHEMBL44030; CHEMBL1171434; 2-amino-3-sulfopro-panoic acid; BDBM85473; CTK8G7889
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| L-873724 | Investigative | [18] | ||
| Synonyms |
UNII-29250PP3ON; 603139-12-4; CHEMBL437501; 29250PP3ON; (2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifluoro-1-[4'-(methylsulfonyl)[1,1'-biphenyl]-4-yl]ethyl]amino]Pentanamide; (2S)-N-(cyanomethyl)-4-methyl-2-[[(1S)-2,2,2-trifluoro-1-[4-(4-methylsulfonylphenyl)phenyl]ethyl]amino]pentanamide; VYFDSJLOCIGIKP-SFTDATJTSA-N; GTPL7860; SCHEMBL2157182; BDBM19489; (+)-L-873724; ZINC34802820; CS-6814; HY-50887; Pentanamide, N-(cyanomethyl)-4-methyl-2-(((1S)-2,2,2-trifluoro-1-(4'-(methylsulfonyl)(1,1'-biphenyl)-4-yl)eth
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1221 nM | |||
| External Link | ||||
| WD repeat-containing protein 5 (WDR5) | 1 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| OICR-9429 | Investigative | [19] | ||
| Synonyms |
1801787-56-3; OICR9429; CHEMBL3798846; N-(4-(4-Methylpiperazin-1-Yl)-3'-(Morpholinomethyl)-[1,1'-Biphenyl]-3-Yl)-6-Oxo-4-(Trifluoromethyl)-1,6-Dihydropyridine-3-Carboxamide; N-[2-(4-methylpiperazin-1-yl)-5-[3-(morpholin-4-ylmethyl)phenyl]phenyl]-6-oxo-4-(trifluoromethyl)-1,6-dihydropyridine-3-carboxamide; GTPL8231; OICR 9429; MolPort-039-101-294; EX-A2417; BCP18185; BDBM50164794; s7833; AKOS025147341; ZINC231558892; SB19642; CS-5776; NCGC00371263-02; AK468854; HY-16993; J3.618.049H
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
References