m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03445
|
[1], [2] | |||
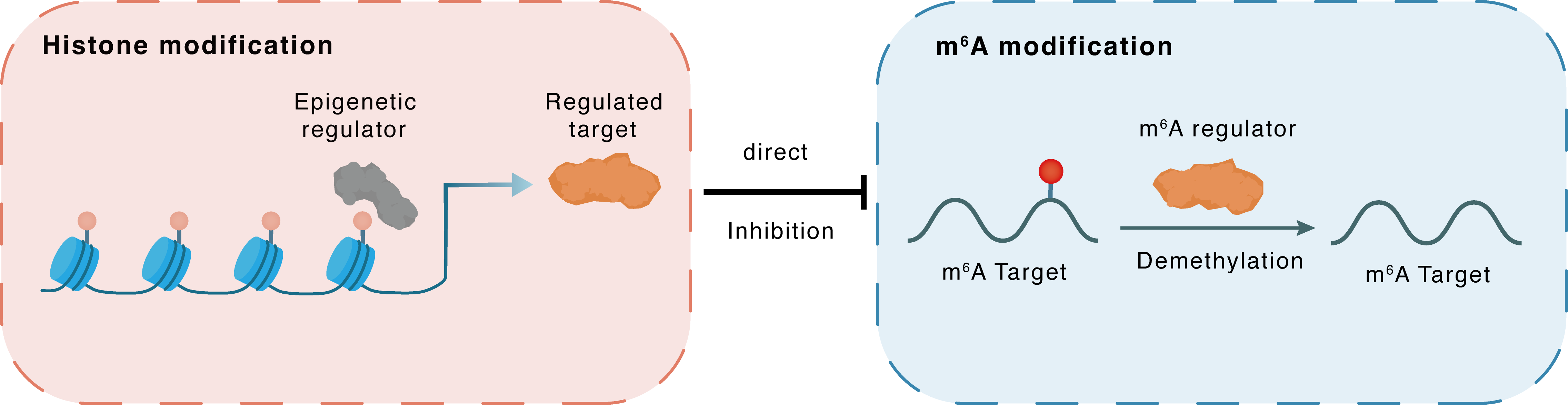 Histone modification
H3K27ac
HDAC1
FTO
Direct
Inhibition
m6A modification
PARP1
PARP1
FTO
Demethylation
Histone modification
H3K27ac
HDAC1
FTO
Direct
Inhibition
m6A modification
PARP1
PARP1
FTO
Demethylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | |||
| m6A Target | Poly [ADP-ribose] polymerase 1 (PARP1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone deacetylase 1 (HDAC1) | ERASER | View Details | ||
| Regulated Target | Histone H3 lysine 27 acetylation (H3K27ac) | View Details | |||
| Downstream Gene | FTO | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Inhibition | |||
| Crosstalk Mechanism | histone modification directly impacts m6A modification through modulating the level of m6A regulator | ||||
| Crosstalk Summary | HDACi reduces ferroptosis suppressor protein (FSP1) by promoting its mRNA degradation. Specifically, it is confirmed that HDACi specifically targets HDAC1 and promotes the Histone H3 lysine 27 acetylation (H3K27ac) modification of fat mass- and obesity-associated gene (FTO) and AlkB Homolog 5, RNA Demethylase (ALKBH5), which results in significant activation of FTO and ALKBH5. The activation of FTO and ALKBH5 reduces N6-methyladenosine (m6A) modification on FSP1 mRNA, leading to its degradation. Crucially, lactylation of HDAC1K412is essential for ferroptosis regulation. Both Vorinostat (SAHA) and Trichostatin A (TSA) notably diminish HDAC1K412lactylation in comparison to other HDAC1 inhibitors, exhibiting a consistent trend of increasing susceptibility to ferroptosis. Targeting FTO significantly suppresses cancer cell growth and enhances chemotherapy sensitivity, which not only mediating the balance of intracellular ROS by regulating G6PD expression, but also maintaining genome instability by regulating Poly [ADP-ribose] polymerase 1 (PARP1) expression. These findings shed light on new molecular mechanisms of CRC development and treatments mediated by m6A modification. | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Pathway Response | Glutathione metabolism | hsa00480 | |||
In-vitro Model |
LoVo | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | |
| HCT 116 | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| HCT 8 | Colon adenocarcinoma | Homo sapiens | CVCL_2478 | ||
| SW620 | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| In-vivo Model | For CDX model, nude mice (female, 4-6-week-old) were subcutaneously injected with 5 × 106 HCT116 cells on the both flank. For PDX model, the patient tumors were divided into small pieces and then inoculated on both flank of nude mice. For knockdown FTO mice model, FTO mice model, two weeks after inoculation, the shFTO#3 lenti-virus injected into the tumor for three consecutive days. For combined medication mice model, intraperitoneal injection of Rhein and Olaparib was started one week after inoculation. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Histone deacetylase 1 (HDAC1) | 250 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Panobinostat | Approved | [3] | ||
| Synonyms |
Faridak; LBH 589; LBH589; LBH-589; LBH-589B; NVP-LBH589; NVP-LBH-589; Panobinostat, NVP-LBH589, LBH589; (E)-N-HYDROXY-3-(4-{[2-(2-METHYL-1H-INDOL-3-YL)-ETHYLAMINO]-METHYL}-PHENYL)-ACRYLAMIDE; (E)-N-hydroxy-3-[4-[[2-(2-methyl-1H-indol-3-yl)ethylamino]methyl]phenyl]prop-2-enamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 0.54 nM | |||
| External Link | ||||
| Vorinostat | Approved | [3] | ||
| Synonyms |
NHNPODA; SAHA; SHH; Zolinza; Merck brand of Vorinostat; OCTANEDIOIC ACID HYDROXYAMIDE PHENYLAMIDE; SAHA cpd; Suberanilohydroxamic acid; Suberoylanilide hydroxamic acid; Vorinostat MSD; Vorinostat [USAN]; M344; MK0683; SKI390; WIN64652; MK-0683; SAHA, Suberoylanilide hydroxamic acid; SW-064652; Zolinza (TN); Vorinostat (JAN/USAN); N1-hydroxy-N8-phenyloctanediamide; Zolinza, MK-0683, SAHA; N'-hydroxy-N-phenyloctanediamide; N-Hydroxy-N'-phenyl octanediamide; N-Hyrdroxy-N'-phenyloctanediamide; N-hydroxy-N'-phenyloctanediamide; N-hydroxy-N'-phenyl-octane-1,8-diotic acid diamide; Vorinostat (HDAC inhibitor)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 1.3 nM | |||
| External Link | ||||
| Romidepsin | Approved | [4] | ||
| Synonyms |
Chromadax; Istodax; Antibiotic FR 901228; FK 228; FK228; FR 901228; FR901228; HDInhib_000006; Chromadax (TN); FK-228; FK-901228; FR-901228; Istodax (TN); Romidepsin (USAN); Cyclo((2Z)-2-amino-2-butenoyl-L-valyl-(3S,4E)-3-hydroxy-7-mercapto-4-heptenoyl-D-valyl-D-cysteinyl), cyclic (35)-disulfide; L-Valine, N-((3S,4E)-3-hydroxy-7-mercapto-1-oxo-4-heptenyl)-D-valyl-D-cysteinyl-(2Z)-2-amino-2-butenoxyl-, (4-1)-lactone, cyclic (1-2)-disulfide; (1S,4S,7Z,10S,16E,21R)-7-Ethylidene-4,21-bis(1-methylethyl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo(8.7.6)tricos-16-ene-3,6,9,19,22-pentone; (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone; (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetrazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 0.0015 nM | |||
| External Link | ||||
| HBI-8000 | Phase 1/2 | [5] | ||
| Synonyms |
CS055; SCHEMBL5500152; GTPL8305; AKOS026750315; N-(2-amino-5-fluorophenyl)-4-{[3-(pyridin-3-yl)prop-2-enamido]methyl}benzamide
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 167 nM | |||
| External Link | ||||
| NVP-LAQ824 | Phase 3 | [6] | ||
| Synonyms |
Dacinostat; 404951-53-7; LAQ824; LAQ-824; LAQ824 (Dacinostat); UNII-V10P524501; (E)-N-hydroxy-3-[4-[[2-hydroxyethyl-[2-(1H-indol-3-yl)ethyl]amino]methyl]phenyl]prop-2-enamide; CHEMBL356066; V10P524501; (2E)-N-hydroxy-3-[4-({(2-hydroxyethyl)[2-(1H-indol-3-yl)ethyl]amino}methyl)phenyl]prop-2-enamide; Dacinostat [INN]; (E)-N-Hydroxy-3-[4-[[2-hydroxyethyl-[2-(1H-indol-3-yl)ethyl]amino]meth yl]phenyl]prop-2-enamide; (2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol-3-yl)ethyl]amino]methyl}phenyl)prop-2-enamide; NVP-LAQ 824; NVP-LAQ824, Dacinostat, LAQ824; LBH539
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 0.55 nM | |||
| External Link | ||||
| SNDX-275 | Phase 3 | [7] | ||
| Synonyms |
Entinostat; Histone Deacetylase Inhibitor I; IN1470; MS 275; SNDX 275; MS 27-275; Ms-275; Entinostat (USAN/INN); MS-27-275; Pyridin-3-ylmethyl 4-(2-aminophenylcarbamoyl)benzylcarbamate; Pyridin-3-ylmethyl N-[[4-[(2-aminophenyl)carbamoyl]phenyl]methyl]carbamate; Pyridin-3-ylmethyl {4-[(2-aminophenyl)carbamoyl]benzyl}carbamate; Pyridin-3-ylmethyl{4-[(2-aminophenyl)carbamoyl]benzyl}carbamate; Carbamic acid, [[4-[[(2-aminophenyl)amino]carbonyl]phenyl] methyl]-, 3-pyridinylmethyl ester; Carbamic acid, [[4-[[(2-aminophenyl)carbaonyl]phenyl]methyl]-, 3-pyridinylmethyl ester; Entinostat, SNDX-275, MS-27-275, MS-275; N-(2-Aminophenyl)-4-[N-(pyridin-3-yl-methoxycarbonyl)aminomethyl]benzamide; N-(2-aminophenyl)-4-(N-(pyridin-3-ylmethoxycarbonyl)aminomethyl)benzamide; Carbamic acid, ((4-(((2-aminophenyl)amino)carbonyl)phenyl)methyl)-, 3-pyridinylmethyl ester; 3-Pyridinylmethyl ((4-(((2-aminophenyl)amino)carbonyl)phenyl)methyl)carbamate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 22 nM | |||
| External Link | ||||
| ITF2357 | Phase 3 | [8] | ||
| Synonyms |
Givinostat; Carbamic acid, N-(4-((hydroxyamino)carbonyl)phenyl)-, (6-((diethylamino)methyl)-2-naphthalenyl)methyl ester, hydrochloride (1:1)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 2 nM | |||
| External Link | ||||
| Resminostat | Phase 2 | [9] | ||
| Synonyms |
864814-88-0; 4SC-201; RAS2410; Resminostat (RAS2410); UNII-1578EUB98L; (E)-3-(1-((4-((dimethylamino)methyl)phenyl)sulfonyl)-1H-pyrrol-3-yl)-N-hydroxyacrylamide; BYK408740; 1578EUB98L; Resminostat [INN]; 4SC 201; Resminostat 4SC-201; Resminostat (4SC-201); SCHEMBL295541; SCHEMBL295540; GTPL7502; EX-A542; DTXSID50235587; MolPort-027-720-936; AOB87187; BCP02538; 4SC201; ZINC13983495; s2693; AKOS030526945; SB16667; DB12392; CS-1521; API0013984; BC261895
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Sodium butyrate | Phase 2 | [10] | ||
| Synonyms |
Butyrate sodium; Sodium butanoate; Sodium propanecarboxylate; OR8783; Butanoic acid, sodium salt; Butyric Acid, Na; Sodium butyrate (USP); Sodium n-butyrate; TPA/BA; Tetradecanoyl phorbol acetate/ sodium butyrate; Butanoic acid, sodium salt (1:1); Tetradecanoyl phorbol acetate (TPA)/ sodium butyrate (BA)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SB-623 | Phase 2 | [11] | ||
| Synonyms |
(E)-3-[1-(2-diethylaminoethyl)-2-phenethylbenzimidazol-5-yl]-N-hydroxyprop-2-enamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 52 nM | |||
| External Link | ||||
| MGCD-0103 | Phase 2 | [3] | ||
| Synonyms |
Mocetinostat; MG 0103; MG 4230; MG 4915; MG 5026; MG0103; MG4230; MG4915; MG5206; MGCD 0103; MGCD0103; MG-0103; MG-4230; MG-4915; MG-5026; Mocetinostat, MGCD0103; N-(2-aminophenyl)-4-[[(4-pyridin-3-ylpyrimidin-2-yl)amino]methyl]benzamide; N-(2-Aminophenyl)-4-((4-pyridin-3-ylpyrimidin-2-ylamino)methyl)benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 9 nM | |||
| External Link | ||||
| Phenylbutyrate | Phase 2 | [12] | ||
| Synonyms |
Benzenebutyric acid; Phenyl butanoate; Phenyl butyrate; HDInhib_000004; Butanoic acid, phenyl ester; Butyric acid, phenyl ester; FR-2080; Gamma-Phenylbutyric acid; Omega-Phenylbutanoic acid; GAMMA-PHENYL-BUTYRIC ACID; Butyric acid, 4-phenyl-(8CI); 1-Phenylbutyric acid; 4-PHENYL-BUTANOIC ACID; 4-PHENYLBUTYRIC ACID; 4-Phenylbutanoic acid; 4-phenylbutans; 4-phenylbutyrate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 64000 nM | |||
| External Link | ||||
| CHR-3996 | Phase 1/2 | [13] | ||
| Synonyms |
CCT-075453; CHR-2504; HDAC inhibitors, Chroma Therapeutics; Histone deacetylase inhibitors, Chroma
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3 nM | |||
| External Link | ||||
| SB-639 | Phase 1 | [11] | ||
| Synonyms |
CHEMBL491316; AC1OCG09; SCHEMBL13118363; BDBM50248476; US8551988, 72; N-hydroxy-3-(2-phenethyl-1-(2-(pyrrolidin-1-yl)ethyl)-1H-benzo[d]imidazol-5-yl)acrylamide; (E)-N-hydroxy-3-[2-phenethyl-1-(2-pyrrolidin-1-ylethyl)benzimidazol-5-yl]prop-2-enamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 11 nM | |||
| External Link | ||||
| RG-2833 | Phase 1 | [5] | ||
| Synonyms |
RG-FA; HDAC-1 inhibtors (Friedreich ataxia), RepliGen
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | Ki = 32 nM | |||
| External Link | ||||
| OBP-801 | Phase 1 | [14] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 3.3 nM | |||
| External Link | ||||
| PMID29671355-Compound-42 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 to 100 nM | |||
| External Link | ||||
| PMID28092474-Compound-33d | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32u | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-33a | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32a | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32j | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32z | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32g | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-34c | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32x | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-33b | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32b | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32o | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-33g | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-33j | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-33p | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-33m | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32v | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID29671355-Compound-24 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 382 nM | |||
| External Link | ||||
| PMID28092474-Compound-34b | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-33e | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32t | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32c | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-33i | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32r | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32h | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID29671355-Compound-65a | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 13800 nM | |||
| External Link | ||||
| PMID28092474-Compound-32y | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-33h | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID29671355-Compound-38a | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1000 nM | |||
| External Link | ||||
| PMID28092474-Compound-33f | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| Diaryl amine derivative 3 | Patented | [16] | ||
| Synonyms |
PMID28092474-Compound-11
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 460 nM | |||
| External Link | ||||
| PMID28092474-Compound-33c | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID29671355-Compound-39 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 3840 nM | |||
| External Link | ||||
| PMID29671355-Compound-19 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 to 1000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32e | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32m | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32p | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32d | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32n | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-33k | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32k | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-34a | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| Isosteric imidazolyl pyrimidine derivative 1 | Patented | [17] | ||
| Synonyms |
PMID26161698-Compound-37
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 656 nM | |||
| External Link | ||||
| PMID29671355-Compound-22 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2100 nM | |||
| External Link | ||||
| PMID29671355-Compound-26 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 8750 nM | |||
| External Link | ||||
| PMID28092474-Compound-33o | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32f | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| Diaryl amine derivative 2 | Patented | [16] | ||
| Synonyms |
PMID28092474-Compound-10
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 240 nM | |||
| External Link | ||||
| PMID28092474-Compound-33l | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32i | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32q | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID29671355-Compound-18 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 10000 nM | |||
| External Link | ||||
| PMID29671355-Compound-57 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID29671355-Compound-27 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 187 nM | |||
| External Link | ||||
| PMID28092474-Compound-32l | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 1000 nM; IC50 <= 10000 nM | |||
| External Link | ||||
| PMID28092474-Compound-32s | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID29671355-Compound-38b | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 226 nM | |||
| External Link | ||||
| PMID29671355-Compound-28 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 28 nM | |||
| External Link | ||||
| PMID29671355-Compound-59 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 3 nM | |||
| External Link | ||||
| PMID29671355-Compound-55 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID29671355-Compound-73 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1000 nM | |||
| External Link | ||||
| PMID29671355-Compound-13 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 >= 10000 nM | |||
| External Link | ||||
| PMID29671355-Compound-11 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 21 nM | |||
| External Link | ||||
| PMID29671355-Compound-9 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 94 nM | |||
| External Link | ||||
| PMID29671355-Compound-8 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 16900 nM | |||
| External Link | ||||
| PMID29671355-Compound-61 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2.6 nM | |||
| External Link | ||||
| PMID29671355-Compound-23 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 3620 nM | |||
| External Link | ||||
| PMID29671355-Compound-44 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 150 nM | |||
| External Link | ||||
| PMID29671355-Compound-56 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 8700 nM | |||
| External Link | ||||
| PMID29671355-Compound-67 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2900 nM | |||
| External Link | ||||
| PMID29671355-Compound-31 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.3 nM | |||
| External Link | ||||
| PMID29671355-Compound-21 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2120 nM | |||
| External Link | ||||
| PMID29671355-Compound-62 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 500 nM | |||
| External Link | ||||
| PMID29671355-Compound-43 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 23 nM | |||
| External Link | ||||
| PMID29671355-Compound-25 | Patented | [15] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 175 nM | |||
| External Link | ||||
| AN-9 | Discontinued in Phase 2 | [10] | ||
| Synonyms |
Pivanex; Pivalyloxymethyl butyrate; AN 9; AN9; AN 9 (ion exchanger); Butanoyloxymethyl 2,2-dimethylpropanoate; Butanoicacid, (2,2-dimethyl-1-oxopropoxy)methyl ester; N-(5-amino-9,10-dioxoanthracen-1-yl)acetamide; N-(5-amino-9,10-dioxo-9,10-dihydroanthracen-1-yl)acetamide; ((2,2-Dimethylpropanoyl)oxy)methyl butanoate;1,5-BIS[3-(DIETHYLAMINO)PROPIONAMIDO]ANTHRACENE-9,10-DIONE
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Tacedinaline | Discontinued in Phase 2 | [11] | ||
| Synonyms |
Acetyldinaline; Tacedinalina; Goe 5549; PD 123654; Ci-994; Goe-5549; PD-123654; Tacedinalina [INN-Spanish]; Tacedinaline [USAN:INN]; C.I. 994; Tacedinaline (USAN/INN); 4-(Acetylamino)-N-(2-aminophenyl)benzamide; 4-acetamido-N-(2-aminophenyl)benzamide; 4-acetamido-n-(2-aminophenyl)benzamid; 4-acetylamino-N-(2'-aminophenyl)benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 50 nM | |||
| External Link | ||||
| Pyroxamide | Discontinued in Phase 1 | [11] | ||
| Synonyms |
POLAR HYBRID COMPOUND; Suberoyl-3-aminopyridineamide hydroxamic acid; N-Hydroxy-N'-3-pyridinyloctanediamide; N'-hydroxy-N-pyridin-3-yloctanediamide; N-hydroxy-n'-(pyridin-3-yl)octanediamide; N-Hydroxy-N'-(3-pyridyl)-1,8-octanediamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 2.7 nM | |||
| External Link | ||||
| M-carboxycinnamic acid bishydroxamide | Preclinical | [11] | ||
| Synonyms |
Cbha; Histone Deacetylase Inhibitor II; HDInhib_000062; M-Carboxycinnamic Acid bis-Hydroxamide; N-hydroxy-3-[(E)-3-(hydroxyamino)-3-oxoprop-1-enyl]benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| HC-Toxin | Preclinical | [11] | ||
| Synonyms |
HC Toxin; Cyclo(aoe-pro-ala-ala); Cyclo(2-amino-8-oxo-9,10-epoxydecanoic acid-prolyl-alanyl-alanine); Cyclic(L-alanyl-D-alanyl-eta-oxo-L-alpha-aminooxiraneoctanoyl-D-prolyl); Cyclo(L-alanyl-D-alanyl-(alphaS,2S)-alpha-amino-eta-oxooxiraneoctanoyl-D-prolyl); (3S,6R,9S,12R)-6,9-dimethyl-3-[6-(oxiran-2-yl)-6-oxohexyl]-1,4,7,10-tetrazabicyclo[10.3.0]pentadecane-2,5,8,11-tetrone
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SK-7068 | Preclinical | [11] | ||
| Synonyms |
N-[[4-[(E)-2-(hydroxycarbamoyl)ethenyl]phenyl]methyl]-4-pyrrolidin-1-yl-benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Scriptaid | Preclinical | [11] | ||
| Synonyms |
CGK1026; IN1099; SB-556629; GNF-PF-2024; N-Hydroxy-1,3-dioxo-1H-benz(de)isoquinoline-2(3H)-hexan amide; 6-(1,3-Dioxo-1H,3H-benzo[de]isoquinolin-2-yl)-hexanoic acid hydroxyamide; 6-(1,3-Dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)-N-hydroxyhexanamide; 6-(1,3-dioxobenzo[de]isoquinolin-2-yl)-N-hydroxyhexanamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 1.5 nM | |||
| External Link | ||||
| 4SC-202 | Preclinical | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Chlamydocin | Preclinical | [11] | ||
| Synonyms |
HDInhib_000038; Cyclic(2-methylalanyl-L-phenylalanyl-D-propyl-L-alpha-amino-eta-oxooxiraneoctanoyl); (3S,9S,12R)-3-benzyl-6,6-dimethyl-9-[6-[(2S)-oxiran-2-yl]-6-oxohexyl]-1,4,7,10-tetrazabicyclo[10.3.0]pentadecane-2,5,8,11-tetrone; (3s,9s,14ar)-9-benzyl-6,6-dimethyl-3-{6-[(2s)-oxiran-2-yl]-6-oxohexyl}decahydropyrrolo[1,2-a][1,4,7,10]tetraazacyclododecine-1,4,7,10-tetrone
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SK-7041 | Preclinical | [11] | ||
| Synonyms |
IN-2001; 4-(dimethylamino)-N-[[4-[(E)-3-(hydroxyamino)-3-oxoprop-1-enyl]phenyl]methyl]benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Depudecin | Preclinical | [11] | ||
| Synonyms |
(1R)-1-[(2S,3S)-3-[(E)-2-[(3S)-3-[(1R)-1-hydroxyethyl]oxiran-2-yl]ethenyl]oxiran-2-yl]prop-2-en-1-ol; 139508-73-9
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Oxamflatin | Terminated | [11] | ||
| Synonyms |
NSC729360; CHEBI:258842; I14-11718; NCGC00165855-01; (2E)-5-(3-(phenylsulfonylamino)phenyl)pent-2-ene-4-ynohydroxamic acid; (2E)-5-[3-(Phenylsulfonylamino)phenyl]pent-2-en-4-ynohydroxamic Acid; (E)-5-[3-(benzenesulfonamido)phenyl]-N-hydroxypent-2-en-4-ynamide; 151720-43-3
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-(3-Benzoyl-ureido)-hexanoic acid hydroxyamide | Investigative | [18] | ||
| Synonyms |
UNII-2OJV8MB11B; EX-2; 2OJV8MB11B; CHEMBL1083439; 851365-34-9; Benzamide, N-(((6-(hydroxyamino)-6-oxohexyl)amino)carbonyl)-; Benzamide, N-[[[6-(hydroxyamino)-6-oxohexyl]amino]carbonyl]-; SCHEMBL4258321; CTK2I4390; DTXSID80234280; VQLQZMGNGMOMPU-UHFFFAOYSA-N; BDBM50319235
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 16 nM | |||
| External Link | ||||
| N,8-dihydroxy-8-(naphthalen-2-yl)octanamide | Investigative | [19] | ||
| Synonyms |
CHEMBL319738; SCHEMBL3382032; JWCSCYWHCCHTEF-UHFFFAOYSA-N; BDBM50114820; N-Hydroxy-8-hydroxy-8-(2-naphthyl)octanamide; 8-Hydroxy-8-naphthalen-2-yl-octanoic acid hydroxyamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 35 nM | |||
| External Link | ||||
| N-hydroxy-5-(pyridin-3-yl)thiophene-2-carboxamide | Investigative | [20] | ||
| Synonyms |
CHEMBL216292; SCHEMBL5903988
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1130 nM | |||
| External Link | ||||
| N-hydroxy-8-oxo-8-(pyridin-3-yl)octanamide | Investigative | [19] | ||
| Synonyms |
CHEMBL98911; SCHEMBL3378753; BDBM50114831; 8-Oxo-8-pyridin-3-yl-octanoic acid hydroxyamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 153 nM | |||
| External Link | ||||
| NSC-746457 | Investigative | [21] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 104 nM | |||
| External Link | ||||
| 4-Benzenesulfonylamino-N-hydroxy-benzamide | Investigative | [22] | ||
| Synonyms |
CHEMBL98345; SCHEMBL15480538; BDBM50105682; N-hydroxy-4-(phenylsulfonamido)benzamide; 4-(Phenylsulfonylamino)benzohydroximic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 5000 nM | |||
| External Link | ||||
| N-hydroxy-7-(naphthalen-2-yl)-7-oxoheptanamide | Investigative | [19] | ||
| Synonyms |
CHEMBL320909; SCHEMBL1520961; AQLMJRZLPWGPGD-UHFFFAOYSA-N; BDBM50114827; N-Hydroxy-6-(2-naphthoyl)hexanamide; 7-Naphthalen-2-yl-7-oxo-heptanoic acid hydroxyamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 35 nM | |||
| External Link | ||||
| 4-tert-butyl-N-hydroxybenzamide | Investigative | [23] | ||
| Synonyms |
62034-73-5; CHEMBL249284; Benzamide, 4-(1,1-dimethylethyl)-N-hydroxy-; p-tert-butyl benzohydroxamic acid; Oprea1_740446; SCHEMBL5696343; 4-tert-Butylbenzhydroxamic Acid; 4-tert-Butylbenzohydroxamic acid; CTK2C8432; DTXSID80613051; BDBM50215019; 4-(tert-butyl )-benzhydroxamic acid; ZINC19478595; AKOS000181279
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 715 nM | |||
| External Link | ||||
| N-hydroxy-7-(naphthalen-2-yloxy)heptanamide | Investigative | [24] | ||
| Synonyms |
CHEMBL217083
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.9 nM | |||
| External Link | ||||
| 8-(3-Benzoyl-ureido)-octanoic acid hydroxyamide | Investigative | [18] | ||
| Synonyms |
CHEMBL1083441; SCHEMBL4928514; WUKFQTZVXXYEAB-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 8280 nM | |||
| External Link | ||||
| N-hydroxy-8-(naphthalen-2-yl)non-8-enamide | Investigative | [19] | ||
| Synonyms |
CHEMBL95535; SCHEMBL3382075; BDBM50114823; 8-Naphthalen-2-yl-non-8-enoic acid hydroxyamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 8 nM | |||
| External Link | ||||
| N-hydroxy-5-(pyridin-2-yl)thiophene-2-carboxamide | Investigative | [20] | ||
| Synonyms |
CHEMBL216509; SCHEMBL5903774; BDBM50198477
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 243 nM | |||
| External Link | ||||
| N-hydroxy-5-phenylthiophene-2-carboxamide | Investigative | [20] | ||
| Synonyms |
CHEMBL217573; SCHEMBL5903684
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 900 nM | |||
| External Link | ||||
| Azithromycin-N-benzyltriazolyldecahydroxamic Acid | Investigative | [25] | ||
| Synonyms |
CHEMBL496761; SCHEMBL14328159; BDBM27184; triazole-linked azithromycin-based compound, 16h
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(4-(dimethylamino)phenoxy)-N-hydroxyheptanamide | Investigative | [24] | ||
| Synonyms |
CHEMBL265479
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 18.8 nM | |||
| External Link | ||||
| 8-(biphenyl-4-yl)-N-hydroxy-8-oxooctanamide | Investigative | [19] | ||
| Synonyms |
CHEMBL95313; SCHEMBL1520838; BDBM50114816; 8-Biphenyl-4-yl-8-oxo-octanoic acid hydroxyamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| N-hydroxy-5-(pyridin-4-yl)thiophene-2-carboxamide | Investigative | [20] | ||
| Synonyms |
CHEMBL217816; SCHEMBL5903934
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1260 nM | |||
| External Link | ||||
| Gymnochrome E | Investigative | [26] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 10900 nM | |||
| External Link | ||||
| N-hydroxy-8-(naphthalen-2-yl)oct-7-enamide | Investigative | [19] | ||
| Synonyms |
CHEMBL451182; SCHEMBL3382916; SCHEMBL3382922; PGGPUSMJSOKMEA-XBXARRHUSA-N; ZINC13474418; BDBM50114829; (E)-N-Hydroxy-8-(2-naphthyl)-7-octenamide; 8-Naphthalen-2-yl-oct-7-enoic acid hydroxyamide; (E)-8-Naphthalen-2-yl-oct-7-enoic acid hydroxyamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 6 nM | |||
| External Link | ||||
| N-hydroxybenzo[b]thiophene-2-carboxamide | Investigative | [27] | ||
| Synonyms |
CHEMBL245946; 211172-97-3; Benzo[b]thiophene-2-carboxamide, N-hydroxy-; SCHEMBL999904; CTK0J7987; DTXSID40470925; BDBM50216024; benzothiophene-2-carbohydroxamic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 670 nM | |||
| External Link | ||||
| 7-(1H-indol-5-yloxy)-N-hydroxyheptanamide | Investigative | [24] | ||
| Synonyms |
CHEMBL426516
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3.6 nM | |||
| External Link | ||||
| 8-(4-bromophenyl)-N-hydroxy-8-oxooctanamide | Investigative | [19] | ||
| Synonyms |
CHEMBL319070; SCHEMBL3382402; RFYYZRFJBNAHCG-UHFFFAOYSA-N; BDBM50114817; n-hydroxy-7-(4-bromobenzoyl)heptanamide; 8-(4-Bromo-phenyl)-8-oxo-octanoic acid hydroxyamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 45 nM | |||
| External Link | ||||
| N-hydroxy-7-(4-methoxyphenyl)-7-oxoheptanamide | Investigative | [19] | ||
| Synonyms |
CHEMBL95990; SCHEMBL1521117; NIGGAQZXTHMKPL-UHFFFAOYSA-N; BDBM50114822; N-Hydroxy-6-(4-methoxybenzoyl)hexanamide; 7-(4-Methoxy-phenyl)-7-oxo-heptanoic acid hydroxyamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 450 nM | |||
| External Link | ||||
| N-hydroxy-8-(2-methoxyphenyl)-8-oxooctanamide | Investigative | [19] | ||
| Synonyms |
CHEMBL95885; SCHEMBL3382183; BDBM50114815; 8-(2-Methoxy-phenyl)-8-oxo-octanoic acid hydroxyamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 95 nM | |||
| External Link | ||||
| N-hydroxy-6-oxo-6-phenylhexanamide | Investigative | [19] | ||
| Synonyms |
CHEMBL95152; SCHEMBL1521154; BDBM50114819; 6-Oxo-6-phenyl-hexanoic acid hydroxyamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1500 nM | |||
| External Link | ||||
| N-hydroxy-8-(naphthalen-2-yl)octanamide | Investigative | [19] | ||
| Synonyms |
CHEMBL95747; SCHEMBL3378763; KWFDCRKEDDNSLQ-UHFFFAOYSA-N; N-Hydroxy-8-(2-naphthyl)octanamide; BDBM50114833; ZINC13474419; 8-Naphthalen-2-yl-octanoic acid hydroxyamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 25 nM | |||
| External Link | ||||
| ADS-102550 | Investigative | [20] | ||
| Synonyms |
CHEMBL217716; SCHEMBL5903759; BDBM50198218
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 29 nM | |||
| External Link | ||||
| N-hydroxy-8-(4-methoxyphenyl)-8-oxooctanamide | Investigative | [19] | ||
| Synonyms |
CHEMBL95835; SCHEMBL3383312; LIOTZVIDBXLBAC-UHFFFAOYSA-N; n-hydroxy-7-(p-anisoyl)heptanamide; BDBM50114826; 8-(4-Methoxy-phenyl)-8-oxo-octanoic acid hydroxyamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 15 nM | |||
| External Link | ||||
| N-(2-aminophenyl)-4-(chroman-3-ylmethyl)benzamide | Investigative | [28] | ||
| Synonyms |
CHEMBL238569; SCHEMBL1064835
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1000 nM | |||
| External Link | ||||
| N-hydroxy-2,2'-bithiophene-5-carboxamide | Investigative | [20] | ||
| Synonyms |
CHEMBL217750; SCHEMBL5903723; BDBM50198479
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2500 nM | |||
| External Link | ||||
| ADS-100380 | Investigative | [20] | ||
| Synonyms |
CHEMBL216885; SCHEMBL5904027; BDBM50198221
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 750 nM | |||
| External Link | ||||
| N-hydroxy-7-oxo-7-phenylheptanamide | Investigative | [19] | ||
| Synonyms |
CHEMBL95916; SCHEMBL1521257; BDBM50114832; 7-Oxo-7-phenyl-heptanoic acid hydroxyamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| 7-(3-Benzoyl-ureido)-heptanoic acid hydroxyamide | Investigative | [18] | ||
| Synonyms |
CHEMBL1083440; SCHEMBL4922807
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 145 nM | |||
| External Link | ||||
| SK-683 | Investigative | [29] | ||
| Synonyms |
CHEMBL116620; SCHEMBL8089576; BDBM50148757
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1 nM | |||
| External Link | ||||
| N-hydroxy-9-oxo-9-phenylnonanamide | Investigative | [19] | ||
| Synonyms |
CHEMBL99810; SCHEMBL1521077; BDBM50114824; 9-Oxo-9-phenyl-nonanoic acid hydroxyamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 135 nM | |||
| External Link | ||||
| N-Hydroxy-N'-(4-methylphenyl)octanediamide | Investigative | [30] | ||
| Synonyms |
CHEMBL1092762; BDBM50314138
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 76 nM | |||
| External Link | ||||
| N-(2,3-Dimethylphenyl)-N'-hydroxyoctanediamide | Investigative | [30] | ||
| Synonyms |
CHEMBL1093043; BDBM50314143
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(2-aminophenyl)quinoxaline-6-carboxamide | Investigative | [31] | ||
| Synonyms |
benzamide-type inhibitor, 20; CHEMBL236060; BDBM19424
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2000 nM | |||
| External Link | ||||
| 7-Biphenyl-4-yl-heptanoic acid hydroxyamide | Investigative | [32] | ||
| Synonyms |
CHEMBL125098; BDBM50222335; 7-(4-Biphenylyl)heptanehydroximic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(2-Ethylphenyl)-N'-hydroxyoctanediamide | Investigative | [30] | ||
| Synonyms |
CHEMBL1093040; BDBM50314142
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-(Biphenyl-4-yloxy)-2-oxo-octanoic acid | Investigative | [33] | ||
| Synonyms |
CHEMBL115049; 436150-72-0; SCHEMBL7368556; CTK1D2674; DTXSID40658342; BDBM50221807; 8-[([1,1'-Biphenyl]-4-yl)oxy]-2-oxooctanoic acid; Octanoic acid, 8-([1,1'-biphenyl]-4-yloxy)-2-oxo-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1,1,1-Trifluoro-8-phenoxy-octan-2-one | Investigative | [34] | ||
| Synonyms |
CHEMBL114796; BDBM50217940
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Biphenyl-4-yloxy)-heptanoic acid hydroxyamide | Investigative | [33] | ||
| Synonyms |
CHEMBL114184; SCHEMBL3383144
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(2,4-Dimethylphenyl)-N'-hydroxyoctanediamide | Investigative | [30] | ||
| Synonyms |
CHEMBL1092032; BDBM50314141
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Phenoxy-heptanoic acid hydroxyamide | Investigative | [32] | ||
| Synonyms |
CHEMBL124322; N-hydroxy-7-phenoxyheptanamide; 7-Phenoxyheptanehydroximic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1,1,1-Trifluoro-8-(4-phenoxy-phenoxy)-octan-2-one | Investigative | [34] | ||
| Synonyms |
CHEMBL117916; SCHEMBL7366611; BDBM50217945
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-(9H-carbazol-9-yl)-N-hydroxyhexanamide | Investigative | [35] | ||
| Synonyms |
CHEMBL1290142; A1-02262; SCHEMBL1004139; SOMDVJCUFVPZKM-UHFFFAOYSA-N; BDBM50331109; 9H-Carbazole-9-hexanamide, N-hydroxy-; 6-Carbazol-9-ylhexanoic acid hydroxyamide; US8748451, 1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3074 nM | |||
| External Link | ||||
| Desclasinose Azithromycinarylalkyl Hydroxamate | Investigative | [25] | ||
| Synonyms |
CHEMBL454025; SCHEMBL14329692; BDBM27176; Desclasinose Azithromycinarylalkyl Hydroxamate, 10
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(4-Ethylphenyl)-N'-hydroxyoctanediamide | Investigative | [30] | ||
| Synonyms |
CHEMBL1093042; BDBM50314139
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(3,5-Dimethylphenyl)-N'-hydroxyoctanediamide | Investigative | [30] | ||
| Synonyms |
CHEMBL1093713; BDBM50314133
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N1-(biphenyl-3-yl)-N8-hydroxyoctanediamide | Investigative | [36] | ||
| Synonyms |
CHEMBL473270
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 9 nM | |||
| External Link | ||||
| N-(2,5-Dimethylphenyl)-N'-hydroxyoctanediamide | Investigative | [30] | ||
| Synonyms |
CHEMBL1093357; BDBM50314144
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Azithromycin-N-benzyltriazolyloctahydroxamic Acid | Investigative | [25] | ||
| Synonyms |
SCHEMBL8976909; CHEMBL455342; BDBM27181; triazole-linked azithromycin-based compound, 16e
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(4-hydroxybiphenyl-3-yl)benzamide | Investigative | [37] | ||
| Synonyms |
CHEMBL269935; SCHEMBL5724398; BDBM50232005
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 58 nM | |||
| External Link | ||||
| AZUMAMIDE E | Investigative | [38] | ||
| Synonyms |
CHEMBL402363
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 50 nM | |||
| External Link | ||||
| Azithromycin-N-benzyltriazolylnonahydroxamic Acid | Investigative | [25] | ||
| Synonyms |
CHEMBL509089; SCHEMBL14329756; BDBM27183; triazole-linked azithromycin-based compound, 16g
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-N'-(3-methylphenyl)octanediamide | Investigative | [30] | ||
| Synonyms |
CHEMBL1089339; BDBM50314136
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 57 nM | |||
| External Link | ||||
| 8-Phenyl-octanoic acid hydroxyamide | Investigative | [32] | ||
| Synonyms |
CHEMBL123624; N-Hydroxy-8-phenyloctanamide; SCHEMBL5807174
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Azithromycin-N-benzyltriazolylhexahydroxamic Acid | Investigative | [25] | ||
| Synonyms |
CHEMBL446811; SCHEMBL14328156; CHEMBL3735805; BDBM27177; triazole-linked azithromycin-based compound, 16a
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(3-Ethylphenyl)-N'-hydroxyoctanediamide | Investigative | [30] | ||
| Synonyms |
CHEMBL1093041; BDBM50314134
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(3,4-Dimethylphenyl)-N'-hydroxyoctanediamide | Investigative | [30] | ||
| Synonyms |
CHEMBL1093359; BDBM50314135
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-N'-(4-methoxyphenyl)octanediamide | Investigative | [30] | ||
| Synonyms |
CHEMBL1091487; BDBM50314137
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 71 nM | |||
| External Link | ||||
| AZUMAMIDE B | Investigative | [38] | ||
| Synonyms |
CHEMBL402727
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1830 nM | |||
| External Link | ||||
| 8-(Biphenyl-3-yloxy)-1,1,1-trifluoro-octan-2-one | Investigative | [34] | ||
| Synonyms |
CHEMBL116023; SCHEMBL7368359; BDBM50218558
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Azithromycinarylalkylhydroxamic Acid | Investigative | [25] | ||
| Synonyms |
CHEMBL510806; SCHEMBL14329660; BDBM27175; Azithromycinarylalkylhydroxamic Acid, 8
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-N'-(2-methylphenyl)octanediamide | Investigative | [30] | ||
| Synonyms |
CHEMBL1076794; BDBM50314140
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 576 nM | |||
| External Link | ||||
| nexturastat A | Investigative | [39] | ||
| Synonyms |
S7473
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 360 nM | |||
| External Link | ||||
| AZUMAMIDE C | Investigative | [38] | ||
| Synonyms |
CHEMBL257972
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 20 nM | |||
| External Link | ||||
| N-(2,6-Dimethylphenyl)-N'-hydroxyoctanediamide | Investigative | [30] | ||
| Synonyms |
CHEMBL1093358; BDBM50314145
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N1-hydroxy-N8-(4-phenylthiazol-2-yl)octanediamide | Investigative | [40] | ||
| Synonyms |
CHEMBL511212; BDBM50258645
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3 nM | |||
| External Link | ||||
| LARGAZOLE | Investigative | [41] | ||
| Synonyms |
CHEMBL1173445; (+)-Largazole; SCHEMBL71330; ZINC56861395
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10.09 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-L-A1in-L-Ala-D-Pro-) | Investigative | [42] | ||
| Synonyms |
CHEMBL393260
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 36 nM | |||
| External Link | ||||
| 7-mercapto-N-(4-phenylthiazol-2-yl)heptanamide | Investigative | [43] | ||
| Synonyms |
CHEMBL419758; NCH-31; JMC505425 Compound 7; BDBM19131; 7-mercapto-N-(4-phenyl-2-thiazolyl)heptanamide; N-(4-phenyl-1,3-thiazol-2-yl)-7-sulfanylheptanamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 48 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-D-A1in-L-Ala-D-Pro-) | Investigative | [42] | ||
| Synonyms |
CHEMBL390991
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2.7 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-A2in-L-Ala-D-Pro-) | Investigative | [42] | ||
| Synonyms |
CHEMBL394261
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Ph5-D-Pro-) | Investigative | [42] | ||
| Synonyms |
CHEMBL391384
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5.3 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-D-2MePhe-L-Ala-D-Pro-) | Investigative | [42] | ||
| Synonyms |
CHEMBL393261
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 170 nM | |||
| External Link | ||||
| N1-(biphenyl-4-yl)-N8-hydroxyoctanediamide | Investigative | [40] | ||
| Synonyms |
CHEMBL512644; SCHEMBL8226957
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 33 nM | |||
| External Link | ||||
| N-(2-aminophenyl)nicotinamide | Investigative | [37] | ||
| Synonyms |
N-(2-Amino-phenyl)-nicotinamide; 436089-31-5; N-(2-aminophenyl)pyridine-3-carboxamide; CHEMBL236678; AC1LMN6K; SCHEMBL18086514; CTK4I7538; DTXSID50360661; CHEBI:125506; ZINC873967; BDBM50220259; 3463AE; AKOS000129725; RTR-042156; MCULE-7933541910; N-(2-aminophenyl)-3-pyridylcarboxamide; ZB014940; ACM436089315; ST086607; ASN 01337807; KB-298440; TR-042156; BC4148434; SR-01000329900; SR-01000329900-1; BRD-K20880473-001-04-6
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2600 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Ser(Bzl)-D-Pro-) | Investigative | [42] | ||
| Synonyms |
CHEMBL241555
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3.2 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Phg-D-Pro-) | Investigative | [42] | ||
| Synonyms |
CHEMBL428737
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 88 nM | |||
| External Link | ||||
| N-(4-aminobiphenyl-3-yl)nicotinamide | Investigative | [37] | ||
| Synonyms |
CHEMBL255805; BDBM50232035
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 48 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Ala-D-Tic-) | Investigative | [42] | ||
| Synonyms |
CHEMBL238587
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4.7 nM | |||
| External Link | ||||
| N-(2-amino-5-(thiophen-2-yl)phenyl)nicotinamide | Investigative | [37] | ||
| Synonyms |
CHEMBL256440; SCHEMBL1066609
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 65 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Ala-D-Pro-) | Investigative | [42] | ||
| Synonyms |
CHEMBL238596; BDBM50222727
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 94 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Ser-D-Pro-) | Investigative | [42] | ||
| Synonyms |
CHEMBL393961
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 6.4 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Ph4-D-Pro-) | Investigative | [42] | ||
| Synonyms |
CHEMBL391383
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 6.2 nM | |||
| External Link | ||||
| N-(2-aminophenyl)-4-methoxybenzamide | Investigative | [31] | ||
| Synonyms |
AC1LFX2W; Cambridge id 5129152; Oprea1_722128; benzamide-type inhibitor, 22; CHEMBL236061; SCHEMBL5226034; BDBM19426; CTK7A1998; MolPort-001-019-504; BDYVCYUXCNZYRW-UHFFFAOYSA-N; ZINC281656; STK156256; AKOS000130378; MCULE-9183453747; N-(2-Amino-phenyl)4-methoxy-benzamide; N-(2-amino-phenyl)-4-methoxy-benzamide; NCGC00240897-01; N1-(4-methoxybenzoyl)-1,2-benzenediamine; N1-(4-methoxy-benzoyl)-1,2-benzenediamine; ST50908739; N-(2-aminophenyl)(4-methoxyphenyl)carboxamide; SR-01000196394
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2000 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-L-2MePhe-L-Ala-D-Pro-) | Investigative | [42] | ||
| Synonyms |
CHEMBL393464
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3.7 nM | |||
| External Link | ||||
| Cyclo(-L-Am7(S2Py)-Aib-L-Phe-D-Pro-) | Investigative | [42] | ||
| Synonyms |
CHEMBL238829; BDBM50222732
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3.9 nM | |||
| External Link | ||||
| 4-Phenylbutyrohydroxamic acid | Investigative | [44] | ||
| Synonyms |
N-Hydroxy-4-phenylbutanamide; 32153-46-1; NSC131300; UNII-QX182FOM5S; QX182FOM5S; 4-phenylbutanehydroxamic acid; CHEMBL55895; Benzenebutanamide, N-hydroxy-; NSC 131300; AC1Q7DIW; AC1L5RDX; Phenylbutyrylhydroxamic Acid; AC1Q5QD1; N-Hydroxy-4-phenyl-butyramide; 4-Phenylbutyryl hydroxamic acid; SCHEMBL1350853; CTK4G8310; DTXSID60185943; MolPort-011-492-164; UPHXPXYRKPCXHK-UHFFFAOYSA-N; ZINC4962622; STL301752; BDBM50015142; AKOS009266186; MCULE-9765156954; NSC-131300; NE28489; BCB03_000829; EN300-68596
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 295 nM | |||
| External Link | ||||
| 8-Oxo-8-phenyl-octanoic acid hydroxyamide | Investigative | [45] | ||
| Synonyms |
CHEMBL95959; SCHEMBL3383197; N-hydroxy-8-oxo-8-phenyloctanamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 65 nM | |||
| External Link | ||||
| ST-3050 | Investigative | [46] | ||
| Synonyms |
CHEMBL472631; SCHEMBL3445133; SCHEMBL3445139; BDBM50278222
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3060 nM | |||
| External Link | ||||
| Octanedioic acid bis-hydroxyamide | Investigative | [47] | ||
| Synonyms |
Suberohydroxamic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 19 nM | |||
| External Link | ||||
| ST-2986 | Investigative | [46] | ||
| Synonyms |
CHEMBL471041; SCHEMBL3444455; BDBM50278219
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5480 nM | |||
| External Link | ||||
| 9,9,9-Trifluoro-8-oxo-nonanoic acid phenylamide | Investigative | [34] | ||
| Synonyms |
9,9,9-Trifluoro-8-Oxo-N-Phenylnonanamide; CHEMBL113537; 2gh6; SCHEMBL2702892; KRCXZGYVOZSCSF-UHFFFAOYSA-N; BDBM50121062; DB07553
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7800 nM | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid phenylamide | Investigative | [48] | ||
| Synonyms |
Thiol-SAHA (t-SAHA); CHEMBL325676; SCHEMBL14821761; BDBM152692
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 218 nM | |||
| External Link | ||||
| 6-benzenesulfinylhexanoic acid hydroxamide | Investigative | [49] | ||
| Synonyms |
6-(benzenesulfinyl)hexanoic acid hydroxyamide; 875737-03-4
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(2-Mercapto-ethyl)-N'-phenyl-succinamide | Investigative | [50] | ||
| Synonyms |
CHEMBL193959
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid biphenyl-4-ylamide | Investigative | [48] | ||
| Synonyms |
CHEMBL112311
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-phenylacetylamino-benzamide | Investigative | [51] | ||
| Synonyms |
CHEMBL356824; 656261-23-3; SCHEMBL675578; CTK1J6158; DTXSID40458440; ZINC13533297; AKOS030583151; Benzeneacetamide, N-[4-[(hydroxyamino)carbonyl]phenyl]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-(2-Bromo-acetylamino)-hexanoic acid phenylamide | Investigative | [48] | ||
| Synonyms |
CHEMBL344920; 651767-99-6; SCHEMBL3736839; CTK1J8444; DTXSID50432973; HWYLREOMBVUGJQ-UHFFFAOYSA-N; BDBM50222416; ZINC13587789; AKOS030603042; N-Phenyl-6-(bromoacetylamino)hexanamide; Hexanamide, 6-[(bromoacetyl)amino]-N-phenyl-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(phenylacetylamino-methyl)-benzamide | Investigative | [52] | ||
| Synonyms |
CHEMBL143674; SCHEMBL673760
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Biphenyl-4-yloxy)-1-oxazol-2-yl-heptan-1-one | Investigative | [53] | ||
| Synonyms |
CHEMBL126355; BDBM50222394
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Octanedioic acid hydroxyamide pyridin-4-ylamide | Investigative | [54] | ||
| Synonyms |
SCHEMBL8082656; CHEMBL165162; ZINC13472304
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(6-Mercapto-hexyl)-benzamide | Investigative | [48] | ||
| Synonyms |
CHEMBL112364; BDBM50223650
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Hydroxy-N-(5-hydroxycarbamoyl-pentyl)-benzamide | Investigative | [54] | ||
| Synonyms |
CHEMBL167455
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-((R)-2-phenyl-butyrylamino)-benzamide | Investigative | [51] | ||
| Synonyms |
SCHEMBL675474
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-benzenesulfonylhexanoic acid hydroxamide | Investigative | [49] | ||
| Synonyms |
CHEMBL203207
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 9-(Biphenyl-4-yloxy)-1,1,1-trifluoro-nonan-2-one | Investigative | [34] | ||
| Synonyms |
SCHEMBL7373122; CHEMBL116578
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Thioacetic acid S-(6-phenylcarbamoyl-hexyl) ester | Investigative | [48] | ||
| Synonyms |
CHEMBL111806; SCHEMBL14812153
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Butyrylamino-N-hydroxy-benzamide | Investigative | [52] | ||
| Synonyms |
CHEMBL142254; 656261-22-2; Benzamide, N-hydroxy-4-[(1-oxobutyl)amino]-; SCHEMBL675234; CTK1J6159; DTXSID90461262
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Naphthalen-2-yloxy)-1-oxazol-2-yl-heptan-1-one | Investigative | [53] | ||
| Synonyms |
CHEMBL127328
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid biphenyl-3-ylamide | Investigative | [48] | ||
| Synonyms |
CHEMBL320323
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione | Investigative | [55] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(2-phenyl-butyrylamino)-benzamide | Investigative | [51] | ||
| Synonyms |
SCHEMBL676079
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Dimethylamino-N-(6-mercapto-hexyl)-benzamide | Investigative | [48] | ||
| Synonyms |
CHEMBL324126
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid pyridin-3-ylamide | Investigative | [48] | ||
| Synonyms |
CHEMBL332246; Heptanamide, 7-mercapto-N-3-pyridinyl-; BDBM50223653
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-Phenoxy-hexane-1-thiol | Investigative | [48] | ||
| Synonyms |
CHEMBL109796; 6-phenoxyhexane-1-thiol; 1-Hexanethiol, 6-phenoxy-; SCHEMBL5679745; MolPort-020-180-823; BDBM50223652; AKOS018584222; MCULE-9521857089
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Benzoylamino-N-hydroxy-benzamide | Investigative | [51] | ||
| Synonyms |
SCHEMBL673678; CHEMBL191227
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Chloro-N-(5-hydroxycarbamoyl-pentyl)-benzamide | Investigative | [45] | ||
| Synonyms |
CHEMBL143734; NSC718168; AC1L8L82; SCHEMBL13039735; ZINC5579677; BDBM50082664; NSC-718168; NCI60_040737; 6-(4-Chlorobenzoylamino)hexanehydroxamic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-(Biphenyl-4-yloxy)-1,1,1-trifluoro-octan-2-one | Investigative | [34] | ||
| Synonyms |
CHEMBL112148; SCHEMBL7364383; BDBM50218532
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Biphenyl-3-yloxy)-1-oxazol-2-yl-heptan-1-one | Investigative | [53] | ||
| Synonyms |
CHEMBL127351; SCHEMBL7365180; HWZHDGRMABBYOV-UHFFFAOYSA-N; BDBM50222367; 7-((1,1'-biphenyl)-3-yloxy)-1-(1 ,3-oxazol-2-yl)-1-heptanone
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-Mercapto-hexanoic acid phenylamide | Investigative | [48] | ||
| Synonyms |
CHEMBL109654; Hexanamide, 6-mercapto-N-phenyl-; SCHEMBL14254925; BDBM50027600
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 791 nM | |||
| External Link | ||||
| Cyclostellettamine derivative | Investigative | [56] | ||
| Synonyms |
CHEMBL88332
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(5-Hydroxycarbamoyl-pentyl)-4-nitro-benzamide | Investigative | [45] | ||
| Synonyms |
CHEMBL139999; SCHEMBL1232700; BDBM50082661; ZINC13472309
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-Mercapto-pentanoic acid phenylamide | Investigative | [48] | ||
| Synonyms |
N-Phenyl-5-mercaptovaleramide; CHEMBL114344; Pentanamide, 5-mercapto-N-phenyl-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Octanedioic acid hydroxyamide pyridin-2-ylamide | Investigative | [54] | ||
| Synonyms |
SCHEMBL8090513; CHEMBL164872; ZINC13472303
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(2-Mercapto-ethyl)-N'-phenyl-oxalamide | Investigative | [50] | ||
| Synonyms |
CHEMBL193979
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(methylsulfonylthio)ethyl 2-propylpentanoate | Investigative | [55] | ||
| Synonyms |
CHEMBL271677; SCHEMBL4156413
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (E)-8-Biphenyl-4-yl-1-oxazol-2-yl-oct-7-en-1-one | Investigative | [53] | ||
| Synonyms |
CHEMBL126465; SCHEMBL7368197; SCHEMBL7368201
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-((S)-2-phenyl-butyrylamino)-benzamide | Investigative | [51] | ||
| Synonyms |
SCHEMBL676080
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(3-phenyl-propionylamino)-benzamide | Investigative | [57] | ||
| Synonyms |
N-hydroxy-4-(3-phenylpropanamido)benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(5-phenyl-pentanoylamino)-benzamide | Investigative | [51] | ||
| Synonyms |
SCHEMBL7311087
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-Oxo-8-phenyl-octanoic acid | Investigative | [54] | ||
| Synonyms |
8-Oxo-8-phenyloctanoic acid; 7-Benzoylheptanoic acid; 24314-23-6; Benzeneoctanoic acid, h-oxo-; 7-BENZOYL HEPTANOIC ACID; AC1L6TSB; SCHEMBL3381106; 8-keto-8-phenyl-caprylic acid; CHEMBL162423; 8-Oxo-8-phenyloctanoic acid #; CTK4F3363; DTXSID40305602; UMCSRRHQLAVYRS-UHFFFAOYSA-N; ZINC2168376; 7009f; NSC171230; AKOS016022495; NSC-171230; MCULE-7202530747; ACM24314236; ST50825837
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(4-phenyl-butyrylamino)-benzamide | Investigative | [51] | ||
| Synonyms |
CHEMBL143336; 656261-24-4; SCHEMBL674421; CTK1J6157; DTXSID30433908; ZINC13533300; AKOS030583673; n-hydroxy-4-(4-phenylbutyryl-amino)benzamide; Benzenebutanamide, N-[4-[(hydroxyamino)carbonyl]phenyl]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-phenylsulfanylhexanoic acid hydroxamide | Investigative | [49] | ||
| Synonyms |
Hexanamide, N-hydroxy-6-(phenylthio)-; CHEMBL203028; SCHEMBL7317658
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ST-2987 | Investigative | [46] | ||
| Synonyms |
CHEMBL471042; SCHEMBL3444989; SCHEMBL3444984; BDBM50278220
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 790 nM | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid quinolin-3-ylamide | Investigative | [48] | ||
| Synonyms |
CHEMBL112234
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-(4-Chloro-phenyl)-pentanoic acid hydroxyamide | Investigative | [58] | ||
| Synonyms |
CHEMBL84288
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-Mercapto-octanoic acid phenylamide | Investigative | [48] | ||
| Synonyms |
8-mercapto-N-phenyloctanamide; CHEMBL326433; ZINC13609343
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 294 nM | |||
| External Link | ||||
| N-(6-Hydroxycarbamoyl-hexyl)-benzamide | Investigative | [54] | ||
| Synonyms |
CHEMBL57107; 174664-71-2; SCHEMBL573254; CTK0A7470; DTXSID00433435; BDBM50220823; ZINC13490043; 7-(Benzoylamino)heptanehydroxamic acid; AKOS030580013; Benzamide, N-[7-(hydroxyamino)-7-oxoheptyl]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Biphenyl-4-yloxy)-1,1,1-trifluoro-heptan-2-one | Investigative | [34] | ||
| Synonyms |
CHEMBL326529; SCHEMBL7365237; BDBM50217957
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid benzothiazol-2-ylamide | Investigative | [48] | ||
| Synonyms |
CHEMBL178779
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(pentanoylamino-methyl)-benzamide | Investigative | [52] | ||
| Synonyms |
CHEMBL143102
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PSAMMAPLIN A | Investigative | [45] | ||
| Synonyms |
110659-91-1; Bisprasin; NSC614495; AC1O46WI; SCHEMBL364511; ZINC150352860; NSC-614495; B723735K022; J-002461; Benzenepropanamide, N,N'-(dithiodi-2,1-ethanediyl)bis(3-bromo-4-hydroxy-alpha-(hydroxyimino)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Poly [ADP-ribose] polymerase 1 (PARP1) | 131 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Nicotinamide | Approved | [59] | ||
| Synonyms |
Aminicotin; Amixicotyn; Amnicotin; Benicot; Dipegyl; Endobion; Enduramide; Hansamid; Mediatric; Niacevit; Niacinamide; Niacotinamide; Niamide; Nicamina; Nicamindon; Nicasir; Nicobion; Nicofort; Nicogen; Nicomidol; Nicosylamide; Nicota; Nicotamide; Nicotilamide; Nicotililamido; Nicotinamid; Nicotinamida; Nicotinamidum; Nicotinsaeureamid; Nicotinsaureamid; Nicotol; Nicotylamide; Nicotylamidum; Nicovit; Nicovitina; Nicovitol; Nicozymin; Nikasan; Nikazan; Nikotinamid; Nikotinsaeureamid; Niocinamide; Niozymin; Papulex; Pelmin; Pelmine; Savacotyl; Amid kyseliny nikotinove; Amid kyseliny nikotinove [Czech]; Amide PP; Astra Brand of Niacinamide; Austrovit PP; Delonin amide; Factor pp; Inovitan PP; Jenapharm Brand of Niacinamide; Merck Brand of Niacinamide; Niacinamide Astra Brand; Niacinamide Jenapharm Brand; Niacinamide Merck Brand; Niacinamide Pharmagenix Brand; Niacinamide [USAN]; Niavit PP; Nicotine acid amide; Nicotine amide; Nicotinic acid amide; Nicotinic amide; Nicotinsaureamid Jenapharm; Nicotinsaureamid [German]; Nikotinsaeureamid [German]; Pelonin amide; Pharmagenix Brand of Niacinamide; Vitamin B; Vitamin PP; Witamina PP; Nicosan 2; Vitamin H1; B 3, Vitamin; B3, Vitamin; Beta-Pyridinecarboxamide; Jenapharm, Nicotinsaureamid; Nandervit-N; Niacin-Vitamin B3; Niacinamide (USP); Nicotinamida [INN-Spanish]; Nicotinamide (Niacinamide); Nicotinamidum [INN-Latin]; Niko-tamin; PP-Faktor; Vi-Nicotyl; Vitamin B (VAN); M-(Aminocarbonyl)pyridine; Niacinamide, Nicotinic acid amide, Nicotinamide; Nicotinamide (JP15/INN); Nicotinamide, niacin, vitamin B3; Nicotinamide-carbonyl-14C; Pyridine-3-carboxamide; Pyridine-3-carboxylic acid amide; 3 Pyridinecarboxamide; 3-Carbamoylpyridine; 3-Pyridinecarboxamide; 3-Pyridinecarboxylic acid amide
Click to Show/Hide
|
|||
| MOA | Binder | |||
| Activity | IC50 = 210000 nM | |||
| External Link | ||||
| KU-0058948 | Approved | [60] | ||
| Synonyms |
CHEMBL380648; 4-[3-(1,4-diazepan-1-ylcarbonyl)-4-fluorobenzyl]phthalazin-1(2H)-one; 4-(3-(1,4-diazepane-1-carbonyl)-4-fluorobenzyl)phthalazin-1(2H)-one; Homopiperazine analogue, 14; SCHEMBL864319; BDBM27533; HGEPGGJUGUMFHT-UHFFFAOYSA-N; ZINC3821234; DB08058; NCGC00386677-01; KU-58948; FT-0670691; TL80090044; 4-[3-([1,4]diazepane-1-carbonyl)-4 -fluorobenzyl]-2H-phthalazin-1-one; 4-[3-([1,4]diazepane-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7 nM | |||
| External Link | ||||
| Niraparib Tosylate | Approved | [61] | ||
| Synonyms |
1038915-73-9; MK-4827 (tosylate); MK-4827 tosylate; UNII-75KE12AY9U; MK-4827(Niraparib) tosylate; 75KE12AY9U; MK-4827-tosylate; MK 4827 tosylate; Niraparib(MK-4827) tosylate; KS-00000TSH; MolPort-044-556-849; s7625; HY-10619B; AKOS030632785; CS-2283; AC-30383; KB-335358; AX8326059
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Nicaraven | Phase 3 | [62] | ||
| Synonyms |
AVS; Antevan; Antevas
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| CC-486 | Phase 3 | [63] | ||
| Synonyms |
AG-14361; AG14361; 328543-09-5; UNII-48N0U0K50I; AG 14361; CHEMBL65892; 48N0U0K50I; Imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one, 2-[4-[(dimethylamino)methyl]phenyl]-5,6-dihydro-; AG-014361; 1-(4-((dimethylamino)methyl)phenyl)-8,9-dihydro-2,7,9a-triazabenzo[cd]azulen-6(7H)-one; Imidazo(4,5,1-jk)(1,4)benzodiazepin-7(4H)-one, 2-(4-((dimethylamino)methyl)phenyl)-5,6-dihydro-; 2-[4-[(Dimethylamino)methyl]phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one; SMR000486393; MLS006011157; MLS001065917; Nucleoside analogue
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 5.8 nM | |||
| External Link | ||||
| AG140699 | Phase 2 | [64] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| AZD5305 | Phase 2 | [65] | ||
| Synonyms |
16MZ1V3RBT; 2589531-76-8; 2-Pyridinecarboxamide, 5-(4-((7-ethyl-5,6-dihydro-6-oxo-1,5-naphthyridin-3-yl)methyl)-1-piperazinyl)-N-methyl-; 2-Pyridinecarboxamide, 5-[4-[(7-ethyl-5,6-dihydro-6-oxo-1,5-naphthyridin-3-yl)methyl]-1-piperazinyl]-N-methyl-; 5-(4-((7-Ethyl-6-oxo-5,6-dihydro-1,5-naphthyridin-3-yl)methyl)piperazin-1-yl)-N-methylpicolinamide; 5-[4-[(7-Ethyl-6-oxo-5,6-dihydro-1,5-naphthyridin-3-yl)methyl]-1-piperazinyl]-N-methylpicolinamide; 5-[4-[(7-ethyl-6-oxo-5H-1,5-naphthyridin-3-yl)methyl]piperazin-1-yl]-N-methylpyridine-2-carboxamide; 5-{4-[(7-ethyl-5,6-dihydro-6-oxo-1,5-naphthyridin-3- yl)methyl]piperazin-1-yl}-N-methylpyridine-2- carboxamide; AC-37130; Azd 5305; AZD 5305 [WHO-DD]; AZD5305; AZD-5305; AZD-5305 [WHO-DD]; CHEMBL5095220; CS-0163534; E80364; EX-A5234; example 4 [WO2021013735]; GTPL11526; HY-132167; MS-26971; NSC834196; NSC-834196; Saruparib; saruparib [INN]; SCHEMBL22912701; SY295016; UNII-16MZ1V3RBT
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27841036-Compound-37 | Phase 2 | [66] | ||
| Synonyms |
2X-121
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2 nM | |||
| External Link | ||||
| Stenoparib | Phase 2 | [67] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| AMXI 5001 | Phase 1/2 | [68] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| NMS-03305293 | Phase 1 | [69] | ||
| Synonyms |
NMS-P293
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-oxo-2,3-dihydro-1H-indazole-4-carboxamide derivative 1 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-6
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 230 nM | |||
| External Link | ||||
| Benzimidazole carboxamide derivative 1 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-I
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 1 nM | |||
| External Link | ||||
| PMID27841036-Compound-33 | Patented | [66] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| 3-oxo-2,3-dihydro-1H-indazole-4-carboxamide derivative 4 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-9
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 44 nM | |||
| External Link | ||||
| Phthalazine ketone derivative 1 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-16
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 6000 nM | |||
| External Link | ||||
| Quinazolinedione derivative 3 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-13
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 140 nM | |||
| External Link | ||||
| 4-Carboxamido-isoindolinone derivative 2 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-3
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 11 nM | |||
| External Link | ||||
| 3-oxo-2,3-dihydro-1H-indazole-4-carboxamide derivative 3 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-8
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 15 nM; Kd < 10 nM | |||
| External Link | ||||
| 7-azaindole derivative 8 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-10
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100 nM | |||
| External Link | ||||
| 3-oxo-2,3-dihydro-1H-indazole-4-carboxamide derivative 5 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-IV
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Phthalazine ketone derivative 3 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-18
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 9000 nM | |||
| External Link | ||||
| Dihydropyrido phthalazinone derivative 1 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-21
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 800 nM | |||
| External Link | ||||
| Phthalazine ketone derivative 2 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-17
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 8000 nM | |||
| External Link | ||||
| Tricyclic indole compound 13 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-XVI
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 to 270 nM | |||
| External Link | ||||
| 3-phenyl isoquinolin-1(2H) derivative 2 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-VII
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Carboxamido-isoindolinone derivative 5 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-III
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Quinazolinedione derivative 2 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-12
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.37 nM | |||
| External Link | ||||
| Quinazolinedione derivative 1 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-11
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.21 nM | |||
| External Link | ||||
| Dihydropyrido phthalazinone derivative 2 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-22
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 800 nM | |||
| External Link | ||||
| 3-oxo-2,3-dihydro-1H-indazole-4-carboxamide derivative 2 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-7
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 26 nM; Kd < 10 nM | |||
| External Link | ||||
| Phthalazine derivative 3 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-19
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| Tetra-hydro-quinoline derivative 1 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-VIII
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1 to 500 nM | |||
| External Link | ||||
| 4-Carboxamido-isoindolinone derivative 1 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-2
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 20 nM | |||
| External Link | ||||
| 4-Carboxamido-isoindolinone derivative 3 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-4
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| 4-Carboxamido-isoindolinone derivative 4 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-5
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 20 nM | |||
| External Link | ||||
| Dihydrodiazepinocarbazolone derivative 1 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-26
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2.9 nM | |||
| External Link | ||||
| Tetra-cyclic pyridophthalazinone derivative 1 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-25
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5.1 nM | |||
| External Link | ||||
| 3-phenyl isoquinolin-1(2H) derivative 1 | Patented | [66] | ||
| Synonyms |
PMID27841036-Compound-20
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 10 nM | |||
| External Link | ||||
| CPH-102 | Preclinical | [70] | ||
| Synonyms |
IABP; INH2BP, Crimson Pharmaceutical; INH2BP, Octamer; PARP inhibitors, Crimson Pharmaceutical; PARP inhibitors, Octamer; CPH-101, Crimson Pharma
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PJ34 | Preclinical | [71] | ||
| Synonyms |
344458-19-1; pj-34; N~2~,N~2~-DIMETHYL-N~1~-(6-OXO-5,6-DIHYDROPHENANTHRIDIN-2-YL)GLYCINAMIDE; CHEMBL372303; P34; 2-(dimethylamino)-N-(6-oxo-5,6-dihydrophenanthridin-2-yl)acetamide; Acetamide, N-(5,6-dihydro-6-oxo-2-phenanthridinyl)-2-(dimethylamino)-; UYJZZVDLGDDTCL-UHFFFAOYSA-N; PJ34(free base); 1xk9; SCHEMBL422317; ZINC8960; AC1L1J45; BDBM27497; CTK1B7701; MolPort-035-395-737; Ibrutinib (PCI32765 pound(c); HMS3651B06; BCP07990; HY-13688A; 2662AH; AKOS030229047; SB19292; DB08348; CS-1463; NCGC00370866-10; DA-42692; BC600341
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10.9 nM | |||
| External Link | ||||
| NU1025 | Terminated | [72] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 48 nM | |||
| External Link | ||||
| 3-Ethynylquinoline-8-carboxamide | Investigative | [73] | ||
| Synonyms |
CHEMBL501330; 8-Quinolinecarboxamide, 3-ethynyl-; BDBM50255268
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2300 nM | |||
| External Link | ||||
| 2-(4-Amino-phenyl)-8-hydroxy-3H-quinazolin-4-one | Investigative | [74] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 289 nM | |||
| External Link | ||||
| N-(4-Phenylthiazol-2-yl)isonicotinamide | Investigative | [75] | ||
| Synonyms |
N-(4-phenyl-1,3-thiazol-2-yl)pyridine-4-carboxamide; CHEMBL482012; 5245-66-9; BAS 03572091; AC1Q5OBS; AC1LG7OX; CBMicro_015073; n-(4-phenyl-1,3-thiazol-2-yl)isonicotinamide; Cambridge id 5245669; Oprea1_303023; Oprea1_553111; SCHEMBL17107144; CTK4J5937; DTXSID30355050; MolPort-001-992-732; ZINC290573; STK483947; BDBM50255300; AKOS000569765; MCULE-9247268363; BIM-0015234.P001; N-(4-Phenyl-thiazol-2-yl)-isonicotinamide; ST50017829; N~4~-(4-phenyl-1,3-thiazol-2-yl)isonicotinamide; Z27772062
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 753 nM | |||
| External Link | ||||
| 5-Chloro-2-methyl-3H-quinazolin-4-one | Investigative | [76] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1200 nM | |||
| External Link | ||||
| BZ3 | Investigative | [77] | ||
| Synonyms |
5-METHOXYINDOLE; 1006-94-6; 5-Methoxy-1H-indole; 1H-Indole, 5-methoxy-; Femedol; 5-Methoxy indole; Indole, 5-methoxy-; Methoxy-5 indole; Indol-5-yl methyl ether; UNII-DQM3AS43PQ; Methoxy-5 indole [French]; 5-Methoxyindole, 99%; EINECS 213-745-3; DQM3AS43PQ; NSC 521752; CHEMBL280311; DWAQDRSOVMLGRQ-UHFFFAOYSA-N; MFCD00005674; 916979-77-6; 5Methoxyindole; 5-methoxyindol; 5-methoxy-indole; 3img; 3imc; PubChem7432; 1,3-dihydro-5-methyl-2H-Indol-2-one; ACMC-1BMAQ; 5-(methyloxy)-1H-indole; AC1Q4F1F; AC1L22NW; SCHEMBL74720; KSC177G3N; WLN: T
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(3'-Methoxyphenyl) Benzimidazole-4-Carboxamide | Investigative | [72] | ||
| Synonyms |
2-(3-methoxyphenyl)-1H-benzimidazole-4-carboxamide; CHEMBL134022; BZC; 1efy; AC1L1BMO; SCHEMBL4321727; CTK7A9229; NVVWVYYHTKCIAE-UHFFFAOYSA-N; ZINC11565446; BDBM50093373; DB04010; 2-(3'-Methoxyphenyl)-1-H-benzimidazole-4-carboxamide; 2-(3-methoxyphenyl)-1H-1,3-benzodiazole-4-carboxamide; 2-(3''-METHOXYPHENYL) BENZIMIDAZOLE-4-CARBOXAMIDE; 2-(3-Methoxy-phenyl)-1H-benzoimidazole-4-carboxylic acid amide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 6 nM | |||
| External Link | ||||
| CEP-6800 | Investigative | [78] | ||
| Synonyms |
UNII-0X7U7SRK9H; 0X7U7SRK9H; CHEMBL247374; 609848-02-4; SCHEMBL12256417; BDBM50197585; 1H-Cyclopenta(a)pyrrolo(3,4-C)carbazole-1,3(2H)-dione, 10-(aminomethyl)-4,5,6,7-tetrahydro-; 8-aminomethyl-1,2,3,11-tetrahydro-5,11-diaza-benzo[a]trindene-4,6-dione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 18 nM | |||
| External Link | ||||
| ANG-2864 | Investigative | [70] | ||
| Synonyms |
PARP inhibitor (ischemia/cancer), Angion Biomedica
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-Methoxybenzamide | Investigative | [79] | ||
| Synonyms |
5813-86-5; m-Methoxybenzamide; m-Anisamide; 3-Methoxy-benzamide; Benzamide, 3-methoxy-; UNII-M8502TLK98; EINECS 227-379-7; NSC 28589; NSC 209527; BRN 2206857; CHEMBL123978; VKPLPDIMEREJJF-UHFFFAOYSA-N; M8502TLK98; 3MB; 3pax; 5-methoxybenzamide; 3-methoxy-benzamid; ACMC-1ASRE; AC1Q5DMC; M-METHOXY BENZAMIDE; bmse000775; 3-Methoxybenzamide, 97%; Oprea1_695428; MLS001066418; 4-10-00-00326 (Beilstein Handbook Reference); cid_98487; SCHEMBL283787; AC1L407F; KS-00000VVU; CTK1H2082; VKPLPDIMEREJJF-UHFFFAOYSA-; DTXSID00206848
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 17000 nM | |||
| External Link | ||||
| 2-Benzyl-2H-indazole-7-carboxamide | Investigative | [80] | ||
| Synonyms |
CHEMBL1094951; SCHEMBL2268172
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 130 nM | |||
| External Link | ||||
| 5-methylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione | Investigative | [81] | ||
| Synonyms |
CHEMBL201723
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5000 nM | |||
| External Link | ||||
| 4-amino-1,8-naphthalimide | Investigative | [72] | ||
| Synonyms |
1742-95-6; 4-Aminonaphthalimide; 6-AMINO-BENZO[DE]ISOQUINOLINE-1,3-DIONE; 6-Amino-1H-benzo[de]isoquinoline-1,3(2H)-dione; 4-Aminonaphthalene-1,8-dicarboximide; Naphthalimide, 4-amino-; DFP 1; 1H-Benz[de]isoquinoline-1,3(2H)-dione, 6-amino-; EINECS 217-110-1; BRN 0177185; 6-aminobenzo[de]isoquinoline-1,3-dione; PARP Inhibitor V, 4-ANI; CHEMBL338790; CHEBI:40071; SSMIFVHARFVINF-UHFFFAOYSA-N; 6-Amino-1H-benz(de)isoquinoline-1,3(2H)-dione; 4-AMINO-1,8 NAPHTHALIMIDE
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 26 nM | |||
| External Link | ||||
| Pyrrolo[3,4-e]indole-1,3(2H,6H)-dione | Investigative | [81] | ||
| Synonyms |
CHEMBL373066; pyrroloisoindoledione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 750 nM | |||
| External Link | ||||
| 8-Amino-6H,11H-indeno[1,2-c]isoquinolin-5-one | Investigative | [82] | ||
| Synonyms |
SCHEMBL4662780; CHEMBL363363; BDBM27514; LQEYAIKMIJUZNT-UHFFFAOYSA-N; indeno[1,2-c]isoquinolinone, 2a; ZINC13652898; 5,6-Dihydro-5-oxo-8-amino-indeno[1,2-c]isoquinoline
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 300 nM | |||
| External Link | ||||
| EB-47 | Investigative | [64] | ||
| Synonyms |
EB 47; 366454-36-6; 5'-Deoxy-5'-[4-[2-[(2,3-dihydro-1-oxo-1H-isoindol-4-yl)amino]-2-oxoethyl]-1-piperazinyl]-5'-oxoadenosine Dihydrochloride; DTXSID40692822; ZINC98052573; NCGC00370771-01; KB-76747; FT-0667818; 4-[1-(6-Amino-9H-purin-9-yl)-1-deoxy-; A-D-ribofuranuronoyl]-N-(2,3-dihydro-1-oxo-1H-isoindol-4-yl)-1-piperazineacetamide Dihydrochloride; 2-{4-[(2R,3R,4S,5S)-5-(6-Amino-9H-purin-9-yl)-3,4-dihydroxyoxolane-2-carbonyl]piperazin-1-yl}-N-(1-oxo-2,3-dihydro-1H-isoindol-4-yl)acetamide (non-preferred name)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BZ6 | Investigative | [77] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(4-methoxyphenyl)quinoline-8-carboxamide | Investigative | [73] | ||
| Synonyms |
CHEMBL481591; 655222-47-2; CTK1J6622; DTXSID70649086; BDBM50255383; AKOS030560276; 8-Quinolinecarboxamide, 2-(4-methoxyphenyl)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1100 nM | |||
| External Link | ||||
| 9-Amino-6H,11H-indeno[1,2-c]isoquinolin-5-one | Investigative | [82] | ||
| Synonyms |
CHEMBL194155; SCHEMBL4078284; BDBM27515; BIBLEFNXUYTZIB-UHFFFAOYSA-N; indeno[1,2-c]isoquinolinone, 2b; ZINC13652899; 5,6-Dihydro-5-oxo-9-amino-indeno[1,2-c]isoquinoline
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 95 nM | |||
| External Link | ||||
| (E)-N-(4-Phenylthiazol-2-yl) cinnamamide | Investigative | [75] | ||
| Synonyms |
CHEMBL452100; 2-Cinnamamido-4-phenylthiazole; (2E)-3-phenyl-N-(4-phenyl-1,3-thiazol-2-yl)prop-2-enamide; 1107621-03-3; AC1LEPJR; ZINC60264; WOJRHCOBUKJCAJ-VAWYXSNFSA-N; MolPort-019-760-060; MolPort-001-931-977; HMS1397P15; STK173781; BDBM50255301; N-(4-phenylthiazol-2-yl)cinnamamide; AKOS000523355; MCULE-8934603681; BAS 00417267; ST4016450; 3-Phenyl-N-(4-phenyl-thiazol-2-yl)-acrylamide; AG-690/11629440; 3-phenyl-N-(4-phenyl-1,3-thiazol-2-yl)acrylamide; F0298-0058; A0793/0037152
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 224 nM | |||
| External Link | ||||
| ANG-2684 | Investigative | [70] | ||
| Synonyms |
ANG-3038; PARP-1 inhibitors (acute pancreatitis/stroke); PARP-1 inhibitors (acute pancreatitis/stroke), Angion Biomedica
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-methylpyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione | Investigative | [81] | ||
| Synonyms |
CHEMBL380940; SCHEMBL5828673
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2000 nM | |||
| External Link | ||||
| 2,3-dihydro-1H-benzo[de]isoquinolin-1-one | Investigative | [83] | ||
| Synonyms |
CHEMBL594596; 2,3-dihydro-benzo[de]isoquinolin-1-one; SCHEMBL832168; ZINC24216; BDBM50306285; FCH1866210
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 540 nM | |||
| External Link | ||||
| 5-amino-3,4-dihydroisoquinolin-1(2H)-one | Investigative | [83] | ||
| Synonyms |
129075-53-2; CHEMBL594759; SCHEMBL7581623; RTPKPVYTPRJRBY-UHFFFAOYSA-N; ZINC45353622; BDBM50306284; AKOS023598631; AB53700; FCH1123505; KS-9128; CM10348; AJ-110485; 5-amino-3,4-dihydro-1(2H)-isoquinolinone; EN300-254419
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 410 nM | |||
| External Link | ||||
| KR-33889 | Investigative | [70] | ||
| Synonyms |
KR-34285; PARP-1 inhibitors (myocardial infarction), KRICT
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-(5-Morpholin-4-yl-pentyl)-2H-phthalazin-1-one | Investigative | [84] | ||
| Synonyms |
CHEMBL194684
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 119 nM | |||
| External Link | ||||
| AG-014376 | Investigative | [85] | ||
| Synonyms |
CHEMBL361489; SCHEMBL7159231; BDBM50154730; 6-(4-Dimethylaminomethyl-phenyl)-3,4-dihydro-2H-[1,4]diazepino[6,7,1-hi]indol-1-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 6.4 nM | |||
| External Link | ||||
| 3-Hydroxy-benzamide | Investigative | [74] | ||
| Synonyms |
3-hydroxybenzamide; 618-49-5; benzamide, 3-hydroxy-; CHEMBL419424; 3-Hydroxy benzamide; NSC379289; 3-hydroxybenzenecarboxamide; AC1Q4ZB3; AC1L7W2U; ACMC-1B71B; Oprea1_435073; SCHEMBL161861; 3-Hydroxybenzamide, AldrichCPR; CTK2F7291; DTXSID90321635; NGMMGKYJUWYIIG-UHFFFAOYSA-N; MolPort-001-791-593; ZINC1590754; KM0548; BDBM50068769; 9282AB; ANW-33964; 3-HYDROXY-BENZOIC ACID,AMIDE; SBB079277; AKOS000207073; VZ26952; NSC-379289; MB00281; MCULE-9599926365; NCGC00323509-01; KB-32185; CJ-25437; AJ-27681; CJ-05592; SC-47787
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 9100 nM | |||
| External Link | ||||
| 8-Methoxy-2-phenyl-3H-quinazolin-4-one | Investigative | [74] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 10000 nM | |||
| External Link | ||||
| Quinoline-8-carboxamide | Investigative | [73] | ||
| Synonyms |
8-Quinolinecarboxamide; 55706-61-1; CCRIS 6967; CHEMBL502330; HPQRQAOVNXWEEQ-UHFFFAOYSA-N; 8-Carbamoylquinoline; 8-Quinolinecarboxamide #; AC1L44SC; SCHEMBL460456; CTK5A4047; quinoline-8-carboxylic acid amide; DTXSID00204243; MolPort-005-722-305; ZINC6095019; BDBM50255266; AKOS008969900; NE38855; MCULE-7978956907; LS-188644; KB-259682
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1900 nM | |||
| External Link | ||||
| 9-Fluoro-6H,11H-indeno[1,2-c]isoquinolin-5-one | Investigative | [82] | ||
| Synonyms |
CHEMBL190895; BDBM27513; indeno[1,2-c]isoquinolinone, 1e; 9-Fluoro-5,6-dihydro-11H-indeno[1,2-c]isoquinoline-5-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 180 nM | |||
| External Link | ||||
| 8-Methyl-2-(4-nitro-phenyl)-3H-quinazolin-4-one | Investigative | [74] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 104 nM | |||
| External Link | ||||
| 2H-Isoquinolin-1-one | Investigative | [74] | ||
| Synonyms |
1-Hydroxyisoquinoline; 491-30-5; Isoquinolin-1(2H)-one; isoquinolin-1-ol; 1-Isoquinolinol; Isocarbostyril; 1(2H)-ISOQUINOLINONE; Isoquinolin-1-one; 489453-23-8; 1(2H)-Isoquinolone; 1,2-dihydroisoquinolin-1-one; 1-hydroxyisoquinolin; isoquinolinol; 87602-67-3; 1(2H)-ISOQUINILONE; Isocarbostyril, 98%; UNII-95EG3HGG1P; 95EG3HGG1P; Isoquinolinone; CHEMBL339695; CHEBI:18350; VDBNYAPERZTOOF-UHFFFAOYSA-N; 1-isoquinolone; oxidoisoquinolinium; EINECS 207-732-1; Isocarbostyril(1-hydroxyisoquinoline); NSC 27273
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 9 nM | |||
| External Link | ||||
| KU-58684 | Investigative | [75] | ||
| Synonyms |
SCHEMBL863338; 623578-11-0
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2,8-Dimethyl-3H-quinazolin-4-one | Investigative | [74] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 490 nM | |||
| External Link | ||||
| 5-aminoisoquinolin-1(2H)-one | Investigative | [73] | ||
| Synonyms |
93117-08-9; 5-amino-2H-isoquinolin-1-one; 5-AMINOISOQUINOLIN-1-OL; 5-amino-1,2-dihydroisoquinolin-1-one; 5-AIQ; CHEMBL446240; 5-Amino-2H-isoquinoin-1-one; 5-AMINO-1(2H)-ISOQUINOLINONE; 5-aminoisoquinolinone; 4pnq; 32X; AC1Q6DVG; AC1L1CUR; 5-Amino-1-isoquinolinol; SCHEMBL215327; 5-Amino-1-hydroxyisoquinoline; BDBM27503; 5-amino-isoquinolin-1(2h)-one; 5-amino isoquinolin-1(2h)-one; CTK5H2122; DTXSID90274354; 1(2H)-Isoquinolinone,5-amino-; MolPort-008-423-043; MolPort-004-803-197
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 940 nM | |||
| External Link | ||||
| INO-1002 | Investigative | [70] | ||
| Synonyms |
PARP inhibitors, Inotek; PARP inhibitor (erectile dysfunction), Inotek; PARP inhibitor (prostate nerve damage), Inotek; Poly (ADP ribose) polymerase inhibitor (erectile dysfunction), Inotek; Poly (ADP ribose) polymerase inhibitor (prostate nerve damage), Inotek
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-(4-aminophenyl)quinoxaline-5-carboxamide | Investigative | [75] | ||
| Synonyms |
CHEMBL443077; quinoxaline analogue, 3f; SCHEMBL7112515
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 87 nM | |||
| External Link | ||||
| 2-phenylpyrazolo-[1,5-a]pyridine-7-carboxamide | Investigative | [80] | ||
| Synonyms |
2-phenylpyrazolo[1,5-a]pyridine-7-carboxamide; 1196713-16-2; ZINC64337832; DA-47424
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-benzylphthalazin-1(2H)-one | Investigative | [75] | ||
| Synonyms |
4-Benzyl-1(2H)-phthalazinone; 32003-14-8; 4-Benzyl-2H-phthalazin-1-one; 4-benzyl-1,2-dihydrophthalazin-1-one; CHEMBL66761; JUCCMEHWBGPJKS-UHFFFAOYSA-N; benzylphthalazinone; phthalazinone, 1; 4-benzyl-phthalazone; AC1LDDNC; SMR000135223; AC1Q6GZZ; ChemDiv2_000142; Cambridge id 5241846; Oprea1_151142; Oprea1_623913; CBDivE_015258; MLS000530246; SCHEMBL863462; CTK4G8063; BDBM27660; DTXSID30346948; MolPort-001-796-654; HMS2379K10; HMS1369G10; 4-benzyl-2-hydrophthalazin-1-one; 4-Benzyl-1(2H)-phthalazinone #
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 770 nM | |||
| External Link | ||||
| 3-Methylquinoline-8-carboxamide | Investigative | [73] | ||
| Synonyms |
CHEMBL504998; 8-Quinolinecarboxamide, 3-methyl-; BDBM50255267
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3400 nM | |||
| External Link | ||||
| 2-(4-Chlorophenyl)-5-Quinoxalinecarboxamide | Investigative | [79] | ||
| Synonyms |
3-(4-CHLOROPHENYL)QUINOXALINE-5-CARBOXAMIDE; 4tju; 1wok; AC1LCVX7; quinoxaline analogue, 3b; SCHEMBL424209; 3-(4-chloro-phenyl)-quinoxaline-5-carboxylic acid amide; CTK8F4675; BDBM27720; ZINC1489510; DB03509; 489457-67-2
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(4-Methoxy-phenyl)-8-methyl-3H-quinazolin-4-one | Investigative | [74] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 891 nM | |||
| External Link | ||||
| HYDAMTIQ | Investigative | [70] | ||
| Synonyms |
PARP-1 inhibitor (brain ischemia), University of Perugia
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-Nitro-6H,11H-indeno[1,2-c]isoquinolin-5-one | Investigative | [82] | ||
| Synonyms |
SCHEMBL4661629; CHEMBL370673; BDBM27510; SWBDUXDIPMGDNO-UHFFFAOYSA-N; indeno[1,2-c]isoquinolinone, 1b; ZINC13652894; 5,6-dihydro-5-oxo-8-nitro-indeno[1,2-c]isoquinoline; 8-Nitro-5,6-dihydro-11H-indeno[1,2-c]isoquinoline-5-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| PD-128763 | Investigative | [79] | ||
| Synonyms |
5-Methyl-3,4-dihydroisoquinolin-1(2H)-one; 129075-56-5; 3,4-Dihydro-5-methyl-1(2H)-isoquinolinone; 3,4-DIHYDRO-5-METHYL-ISOQUINOLINONE; 1(2H)-Isoquinolinone, 3,4-dihydro-5-methyl-; CHEBI:41928; PD128763; CHEMBL125200; 5-methyl-3,4-dihydro-2H-isoquinolin-1-one; 3,4-dihydro-5-methylisoquinolinone; 1(2H)-Isoquinolinone,3,4-dihydro-5-methyl-; 5-methyl-1,2,3,4-tetrahydroisoquinolin-1-one; DHQ; PD 128763; dihydroisoquinolinone, 1; AC1L3WIO; ACMC-1C7L4; SCHEMBL831538; AMBZ0075; KS-00000QFP; CTK4B6146; BDBM27682
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 70 nM | |||
| External Link | ||||
| 8-Methyl-2-phenyl-3H-quinazolin-4-one | Investigative | [74] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 795 nM | |||
| External Link | ||||
| TI3 | Investigative | [77] | ||
| Synonyms |
RB106; CHEMBL419499; [(2S)-2-SULFANYL-3-PHENYLPROPANOYL]-GLY-(5-PHENYLPROLINE); 1qf2; AC1L9LL2; BDBM50051785; DB02669; N-[(S)-2-Mercapto-3-phenylpropionyl]-Gly-[(5R)-5-phenyl-L-Pro-]-OH; N-[(2S)-3-phenyl-2-sulfanylpropanoyl]glycyl-(5R)-5-phenyl-L-proline; (2S,5R)-5-phenyl-1-[2-[[(2S)-3-phenyl-2-sulfanylpropanoyl]amino]acetyl]pyrrolidine-2-carboxylic acid; (2S,5R)-1-[2-((S)-2-Mercapto-3-phenyl-propionylamino)-acetyl]-5-phenyl-pyrrolidine-2-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-aminobenzo[c][1,5]naphthyridin-6(5H)-one | Investigative | [75] | ||
| Synonyms |
CHEMBL106154; Benzo[c]-1,5-naphthyridin-6(5H)-one, 3-amino-; SCHEMBL12750402; BDBM50130580; 433726-73-9
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 180 nM | |||
| External Link | ||||
| S-111 | Investigative | [70] | ||
| Synonyms |
PARP1 inhibitor (cancer), Sentinel Oncology
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Thieno-phenanthridin-6-one | Investigative | [64] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-Morpholin-4-ylmethyl-5H-phenanthridin-6-one | Investigative | [84] | ||
| Synonyms |
CHEMBL194535
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 23 nM | |||
| External Link | ||||
| 1,2,3,4,4a,5-hexahydrophenanthridin-6(10bH)-one | Investigative | [75] | ||
| Synonyms |
CHEMBL84044; 7403-93-2; NSC403412; AC1L83AN; SCHEMBL6645911; DTXSID70323238; BDBM50131013; NSC-403412; 2,3,4,4a,5,10b-hexahydro-1H-phenanthridin-6-one; 1,3,4,4a,5,10b-Hexahydro-2H-phenanthridin-6-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7500 nM | |||
| External Link | ||||
| 8-Hydroxy-2-(4-nitro-phenyl)-3H-quinazolin-4-one | Investigative | [74] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 105 nM | |||
| External Link | ||||
| 2-(4-Amino-phenyl)-8-methyl-3H-quinazolin-4-one | Investigative | [74] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 504 nM | |||
| External Link | ||||
| 4-(4-Morpholin-4-yl-butyl)-2H-phthalazin-1-one | Investigative | [84] | ||
| Synonyms |
CHEMBL373210
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 950 nM | |||
| External Link | ||||
| 2-phenyl-2H-benzo[d][1,2,3]triazole-4-carboxamide | Investigative | [80] | ||
| Synonyms |
CHEMBL1096560; SCHEMBL2265205
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 71 nM | |||
| External Link | ||||
| 2-phenyl-2H-indazole-7-carboxamide | Investigative | [80] | ||
| Synonyms |
CHEMBL594298; SCHEMBL1422404; BDBM50306166
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 24 nM | |||
| External Link | ||||
| Carba-Nicotinamide-Adenine-Dinucleotide | Investigative | [79] | ||
| Synonyms |
Carba-NAD; Carbanicotinamide adenine dinucleotide; 112345-60-5; AC1L4TS7; AC1Q5J0L; SCHEMBL16445201; 5'-o-{[({[(1r,2r,3s,4r)-4-(3-carbamoylpyridinium-1-yl)-2,3-dihydroxycyclopentyl]methoxy}phosphinato)oxy](hydroxy)phosphoryl}adenosine; [[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl] [(1R,2R,3S,4R)-4-(3-carbamoylpyridin-1-ium-1-yl)-2,3-dihydroxycyclopentyl]methyl phosphate; Adenosine 5'-(trihydrogen diphosphate), 5'-((4-(3-(aminocarbonyl)pyridinio)-2,3-dihydroxycyclopentyl)m
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-aminobenzamide | Investigative | [71] | ||
| Synonyms |
3544-24-9; m-Aminobenzamide; Benzamide, 3-amino-; 3-Amino-benzamide; Benzamide, m-amino-; meta-aminobenzamide; 3-ABA; 3-Aminobenzimide; 3-amino benzamide; aniline-3-carboxamide; 3-aminobenzoic acid amide; UNII-8J365YF1YH; CCRIS 3925; EINECS 222-586-9; NSC 36962; BRN 2802373; PARP Inhibitor I, 3-ABA; 3-H2NC6H4CONH2; CHEMBL81977; 3-AB; 8J365YF1YH; CHEBI:64042; GSCPDZHWVNUUFI-UHFFFAOYSA-N; MFCD00007989; 3-Aminobenzamide, 98%; SR-01000075657; HSDB 7581; 3-azanylbenzamide; m-amino benzamide; 4pml; 3-Aminobenzaminde
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 3100 nM | |||
| External Link | ||||
| 3-Ethenylquinoline-8-carboxamide | Investigative | [73] | ||
| Synonyms |
CHEMBL481793; 8-Quinolinecarboxamide, 3-ethenyl-; BDBM50255270
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5800 nM | |||
| External Link | ||||
| 3-Phenylquinoline-8-carboxamide | Investigative | [73] | ||
| Synonyms |
CHEMBL450259; BDBM50255264
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 15000 nM | |||
| External Link | ||||
| 3-(4-methoxyphenyl)quinoxaline-5-carboxamide | Investigative | [75] | ||
| Synonyms |
CHEMBL519443; quinoxaline analogue, 3e; SCHEMBL7045177
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 71 nM | |||
| External Link | ||||
| TI4 | Investigative | [77] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Benzo[c][1,5]naphthyridin-6(5H)-one | Investigative | [75] | ||
| Synonyms |
CHEMBL320031; Benzo[c]-1,5-naphthyridin-6(5H)-one; SCHEMBL12750384; BDBM50130585; 5H-Benzo[c][1,5]naphthyridin-6-one; Benzo[c][1,5]naphthyridine-6(5H)-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 116 nM | |||
| External Link | ||||
| 3-(4-cyanophenyl)quinoxaline-5-carboxamide | Investigative | [75] | ||
| Synonyms |
CHEMBL481603; quinoxaline analogue, 3c; SCHEMBL7108289
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 101 nM | |||
| External Link | ||||
| 1,7,8,9-tetrahydro-1,5-diaza-trindene-4,6-dione | Investigative | [81] | ||
| Synonyms |
CHEMBL201907; SCHEMBL2086780; ZINC28569089
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 40 nM | |||
| External Link | ||||
| 8-Fluoro-6H,11H-indeno[1,2-c]isoquinolin-5-one | Investigative | [82] | ||
| Synonyms |
CHEMBL370045; BDBM27512; indeno[1,2-c]isoquinolinone, 1d; ZINC13652896; 8-Fluoro-5,6-dihydro-11H-indeno[1,2-c]isoquinoline-5-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 225 nM | |||
| External Link | ||||
| 8-Hydroxy-2-phenyl-3H-quinazolin-4-one | Investigative | [74] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1034 nM | |||
| External Link | ||||
| 8-Methoxy-2-(4-nitro-phenyl)-3H-quinazolin-4-one | Investigative | [74] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 10000 nM | |||
| External Link | ||||
| 2-(4-Chlorophenyl)-2H-indazole-7-carboxamide | Investigative | [80] | ||
| Synonyms |
CHEMBL1099295; SCHEMBL2264064
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 24 nM | |||
| External Link | ||||
| BPI-704001 | Investigative | [70] | ||
| Synonyms |
BPI-705001; BPI-715001; PARP-1 inhibitors (cancer); PARP-1 inhibitors (cancer), Beta Pharma
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Pyrrolo[3,4-c]carbazole-1,3(2H,6H)-dione | Investigative | [81] | ||
| Synonyms |
CHEMBL370869; SCHEMBL5381581
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10000 nM | |||
| External Link | ||||
| 2-Methylquinoline-8-carboxamide | Investigative | [73] | ||
| Synonyms |
CHEMBL471966; 8-Quinolinecarboxamide, 2-methyl-; SCHEMBL422282; BDBM50255329; AKOS022882220
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| 8-Methoxy-2-methyl-3H-quinazolin-4-one | Investigative | [74] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-Prop-1-ynylquinoline-8-carboxamide | Investigative | [73] | ||
| Synonyms |
CHEMBL504903; ZINC40829471
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2200 nM | |||
| External Link | ||||
| 2-(2-Chlorophenyl)-2H-indazole-7-carboxamide | Investigative | [80] | ||
| Synonyms |
CHEMBL1094952; SCHEMBL2265265
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| 2-(3-Piperidin-1-yl-propyl)-3H-quinazolin-4-one | Investigative | [76] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1100 nM | |||
| External Link | ||||
| 3-Ethylquinoline-8-carboxamide | Investigative | [73] | ||
| Synonyms |
CHEMBL453989; 8-Quinolinecarboxamide, 3-ethyl-; BDBM50255269
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3700 nM | |||
| External Link | ||||
| A-620223 | Investigative | [70] | ||
| Synonyms |
A-866111; A-966492; ABT-472; ABT-999; PARP inhibitor, Abbott; PARP inhibitors, Abbott; Poly (ADP)ribose polymer inhibitor, Abbott; Poly (ADP)ribose inhibitors (cancer), Abbott
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 3 nM | |||
| External Link | ||||
| DR2313 | Investigative | [64] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(4-Azido-phenyl)-8-methoxy-3H-quinazolin-4-one | Investigative | [74] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(3-Chlorophenyl)-2H-indazole-7-carboxamide | Investigative | [80] | ||
| Synonyms |
CHEMBL1094953; SCHEMBL2265628
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 14 nM | |||
| External Link | ||||
| 2-(4-Hydroxy-phenyl)-8-methyl-3H-quinazolin-4-one | Investigative | [74] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1200 nM | |||
| External Link | ||||
| 2-ethylquinoline-8-carboxamide | Investigative | [73] | ||
| Synonyms |
CHEMBL526128; 8-Quinolinecarboxamide, 2-ethyl-; BDBM50255330
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 800 nM | |||
| External Link | ||||
| 2-phenylquinoline-8-carboxamide | Investigative | [73] | ||
| Synonyms |
CHEMBL480429; SCHEMBL6442515; ZINC3939668
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 900 nM | |||
| External Link | ||||
| BZ5 | Investigative | [77] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| ME0328 | Investigative | [86] | ||
| Synonyms |
ME-0328; compound 5b [PMID 24188023]
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 6300 nM | |||
| External Link | ||||
| [2(R,S)-2-Sulfanylheptanoyl]-Phe-Ala | Investigative | [77] | ||
| Synonyms |
TI1; AC1NRDCJ; 1qf1; DB02597; N-[(2S)-2-sulfanylheptanoyl]-L-phenylalanyl-L-alanine; N-[N-[(S)-2-Mercaptoheptanoyl]-L-phenylalanyl]-L-alanine; (2S)-2-[[(2S)-3-phenyl-2-[[(2S)-2-sulfanylheptanoyl]amino]propanoyl]amino]propanoic acid; (2S)-2-[(2S)-3-phenyl-2-[(2S)-2-sulfanylheptanamido]propanamido]propanoic
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| S-070 | Investigative | [70] | ||
| Synonyms |
S-158; Targeted synergy program (solid tumor), Sentinel Oncology; Chk1/PARP-1 inhibitors (cancer); Chk1/PARP-1 inhibitors (cancer), Sentinel Oncology
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2B91: Colorectal cancer | 25 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Retifanlimab | Approved | [87] | ||
| Synonyms |
INCMGA0012; Retifanlimab
Click to Show/Hide
|
|||
| External Link | ||||
| Aflibercept | Approved | [88] | ||
| Synonyms |
Ziv-Aflibercept; Zaltrap (TN); VEGF Trap; VEGF Trap-Eye
Click to Show/Hide
|
|||
| External Link | ||||
| Regorafenib | Approved | [89] | ||
| Synonyms |
755037-03-7; BAY 73-4506; Regorafenibum; Stivarga; 4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide; BAY73-4506; Regorafenib (BAY 73-4506); UNII-24T2A1DOYB; 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide; BAY-73-4506; 24T2A1DOYB; CHEMBL1946170; CHEBI:68647; Stivarga (TN); BAY73-4506 hydrochloride; Regorafenib [USAN:INN]
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [90] | ||
| Synonyms |
Bevacizumab (ophthalmic slow-release tissue tablet)
Click to Show/Hide
|
|||
| External Link | ||||
| SYM-004 | Phase 3 | [90] | ||
| Synonyms |
Chimeric IgG1 antibody 1024 (cancer), Symphogen; Chimeric IgG1 antibody 992 (cancer), Symphogen; Chimeric IgG1 antibodies992 + 1024 (cancer), Symphogen
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab + Erlotinib | Phase 3 | [91] | ||
| External Link | ||||
| CPI-613 | Phase 3 | [90] | ||
| Synonyms |
95809-78-2; 6,8-bis(benzylthio)octanoic acid; CPI 613; MLS006010202; SCHEMBL1062218; 6,8-Bis(benzylsulfanyl)octanoic acid; Octanoic acid, 6,8-bis((phenylmethyl)thio)-; Octanoic acid, 6,8-bis[(phenylMethyl)thio]-; 6,8-Bis[(phenylmethyl)thio]octanoic acid; CPI613; CHEMBL3186849; QCR-193; AOB1058; MolPort-023-219-128; HMS3656L06; C22H28O2S2; BCP04663; EX-A2043; s2776; AKOS025142095; BCP9000552; DB12109; RL06062; CS-0961; NCGC00344764-01; SMR004701300; AS-16613; BC261916; AK174899; HY-15453; BCP0726000030; KB-293127; AB0035874
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [88] | ||
| External Link | ||||
| AlloStim | Phase 2/3 | [92] | ||
| Synonyms |
AlloStim (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Sibrotuzumab | Phase 2 | [93] | ||
| External Link | ||||
| CV301 | Phase 2 | [94] | ||
| External Link | ||||
| Efatutazone | Phase 2 | [95] | ||
| Synonyms |
Inolitazone; 223132-37-4; 5-[[4-[[6-(4-amino-3,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-yl]methoxy]phenyl]methyl]-2,4-Thiazolidinedione; Efatutazone [INN]; RS5444; CS-7017; SCHEMBL3246054; CHEMBL3545280; JCYNMRJCUYVDBC-UHFFFAOYSA-N; Efatutazone;CS-7017;RS5444; BCP07478; AKOS030526729; DB11894; CS-0778; KB-77905; DA-07988; HY-14792; QC-10456; 4CA-1384; FT-0737589; 5-[4-[6-(4-amino-3 ,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-ylmethoxy]benzyl]thiazolidine-2,4-dione
Click to Show/Hide
|
|||
| External Link | ||||
| LOR-2040 | Phase 2 | [96] | ||
| External Link | ||||
| RG7221 | Phase 2 | [97] | ||
| External Link | ||||
| PEG-SN38 | Phase 2 | [98] | ||
| Synonyms |
EZN-2208
Click to Show/Hide
|
|||
| External Link | ||||
| MEGF0444A | Phase 2 | [99] | ||
| External Link | ||||
| Encapsulated cell therapy | Phase 1/2 | [100] | ||
| External Link | ||||
| AB928 | Phase 1/2 | [101] | ||
| External Link | ||||
| MGD007 | Phase 1 | [97] | ||
| External Link | ||||
| BNC-101 | Phase 1 | [102] | ||
| External Link | ||||
| Navicixizumab | Phase 1 | [90] | ||
| External Link | ||||
| RG7160 | Discontinued in Phase 2 | [103] | ||
| External Link | ||||
| Nimesulide | Terminated | [104] | ||
| Synonyms |
51803-78-2; N-(4-Nitro-2-phenoxyphenyl)methanesulfonamide; Mesulid; Flogovital; Sulidene; Nimed; R-805; 4-NITRO-2-PHENOXYMETHANESULFONANILIDE; Nisulid; Nimesulidum [INN-Latin]; Nimesulida [INN-Spanish]; R 805; UNII-V4TKW1454M; 4-Nitro-2-phenoxy-methanesulfonanilide; 4'-Nitro-2'-phenoxymethanesulfonanilide; Methanesulfonamide, N-(4-nitro-2-phenoxyphenyl)-; EINECS 257-431-4; 4'-Nitro-2'-phenoxymethansulfonanilid; BRN 2421175; CHEMBL56367; MLS000069680; V4TKW1454M; Methanesulfonanilide, 4'-nitro-2'-phenoxy-; CHEBI:44445; Dulanermin
Click to Show/Hide
|
|||
| External Link | ||||
| Saracatinib | Phase 2 | [105] | ||
| External Link | ||||
| G3139 + Irinotecan | Investigative | [106] | ||
| External Link | ||||
References