m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03395
|
[1], [2] | |||
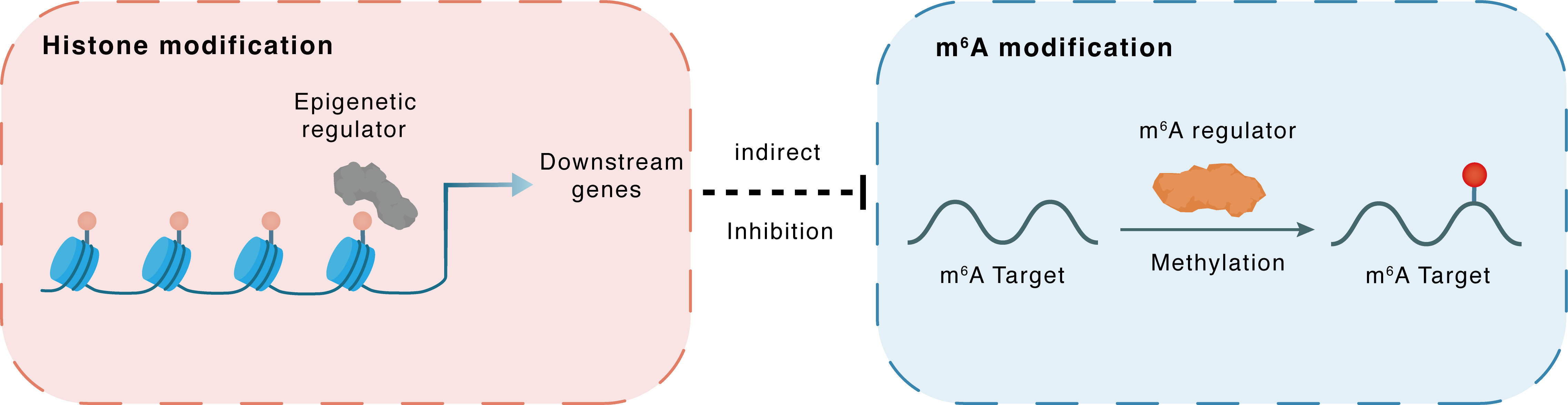 Histone modification
H3K4me1
Epigenetic Regulator
MIR570HG
Indirect
Inhibition
m6A modification
MET
MET
METTL3
Methylation
Histone modification
H3K4me1
Epigenetic Regulator
MIR570HG
Indirect
Inhibition
m6A modification
MET
MET
METTL3
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Hepatocyte growth factor receptor (c-Met/MET) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Regulated Target | Histone H3 lysine 4 monomethylation (H3K4me1) | View Details | |||
| Downstream Gene | MIR570HG | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Inhibition | |||
| Crosstalk Mechanism | Histone modification indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | Gain of Histone H3 lysine 4 monomethylation (H3K4me1) and H3K27ac led to the activation of MIR570HG expression. LINC00969 interacts with EZH2 and METTL3, transcriptionally regulates the level of H3K27me3 in the NLRP3 promoter region, and posttranscriptionally modifies the m6A level of NLRP3 in an m6A-YTHDF2-dependent manner METTL3 combines with Hepatocyte growth factor receptor (c-Met/MET) and causes the PI3K/AKT signalling pathway to be manipulated, which affects the sensitivity of lung cancer cells to gefitinib. METTL3 knockdown promotes apoptosis and inhibits proliferation of lung cancer cells. | ||||
| Responsed Disease | Lung cancer | ICD-11: 2C25 | |||
| Responsed Drug | Gefitinib | ||||
| Cell Process | Pyroptosis | ||||
In-vitro Model |
PC-9 | Lung adenocarcinoma | Homo sapiens | CVCL_B260 | |
| NCI-H3255 | Lung adenocarcinoma | Homo sapiens | CVCL_6831 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Hepatocyte growth factor receptor (c-Met/MET) | 76 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Capmatinib | Approved | [3] | ||
| Synonyms |
1029712-80-8; INCB28060; INC-280; INC280; UNII-TY34L4F9OZ; 2-fluoro-N-methyl-4-(7-(quinolin-6-ylmethyl)imidazo[1,2-b][1,2,4]triazin-2-yl)benzamide; INC28060; INCB-28060; INCB28060(Capmatinib); NVP-INC280; TY34L4F9OZ; Capmatinib (INCB28060); INCB 28060; 2-Fluoro-N-methyl-4-[7-[(quinolin-6-yl)methyl]imidazo[1,2-b]-[1,2,4]triazin-2-yl]benzamide; BenzaMide, 2-fluoro-N-Methyl-4-[7-(6-quinolinylMethyl)iMidazo[1,2-b][1,2,4]triazin-2-yl]-; C23H17FN6O
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 0.13 nM | |||
| External Link | ||||
| Tepotinib | Approved | [4] | ||
| Synonyms |
1100598-32-0; EMD 1214063; UNII-1IJV77EI07; Tepotinib (EMD 1214063); EMD1214063; 1IJV77EI07; MSC-2156119J; Benzonitrile, 3-[1,6-dihydro-1-[[3-[5-[(1-methyl-4-piperidinyl)methoxy]-2-pyrimidinyl]phenyl]methyl]-6-oxo-3-pyridazinyl]-; C29H28N6O2; Tepotinib [INN]; Benzonitrile, 3-[1,6-Dihydro-1-[[3-[5-[(1-Methyl-4-Piperidinyl)Methoxy]-2-PyriMidinyl]Phenyl]Methyl]-6-Oxo-3-Pyridazinyl]; Benzonitrile, 3-(1,6-dihydro-1-((3-(5-((1-methyl-4-piperidinyl)methoxy)-2-pyrimidinyl)phenyl)methyl)-6-oxo
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 < 1 nM | |||
| External Link | ||||
| Cabozantinib | Approved | [5] | ||
| Synonyms |
Cabometyx; Cometriq
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1.3 nM | |||
| External Link | ||||
| Amivantamab | Approved | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Crizotinib | Approved | [7] | ||
| Synonyms |
Xalkori (TN); novel ALK inhibitors
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 0.51 nM | |||
| External Link | ||||
| RG3638 | Phase 3 | [8] | ||
| Synonyms |
Onartuzumab
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Savolitinib | Phase 3 | [9] | ||
| Synonyms |
1313725-88-0; AZD-6094; AZD6094; UNII-2A2DA6857R; CHEMBL3334567; 2A2DA6857R; Savolitinib [INN]; Volitinib(Savolitinib); Savolitinib [USAN:INN]; GTPL9918; SCHEMBL12489208; EX-A845; BDBM50023342; ZINC149738712; AKOS030526403; DB12048; compound 28 [PMID: 25148209]; HY-15959; AS-35250; 1H-1,2,3-Triazolo(4,5-b)pyrazine, 1-((1S)-1-imidazo(1,2-a)pyridin-6-ylethyl)-6-(1-methyl-1H-pyrazol-4-yl)-; KB-333895; FT-0700162; J-690125; 4-{1-[(1S)-1-{imidazo[1,2-a]pyri
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1.995 nM | |||
| External Link | ||||
| Beperminogene perplasmid | Phase 3 | [10] | ||
| MOA | Modulator | |||
| External Link | ||||
| Tivantinib | Phase 3 | [11] | ||
| Synonyms |
905854-02-6; ARQ-197; ARQ197; ARQ 197; Tivantinib (ARQ 197); (3R,4R)-3-(5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1-yl)-4-(1H-indol-3-yl)pyrrolidine-2,5-dione; UNII-PJ4H73IL17; PJ4H73IL17; 1000873-98-2; (3R,4R)-3-(5,6-Dihydro-4H-pyrrolo(3,2,1-ij)quinolin-1-yl)-4-(1H-indol-3-yl)pyrrolidine-2,5-dione; Tivantinib [USAN:INN]; 1228508-24-4; ARQ 197, Tivantinib; Tivantinib (ARQ-197); ARQ 197 (Tivantinib); cc-86; SCHEMBL44944; Tivantinib (JAN/USAN/INN); GTPL7948; CHEMBL2103882; CHEBI:91398; QCR-102
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 355 nM | |||
| External Link | ||||
| MGCD516 | Phase 2/3 | [8] | ||
| Synonyms |
Sitravatinib
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| LY-2875358 | Phase 2 | [12] | ||
| Synonyms |
C-Met mAb (cancer), Eli Lilly; Anti-c-Met antibody (cancer), Lilly
Click to Show/Hide
|
|||
| External Link | ||||
| SAR-125844 | Phase 2 | [13] | ||
| Synonyms |
Met inhibitor (iv, cancer), sanofi-aventis
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4 nM | |||
| External Link | ||||
| Emibetuzumab | Phase 2 | [14] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| AMG 337 | Phase 2 | [15] | ||
| MOA | Modulator | |||
| Activity | IC50 = 1 nM | |||
| External Link | ||||
| AMG 208 | Phase 2 | [16] | ||
| Synonyms |
AMG-208; 1002304-34-8; AMG208; UNII-Y2SR66P7VM; 7-methoxy-4-((6-phenyl-[1,2,4]triazolo[4,3-b]pyridazin-3-yl)methoxy)quinoline; Y2SR66P7VM; CHEBI:90626; 7-methoxy-4-[(6-phenyl[1,2,4]triazolo[4,3-b]pyridazin-3-yl)methoxy]quinoline; 7-Methoxy-4-[(6-phenyl-1,2,4-triazolo[4,3-b]pyridazin-3-yl)methoxy]quinoline; C22H17N5O2; 7-methoxy-4-[(6-phenyl-[1,2,4]triazolo[4,3-b]pyridazin-3-yl)methoxy]quinoline; 7-methoxy-4-({6-phenyl-[1,2,4]triazolo[4,3-b]pyridazin-3-yl}methoxy)quinoline; Triazolopyridazine, 4; 3cd8
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 9 nM | |||
| External Link | ||||
| HM-5016504 | Phase 2 | [8] | ||
| Synonyms |
C-Met inhibitor (cancer), Hutchison
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Telisotuzumab vedotin | Phase 2 | [17] | ||
| Synonyms |
ABBV-399
Click to Show/Hide
|
|||
| External Link | ||||
| APL-101 | Phase 2 | [18] | ||
| Synonyms |
Bozitinib; PLB-1001; 1440964-89-5; Vebreltinib; Vebreltinib [USAN]; UNII-2WZP8A9VFN; 2WZP8A9VFN; Bozitinib (PLB-1001); SCHEMBL15594471; BDBM107096; CBI-3103; s6762; WHO 11677; HY-125017; CS-0088607; US9695175, 44; 1,2,4-Triazolo(4,3-b)pyridazine, 6-(1-cyclopropyl-1H-pyrazol-4-yl)-3-(difluoro(6- fluoro-2-methyl-2H-indazol-5-yl)methyl)-; 6-(1-Cyclopropyl-1H-pyrazol-4-yl)-3-(difluoro(6-fluoro-2-methyl-2H-indazol-5- yl)methyl)-1,2,4-triazolo(4,3-b)pyridazine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CMX-2043 | Phase 2 | [19] | ||
| MOA | Modulator | |||
| External Link | ||||
| LY2801653 | Phase 2 | [20] | ||
| Synonyms |
Merestinib; 1206799-15-6; LY-2801653; UNII-5OGS5K699E; N-(3-fluoro-4-((1-methyl-6-(1H-pyrazol-4-yl)-1H-indazol-5-yl)oxy)phenyl)-1-(4-fluorophenyl)-6-methyl-2-oxo-1,2-dihydropyridine-3-carboxamide; 5OGS5K699E; N-(3-Fluoro-4-{[1-Methyl-6-(1h-Pyrazol-4-Yl)-1h-Indazol-5-Yl]oxy}phenyl)-1-(4-Fluorophenyl)-6-Methyl-2-Oxo-1,2-Dihydropyridine-3-Carboxamide; C30H22F2N6O3; Merestinib [USAN]; SCHEMBL679002; LY2801653 (Merestinib); GTPL9841; CHEMBL3545307; QCR-139; DTXSID20659635; SYN1222; QHADVLVFMKEIIP-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 4.7 nM | |||
| External Link | ||||
| XL880 | Phase 2 | [21] | ||
| Synonyms |
GSK 089; GSK 1363089; GSK1363089; XL 880; GSK1363089, GSK089, foretinib, EXEL-2880, XL880; 88Z; MET inhibitors
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 0.4 nM | |||
| External Link | ||||
| ChronSeal | Phase 1/2 | [22] | ||
| Synonyms |
Antibiotic-free recombinant HGF, Linkoping; Recombinant HGF, Kringle/ChronTech; Recombinant HGF, Kringle/Tripep
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Anti-C-met CAR-T cells | Phase 1/2 | [23] | ||
| MOA | CAR-T-Cell-Therapy | |||
| External Link | ||||
| BMS-777607 | Phase 1/2 | [24] | ||
| Synonyms |
1025720-94-8; BMS 777607; 1196681-44-3; BMS777607; ASLAN-002; UNII-A3MMS6HDO1; N-(4-(2-Amino-3-chloropyridin-4-yloxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide; A3MMS6HDO1; N-[4-[(2-Amino-3-chloropyridin-4-yl)oxy]-3-fluorophenyl]-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide; N-(4-((2-amino-3-chloropyridin-4-yl)oxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 2.512 nM | |||
| External Link | ||||
| Sym015 | Phase 1/2 | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| MK-2461 | Phase 1/2 | [25] | ||
| Synonyms |
MK 2461
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.4 nM | |||
| External Link | ||||
| REGN5093 | Phase 1 | [26] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PF-4217903 | Phase 1 | [27] | ||
| Synonyms |
2-(4-(1-(Quinolin-6-ylmethyl)-1H-[1,2,3]triazolo[4,5-b]pyrazin-6-yl)-1H-pyrazol-1-yl)ethanol; PF-04217903; 956905-27-4; PF04217903; PF 04217903; UNII-CYJ9ATV1IJ; CYJ9ATV1IJ; CHEMBL2001019; 1159490-85-3; aka PF-04217903; C19H16N8O; 2-[4-(3-Quinolin-6-ylmethyl-3H-[1,2,3]triazolo[4,5-b]pyrazin-5-yl)-pyrazol-1-yl]-ethanol; 2-(4-(1-(Quinolin-6-ylmethyl)-1H-(1,2,3)triazolo(4,5-b)pyrazin-6-yl)-1H-pyrazol-1-yl)ethanol; 3zxz; PDMUGYOXRHVNMO-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 0.3 nM | |||
| External Link | ||||
| ABBV-399 | Phase 1 | [14] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| CBT-101 | Phase 1 | [9] | ||
| Synonyms |
L-Asparaginyl-L-leucyl-glycyl-L-valyl-L-[S-(acetamidomethyl)]cysteinamide hydrochloride
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MK-8033 | Phase 1 | [28] | ||
| Synonyms |
1001917-37-8; UNII-350H6PBQ5Q; 3-(1-Methyl-1H-pyrazol-4-yl)-5-oxo-N-(2-pyridinylmethyl)-5H-benzo[4,5]cyclohepta[1,2-b]pyridine-7-methanesulfonamide; 350H6PBQ5Q; CHEMBL2323775; MK8033; 1-(3-(1-Methyl-1H-pyrazol-4-yl)-5-oxo-5H-benzo(4,5)cyclohepta(1,2-b)pyridin-7-yl)-N-(pyridin-2-ylmethyl)methanesulfonamide; 1-[2-(1-methylpyrazol-4-yl)-11-oxobenzo[1,2]cyclohepta[2,4-b]pyridin-9-yl]-N-(pyridin-2-ylmethyl)methanesulfonamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.6 nM | |||
| External Link | ||||
| Autologous T Cells Expressing MET scFv CAR | Phase 1 | [29] | ||
| MOA | CAR-T-Cell-Therapy | |||
| External Link | ||||
| ABT-700 | Phase 1 | [30] | ||
| MOA | Modulator | |||
| External Link | ||||
| Hepapoietin | Phase 1 | [31] | ||
| Synonyms |
APS-1010; Hepapoietin, SnowBrand; Hepapoietin (liver/kidney disease); Hepapoietin (liver/kidney disease), Atlas Pharmaceuticals
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| EMD-1204831 | Phase 1 | [32] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| JNJ-38877605 | Phase 1 | [33] | ||
| Synonyms |
C-met inhibitor, Ortho Biotech Oncology Research & Development; C-met inhibitor (solid tumors), ORD/J&J PRD
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.95 nM | |||
| External Link | ||||
| SGX523 | Phase 1 | [34] | ||
| Synonyms |
SGX-523; SGX523, SGX-523
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3.1 nM | |||
| External Link | ||||
| TR1801-ADC | Phase 1 | [35] | ||
| External Link | ||||
| LY3164530 | Phase 1 | [36] | ||
| MOA | Modulator | |||
| External Link | ||||
| C-Met/PD-L1 CAR-T Cell | Phase 1 | [37] | ||
| MOA | CAR-T-Cell-Therapy(Dual specific) | |||
| External Link | ||||
| E-7050 | Phase 1 | [38] | ||
| Synonyms |
C-Met and VEGF-2 tyrosine kinase inhibitor (oral, cancer), Eisai
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 14 nM | |||
| External Link | ||||
| XL092 | Phase 1 | [39] | ||
| Synonyms |
SCHEMBL21200856; NSC828252; XL-092; NSC-828252; 2367004-54-2
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BPI-9016 M | Phase 1 | [40] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| AZD9592 | Phase 1 | [41] | ||
| External Link | ||||
| TPX-0022 | Phase 1 | [42] | ||
| Synonyms |
CSF1R-IN-2; SCHEMBL20694441; TPX0022; NSC820832; NSC-820832; HY-111787; CS-0091874; 2271119-26-5
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RXDX-106 | Phase 1 | [43] | ||
| Synonyms |
CEP-40783; 1437321-24-8; CEP40783; UNII-1969ZJE05Q; 1969ZJE05Q; N-(4-((6,7-dimethoxyquinolin-4-yl)oxy)-3-fluorophenyl)-3-(4-fluorophenyl)-1-isopropyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidine-5-carboxamide; N-[4-(6,7-dimethoxyquinolin-4-yl)oxy-3-fluorophenyl]-3-(4-fluorophenyl)-2,4-dioxo-1-propan-2-ylpyrimidine-5-carboxamide; RXDX-106 (CEP-40783); SCHEMBL16089863; BCP25839; EX-A2540; MFCD28502441; NSC797770; s8570; AKOS032960472; ZINC205893112; CCG-270157; CS-6371; NSC-797770; SB18930; AC-31425; AS-35141; HY-100946; N-(4-((6,7-Dimethoxy-4-quinolinyl)oxy)-3-fluorophenyl)-3-(4-fluorophenyl)-1,2,3,4-tetrahydro-1-(1-methylethyl)-2,4-dioxo-5-pyrimidinecarboxamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Altiratinib | Phase 1 | [44] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2.7 nM | |||
| External Link | ||||
| Mteron-F1 | Preclinical | [45] | ||
| MOA | Stimulator | |||
| External Link | ||||
| NPS-1034 | Preclinical | [46] | ||
| Synonyms |
1221713-92-3; CHEMBL3810063; 1-(4-fluorophenyl)-N-[3-fluoro-4-[(3-phenyl-1H-pyrrolo[2,3-b]pyridin-4-yl)oxy]phenyl]-2,3-dimethyl-5-oxopyrazole-4-carboxamide; N-(3-fluoro-4-((3-phenyl-1H-pyrrolo[2,3-b]pyridin-4-yl)oxy)phenyl)-2-(4-fluorophenyl)-1,5-dimethyl-3-oxo-2,3-dihydro-1H-pyrazole-4-carboxamide.; SCHEMBL1963111; BCP15998; NPS1034;NPS 1034; BDBM50172077; s7669; ZINC68204845; CCG-270007; NCGC00481564-01; AC-31427; AK685795; BS-14709; HY-100509; CS-0019643; N-(3-fluoro-4-((3-phenyl-1H-pyrrolo[2,3-b]pyridin-4-yl)oxy)phenyl)-2-(4-fluorophenyl)-1,5-dimethyl-3-oxo-2,3-dihydro-1H-pyrazole-4-carboxamide; S4K
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ALD-805 | Investigative | [8] | ||
| External Link | ||||
| PHA-665752 | Investigative | [8] | ||
| Synonyms |
477575-56-7; PHA 665752; PHA665752; TCMDC-125885; UNII-0VXU5T5R3J; (2R)-1-[[5-[(Z)-[5-[[(2,6-DICHLOROPHENYL)METHYL]SULFONYL]-1,2-DIHYDRO-2-OXO-3H-INDOL-3-YLIDENE]METHYL]-2,4-DIMETHYL-1H-PYRROL-3-YL]CARBONYL]-2-(1-PYRROLIDINYLMETHYL)PYRROLIDINE; 0VXU5T5R3J; CHEMBL450786; CHEBI:90197; (R,Z)-5-(2,6-dichlorobenzylsulfonyl)-3-((3,5-dimethyl-4-(2-(pyrrolidin-1-ylmethyl)pyrrolidine-1-carbonyl)-1H-pyrrol-2-yl)methylene)indolin-2-one; PHA-665752 hydrate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 9 nM | |||
| External Link | ||||
| APS-3010 | Investigative | [8] | ||
| Synonyms |
Hepatic growth factor inhibitor (cancer), Atlas Pharmaceuticals
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| LA-480 | Investigative | [8] | ||
| Synonyms |
Bispecific c-Met monoclonal antibody (cancer), Lilly
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-cMET mab | Investigative | [8] | ||
| Synonyms |
Anti-cMET mAb (undisclosed indication); Anti-cMET mAb (undisclosed indication), PharmAbcine; CMET inhibitor (mAb, undisclosed indication), PharmAbcine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| AM7 | Investigative | [47] | ||
| Synonyms |
pyrimidone, 22; AM 7; AM-7
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 8.9 nM | |||
| External Link | ||||
| mab 224G11 | Investigative | [8] | ||
| Synonyms |
C-Met antagonist monoclonal antibody (cancer); C-Met antagonist monoclonal antibody (cancer), Pierre Fabre/Abbott
Click to Show/Hide
|
|||
| External Link | ||||
| TP-801 | Investigative | [8] | ||
| Synonyms |
C-Met tyrosine kinase inhibitor (oral, cancer), Tiger Pharmatech
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SU11274 | Investigative | [48] | ||
| Synonyms |
Met kinase Inhibitor; SU-11274
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| PF-00614435 | Investigative | [8] | ||
| Synonyms |
PF-00658968; PF-00851623; PF-02311803; PF-4254644; PF-851623; PF-899555; C-Met (HGFR) inhibitors (cancer), Pfizer
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CMET Avimer polypeptides | Investigative | [8] | ||
| Synonyms |
CMET Avimer polypeptides (cancer); MEDI-555; CMET Avimer polypeptides (cancer), MedImmmue; CMET-targeting anticancer avimers, MedImmune; CMET-targeting avimers (cancer), Avidia/MedImmune
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PRS-110 | Investigative | [8] | ||
| Synonyms |
C-Met inhibitor (cancer), Pieris; C-mesenchymal-epithelial transition factor (lipoprotein/protein recombinant, cancer), Pieris AG
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1-benzyl-1H-pyrrolo[3,2-b]pyridine | Investigative | [49] | ||
| Synonyms |
CHEMBL561256; 1-benzyl-4-azaindole; 1h-pyrrolo[3,2-b]pyridine,1-(phenylmethyl)-; SCHEMBL4716978; LOGFFHFLSCMKJF-UHFFFAOYSA-N; BDBM50295764; ZINC43079815; 50426-35-2
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 19 nM | |||
| External Link | ||||
| RP-1040 | Investigative | [8] | ||
| Synonyms |
RP-1086; RP-1087; RP-1088; RP-1101; RP-1103; RP-1105; RP-1109; RP-1110; RP-1111; RP-1112; C-Met kinase inhibitors (oral,cancer); C-Met kinase inhibitors (oral, cancer), Incozen Therapeutics/Rhizen Pharmaceuticals
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BAY-85-3474 | Investigative | [8] | ||
| Synonyms |
Met inhibitor (cancer), Bayer Schering
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| GE-137 | Investigative | [50] | ||
| MOA | Modulator | |||
| External Link | ||||
| 3-(phenylsulfonyl)-1H-pyrrolo[2,3-b]pyridine | Investigative | [49] | ||
| Synonyms |
CHEMBL538932; 633303-90-9; SCHEMBL3889418; CTK2A9458; DTXSID00621754; XIVJYIWQYBHEBJ-UHFFFAOYSA-N; BDBM50295740; 3-(Benzenesulfonyl)-1H-pyrrolo[2,3-b]pyridine; 1H-Pyrrolo[2,3-b]pyridine, 3-(phenylsulfonyl)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1175 nM | |||
| External Link | ||||
| 1-(2-nitrophenethyl)-1H-pyrrolo[3,2-b]pyridine | Investigative | [49] | ||
| Synonyms |
CHEMBL540744
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 250 nM | |||
| External Link | ||||
| ALD-806 | Investigative | [8] | ||
| Synonyms |
Anti-HGF mAb (cancer), Alder Biopharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| 1-(phenylsulfonyl)-1H-pyrrolo[3,2-b]pyridine | Investigative | [49] | ||
| Synonyms |
1-(Phenylsulfonyl)-4-azaindole; 677302-44-2; CHEMBL556118; SCHEMBL3705420; HGOGRVVSSAWHTO-UHFFFAOYSA-N; BDBM50295729; ZINC42923799; AKOS032961360; KB-3354225
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3500 nM | |||
| External Link | ||||
| DP-3590 | Investigative | [8] | ||
| Synonyms |
DP-4157; DP-4693; DP-4756; C-Met kinase inhibitors (solid tumor/metastatic cancer); C-Met kinase inhibitors (solid tumor/metastatic cancer), Deciphera
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| HuMax-cMet | Investigative | [8] | ||
| Synonyms |
CMet-targeting human IgG1 antibody (cancer), Genmab
Click to Show/Hide
|
|||
| External Link | ||||
| YH013 | Investigative | [51] | ||
| External Link | ||||
| PMID24210504C1o | Investigative | [52] | ||
| Synonyms |
GTPL8143; BDBM50444090
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 8 nM | |||
| External Link | ||||
| PMID21123062C27 | Investigative | [53] | ||
| Synonyms |
GTPL8210; BDBM50334085
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 44 nM | |||
| External Link | ||||
| PMID21967808CR-16 | Investigative | [54] | ||
| Synonyms |
GTPL8213; BDBM50361564
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 26 nM | |||
| External Link | ||||
| BMS-536924 | Investigative | [55] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 4870 nM | |||
| External Link | ||||
| 2C25: Lung cancer | 52 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Adagrasib | Approved | [56] | ||
| Synonyms |
2326521-71-3; MRTX-849; UNII-8EOO6HQF8Y; 8EOO6HQF8Y; 2-((S)-4-(7-(8-Chloronaphthalen-1-yl)-2-(((S)-1-methylpyrrolidin-2-yl)methoxy)-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4-yl)-1-(2-fluoroacryloyl)piperazin-2-yl)acetonitrile; CHEMBL4594350; SCHEMBL20974691; GTPL10888; Kras G12C inhibitor MRTX849; BCP31538; EX-A3258; MRTX 849; MFCD32263433; s8884; compound 20 [PMID: 32250617]; BS-16211; HY-130149; CS-0105265; 2-Piperazineacetonitrile, 4-(7-(8-chloro-1-naphthalenyl)-5,6,7,8-tetrahydro-2-(((2S)-1-methyl-2-pyrrolidinyl)methoxy)pyrido(3,4-d)pyrimidin-4-yl)-1-(2-fluoro-1-oxo-2-propen-1-yl)-, (2S)-
Click to Show/Hide
|
|||
| External Link | ||||
| Amivantamab | Approved | [57] | ||
| External Link | ||||
| Mobocertinib | Approved | [58] | ||
| Synonyms |
1847461-43-1; TAK-788; TAK788; AP32788; UNII-39HBQ4A67L; 39HBQ4A67L; propan-2-yl 2-[4-{[2-(dimethylamino)ethyl](methyl)amino}-2-methoxy-5-(prop-2-enamido)anilino]-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Mobocertinib [INN]; Mobocertinib [USAN]; AP-32788; Mobocertinib (TAK788); Mobocertinib(TAK-788); SCHEMBL17373133; GTPL10468; BDBM368374; BCP31045; EX-A3392; US10227342, Example 10; MFCD32669806; NSC825519; s6813; TAK-788;AP32788; WHO 11183; NSC-825519; example 94 [WO2015195228A1]; HY-135815; CS-0114256; TAK-788;TAK 788; AP 32788; 5-Pyrimidinecarboxylic acid, 2-((4-((2-(dimethylamino)ethyl)methylamino)-2-methoxy-5-((1-oxo-2-propen-1-yl)amino)phenyl)amino)-4-(1-methyl-1H-indol-3-yl)-, 1-methylethyl ester; C(C=C)(=O)NC=1C(=CC(=C(C=1)NC1=NC=C(C(=N1)C1=CN(C2=CC=CC=C12)C)C(=O)OC(C)C)OC)N(C)CCN(C)C; Isopropyl 2-((5-acrylamido-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenyl)amino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Isopropyl 2-(5-acrylamido-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenylamino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Propan-2-yl 2-(5-(acryloylamino)-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyanilino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; propan-2-yl 2-[4-[2-(dimethylamino)ethyl-methylamino]-2-methoxy-5-(prop-2-enoylamino)anilino]-4-(1-methylindol-3-yl)pyrimidine-5-carboxylate
Click to Show/Hide
|
|||
| External Link | ||||
| Sugemalimab | Approved in China | [59] | ||
| External Link | ||||
| Sacituzumab govitecan | Approved | [60] | ||
| Synonyms |
1491917-83-9; 1535963-91-7; 1796566-95-4; CYSTEINYL CL2A-SN-38; DA64T2C2IO; DTXSID401335985; EX-A4354; F82944; GOVITECAN CYSTEINYL CONJUGATE; hRS 7SN38; hRS7-SN38; IMMU 132; IMMU-132; M9BYU8XDQ6; Sacituzumab govitecan; Sacituzumab govitecan [USAN]; sacituzumab-govitecan; Satralizumab linker; SN-38 CYSTEINYL CONJUGATE; UNII-M9BYU8XDQ6
Click to Show/Hide
|
|||
| External Link | ||||
| Atezolizumab | Approved | [61] | ||
| External Link | ||||
| Tepotinib | Approved | [4] | ||
| Synonyms |
1100598-32-0; EMD 1214063; UNII-1IJV77EI07; Tepotinib (EMD 1214063); EMD1214063; 1IJV77EI07; MSC-2156119J; Benzonitrile, 3-[1,6-dihydro-1-[[3-[5-[(1-methyl-4-piperidinyl)methoxy]-2-pyrimidinyl]phenyl]methyl]-6-oxo-3-pyridazinyl]-; C29H28N6O2; Tepotinib [INN]; Benzonitrile, 3-[1,6-Dihydro-1-[[3-[5-[(1-Methyl-4-Piperidinyl)Methoxy]-2-PyriMidinyl]Phenyl]Methyl]-6-Oxo-3-Pyridazinyl]; Benzonitrile, 3-(1,6-dihydro-1-((3-(5-((1-methyl-4-piperidinyl)methoxy)-2-pyrimidinyl)phenyl)methyl)-6-oxo
Click to Show/Hide
|
|||
| External Link | ||||
| Sotorasib | Approved | [62] | ||
| Synonyms |
AMG-510; AMG510; AMG-510 racemate; 2252403-56-6; AMG 510; Kras G12C inhibitor 9; 2296729-00-3; UNII-2B2VM6UC8G; 2B2VM6UC8G; CHEMBL4535757; 2296729-00-3 (racemate); 4-((S)-4-Acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(2-isopropyl-4-methylpyridin-3-yl)pyrido[2,3-d]pyrimidin-2(1H)-one; 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-[(2S)-2-methyl-4-prop-2-enoylpiperazin-1-yl]pyrido[2,3-d]pyrimidin-2-one; Sotorasib [INN]; 6-Fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-((2S)-2-methyl-4-prop-2-enoylpiperazin-1-yl)pyrido(2,3-d)pyrimidin-2-one; AMG510 racemate; Sotorasib [USAN]; AMG-510(racemate); Kras mutant-targeting AMG 510; SCHEMBL20560375; GTPL10678; AMG 510 pound>>AMG-510; AMY16918; BCP30452; BCP33368; EX-A3538; BDBM50514402; NSC818433; s8830; WHO 11370; DB15569; NSC-818433; BS-16684; HY-114277; CS-0081316; compound (R)-38 [PMID: 31820981]; (1m)-6-Fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-(propan-2-yl)pyridin-3-yl)-4-((2S)-2-methyl-4-(prop-2-enoyl)piperazin-1-yl)pyrido(2,3-d)pyrimidin-2(1H)-one; (1S)-4-((S)-4-Acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(2-isopropyl-4-methylpyridin-3-yl)pyrido[2,3-d]pyrimidin-2(1H)-one; 2296729-66-1; Pyrido(2,3-d)pyrimidin-2(1H)-one, 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-(1-methylethyl)-3-pyridinyl)-4-((2S)-2-methyl-4-(1-oxo-2-propen-1-yl)-1-piperazinyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| Entrectinib | Approved | [63] | ||
| Synonyms |
1108743-60-7; RXDX-101; UNII-L5ORF0AN1I; Entrectinib (RXDX-101); L5ORF0AN1I; Benzamide, N-[5-[(3,5-difluorophenyl)methyl]-1H-indazol-3-yl]-4-(4-methyl-1-piperazinyl)-2-[(tetrahydro-2H-pyran-4-yl)amino]-; Benzamide, N-(5-((3,5-difluorophenyl)methyl)-1H-indazol-3-yl)-4-(4-methyl-1-piperazinyl)-2-((tetrahydro-2H-pyran-4-yl)amino)-; Entrectinib [USAN:INN]; YMX; Kinome_2659; Entrectinib(rxdx-101); Entrectinib (USAN/INN); SCHEMBL3512601; GTPL8290; CHEMBL1983268; KS-00000TSK
Click to Show/Hide
|
|||
| External Link | ||||
| Repotrectinib | Approved | [64] | ||
| Synonyms |
FIKPXCOQUIZNHB-RRKGBCIJSA-N; SCHEMBL20438940; TPX 0005; BCP19778
Click to Show/Hide
|
|||
| External Link | ||||
| MYL-1402O | Phase 3 | [65] | ||
| Synonyms |
bevacizumab biosimilar
Click to Show/Hide
|
|||
| External Link | ||||
| AB154 | Phase 3 | [66] | ||
| Synonyms |
Domvanalimab
Click to Show/Hide
|
|||
| External Link | ||||
| Datopotamab deruxtecan | Phase 3 | [67] | ||
| External Link | ||||
| CS1001 | Phase 3 | [68] | ||
| External Link | ||||
| JDQ443 | Phase 3 | [69] | ||
| Synonyms |
(S)-JDQ-443; 1-(6-((4S)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl)-2-azaspiro[3.3]heptan-2-yl)prop-2-en-1-one; 1-[6-[(4R)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-inda zol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]- 2-Propen-1-one; 1-[6-[(4R)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]-2-propen-1-one; 1-[6-[4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methylindazol-5-yl)pyrazol-1-yl]-2-azaspiro[3.3]heptan-2-yl]prop-2-en-1-one; 1-{6-[(4M)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5- methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2- azaspiro[3.3]heptan-2-yl}prop-2-en-1-one; 1-{6-[(4M)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]heptan-2-yl}prop-2-en-1-one; 2653994-08-0; 2653994-10-4; 2-Propen-1-one, 1-[6-[(4R)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]-; AKOS040757949; AT36708; BDBM50579985; CHEMBL5077861; compound 5 [PMID: 35404998]; CS-0226220; CS-0311034; EX-A5693; example 1a [WO2021120890A1]; GLXC-25533; GTPL11715; HY-139612A; JDQ 443; JDQ 443 [WHO-DD]; JDQ443; JDQ-443; MS-29737; NSC846146; NSC-846146; NVP-JDQ443; NVP-JDQ-443; Opnurasib; opnurasib [INN]; -PROPEN-1-ONE, 1-(6-((4R)-4-(5-CHLORO-6-METHYL-1H-INDAZOL-4-YL)-5-METHYL-3-(1-METHYL-1H-INDAZOL-5-YL)-1H-PYRAZOL-1-YL)-2-AZASPIRO(3.3)HEPT-2-YL)-; Q3W0H3V1LQ; SCHEMBL23533580
Click to Show/Hide
|
|||
| External Link | ||||
| TRS003 | Phase 3 | [70] | ||
| External Link | ||||
| Tusamitamab ravtansine | Phase 3 | [71] | ||
| Synonyms |
SAR408701
Click to Show/Hide
|
|||
| External Link | ||||
| RG6058 | Phase 3 | [72] | ||
| Synonyms |
Tiragolumab
Click to Show/Hide
|
|||
| External Link | ||||
| GSK4069889 | Phase 2 | [73] | ||
| Synonyms |
TSR-022
Click to Show/Hide
|
|||
| External Link | ||||
| APL-101 | Phase 2 | [74] | ||
| Synonyms |
Bozitinib; PLB-1001; 1440964-89-5; Vebreltinib; Vebreltinib [USAN]; UNII-2WZP8A9VFN; 2WZP8A9VFN; Bozitinib (PLB-1001); SCHEMBL15594471; BDBM107096; CBI-3103; s6762; WHO 11677; HY-125017; CS-0088607; US9695175, 44; 1,2,4-Triazolo(4,3-b)pyridazine, 6-(1-cyclopropyl-1H-pyrazol-4-yl)-3-(difluoro(6- fluoro-2-methyl-2H-indazol-5-yl)methyl)-; 6-(1-Cyclopropyl-1H-pyrazol-4-yl)-3-(difluoro(6-fluoro-2-methyl-2H-indazol-5- yl)methyl)-1,2,4-triazolo(4,3-b)pyridazine
Click to Show/Hide
|
|||
| External Link | ||||
| SGN-LIV1A | Phase 2 | [75] | ||
| Synonyms |
Ladiratuzumab Vedotin
Click to Show/Hide
|
|||
| External Link | ||||
| BNT116 | Phase 2 | [76] | ||
| External Link | ||||
| AZD7789 | Phase 2 | [77] | ||
| External Link | ||||
| L-DOS47 | Phase 1/2 | [78] | ||
| External Link | ||||
| NC318 | Phase 2 | [79] | ||
| External Link | ||||
| Vorolanib | Phase 2 | [80] | ||
| Synonyms |
UNII-YP8G3I74EL; YP8G3I74EL; 1013920-15-4; (S,Z)-N-(1-(Dimethylcarbamoyl)pyrrolidin-3-yl)-5-((5-fluoro-2-oxoindolin-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide; Vorolanib [INN]; SCHEMBL2439528; CHEMBL4297587; N-((3S)-1-(dimethylcarbamoyl)pyrrolidin-3-yl)-5-((Z)-(5-fluoro-2-oxo-1H-indol-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide; s6843; DB15247; HY-109019; CS-0030517; Q27294638; 1H-Pyrrole-3-carboxamide, N-((3S)-1-((dimethylamino)carbonyl)-3-pyrrolidinyl)-5-((Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidene)methyl)-2,4-dimethyl-
Click to Show/Hide
|
|||
| External Link | ||||
| Xofigo | Phase 2 | [81] | ||
| External Link | ||||
| Bintrafusp alfa | Phase 2 | [82] | ||
| External Link | ||||
| Voyager-V1 | Phase 2 | [83] | ||
| Synonyms |
VSV-IFNBeta-NIS
Click to Show/Hide
|
|||
| External Link | ||||
| AB-106 | Phase 2 | [84] | ||
| Synonyms |
DS6051b; GTPL11198; AB106
Click to Show/Hide
|
|||
| External Link | ||||
| RO-5126766 | Phase 2 | [85] | ||
| Synonyms |
VS-6766; CH-5126766; Dual Raf/MEK protein kinase inhibitor (cancer), Roche
Click to Show/Hide
|
|||
| External Link | ||||
| TC-210 | Phase 1/2 | [86] | ||
| External Link | ||||
| EMB-01 | Phase 1/2 | [87] | ||
| External Link | ||||
| NM21-1480 | Phase 1/2 | [88] | ||
| External Link | ||||
| MRx0518 | Phase 1/2 | [89] | ||
| External Link | ||||
| DZD9008 | Phase 1/2 | [90] | ||
| External Link | ||||
| BGB-A425 | Phase 1/2 | [91] | ||
| External Link | ||||
| Rilvegostomig | Phase 1/2 | [92] | ||
| Synonyms |
AZD2936
Click to Show/Hide
|
|||
| External Link | ||||
| IK-007 | Phase 1/2 | [93] | ||
| Synonyms |
grapiprant
Click to Show/Hide
|
|||
| External Link | ||||
| IBI318 | Phase 1 | [94] | ||
| External Link | ||||
| GEN-011 | Phase 1 | [95] | ||
| External Link | ||||
| ENV-105 | Phase 1 | [96] | ||
| Synonyms |
Carotuximab
Click to Show/Hide
|
|||
| External Link | ||||
| AMG 160 | Phase 1 | [97] | ||
| External Link | ||||
| ADP-A2M10 | Phase 1 | [98] | ||
| External Link | ||||
| MEDI5752 | Phase 1 | [99] | ||
| External Link | ||||
| PF-07104091 | Phase 1 | [100] | ||
| External Link | ||||
| PF-06936308 | Phase 1 | [101] | ||
| External Link | ||||
| GEM3PSCA | Phase 1 | [102] | ||
| External Link | ||||
| PF-07257876 | Phase 1 | [103] | ||
| External Link | ||||
| Cosibelimab | Phase 1 | [104] | ||
| Synonyms |
CK-301/TG-1501
Click to Show/Hide
|
|||
| External Link | ||||
| Gemcitabine | Approved | [7] | ||
| External Link | ||||
| SMI-4a | Investigative | [105] | ||
| Synonyms |
438190-29-5; SMI 4a; TCS PIM-1 4a; (Z)-SMI-4a; (Z)-5-(3-(trifluoromethyl)benzylidene)thiazolidine-2,4-dione; (5Z)-5-[3-(trifluoromethyl)benzylidene]-1,3-thiazolidine-2,4-dione; CHEMBL183906; (5Z)-5-[[3-(TRIFLUOROMETHYL)PHENYL]METHYLENE]-2,4-THIAZOLIDINEDIONE; (5Z)-5-[[3-(trifluoromethyl)phenyl]methylidene]-1,3-thiazolidine-2,4-dione; 327033-36-3; C11H6F3NO2S; (Z)-5-(3-(Trifluoromethyl)benzylidene)-thiazolidine-2,4-dione; (5Z)-5-{[3-(trifluoromethyl)phenyl]methylidene}-1,3-thiazolidine-2,4-dione; Pim inhibitor 4a; 3vc4; SMI-4q; TCS PIM-1-4a; 5-(3-(Trifluoromethyl)benzylidene)thiazolidine-2,4-dione; 5-[3-(Trifluoromethyl)benzylidene]thiazolidine-2,4-dione; cc-717; thiazolidine-2,4-dione, 4a; SCHEMBL2541382; SCHEMBL2541388; BDBM26626; AOB6260; EX-A111; SYN1113; BDBM138364; HMS3229J21; 2720AH; HY-16576A; MFCD01152003; s8005; ZINC12576047; AKOS001314163; SMI-4a, >=98% (HPLC); CCG-265027; NCGC00345836-02; NCGC00345836-14; AC-32861; HY-15474; AB0165836; EC-000.2291; J3.561.866J; A11945; W-5256; US8877795, 12; Q27451064; 5-[[3-(trifluoromethyl)phenyl]methylene]-2,4-thiazolidinedione
Click to Show/Hide
|
|||
| External Link | ||||
References