m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03384
|
[1], [2] | |||
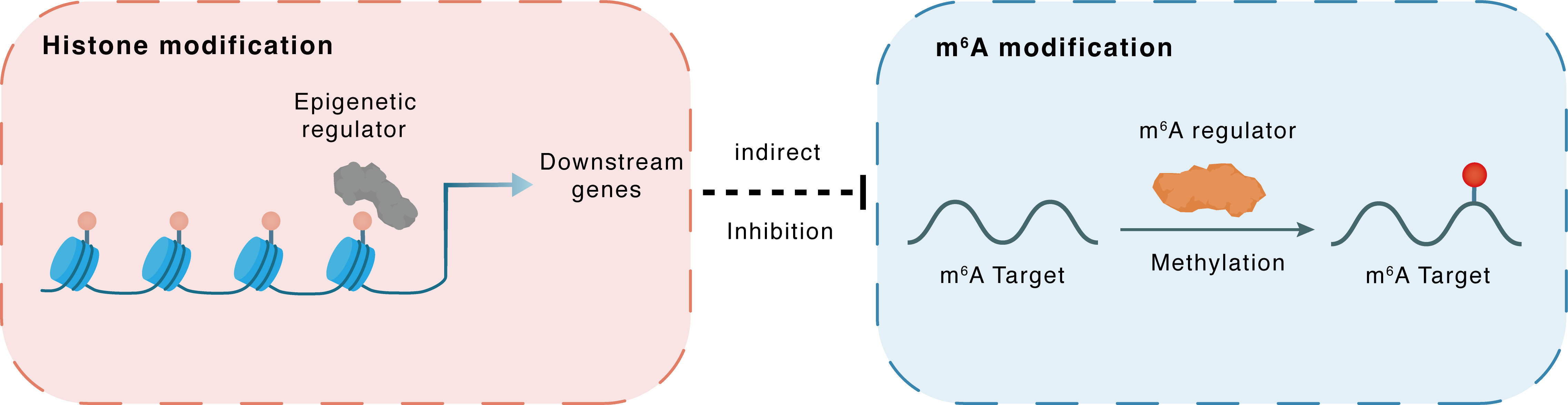 Histone modification
H3K27ac
Epigenetic Regulator
MIR570HG
Indirect
Inhibition
m6A modification
PTEN
PTEN
METTL3
Methylation
Histone modification
H3K27ac
Epigenetic Regulator
MIR570HG
Indirect
Inhibition
m6A modification
PTEN
PTEN
METTL3
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Mutated in multiple advanced cancers 1 (PTEN) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Regulated Target | Histone H3 lysine 27 acetylation (H3K27ac) | View Details | |||
| Downstream Gene | MIR570HG | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Inhibition | |||
| Crosstalk Mechanism | Histone modification indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | Gain of H3K4me1 and Histone H3 lysine 27 acetylation (H3K27ac) led to the activation of MIR570HG expression. LINC00969 interacts with EZH2 and METTL3, transcriptionally regulates the level of H3K27me3 in the NLRP3 promoter region, and posttranscriptionally modifies the m6A level of NLRP3 in an m6A-YTHDF2-dependent manner Bete-elemene exerted the restrictive impacts on the cell growth of lung cancer in vivo and in vitro through targeting METTL3. Bete-elemene contributed to the augmented Mutated in multiple advanced cancers 1 (PTEN) expression via suppressing its m6A modification. | ||||
| Responsed Disease | Lung cancer | ICD-11: 2C25 | |||
| Responsed Drug | Gefitinib | ||||
| Cell Process | Pyroptosis | ||||
In-vitro Model |
A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| In-vivo Model | Four-week-old BALB/c nude mice were randomly divided into three groups: (1) vector group, (2) vector + Bete-elemene group, and (3) Bete-elemene + METTL3 group. Nude mice were raised in an SPF level animal house and were free to eat and drink. Mice in the vector group were subcutaneously injected with lung cancer cells transfected with empty vector and did not receive Bete-elemene administration, and this group was implemented as the negative control. Following establishing orthotopic xenografts by using A549 or H1299 cells transfected with empty vector, mice in the vector + Bete-elemene group underwent intraperitoneal injection with Bete-elemene once a day. For the subcutaneous transplanted model, A549 or H1299 cells transfected with METTL3-overexpressing vector were inoculated into mice from the Bete-elemene + METTL3 group. Then, mice were intraperitoneally administrated with Bete-elemene once a day. Three weeks later, all the animals were euthanized with CO2. Xenografts were removed and weighted after mice were euthanatized. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| 2C25: Lung cancer | 52 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Adagrasib | Approved | [3] | ||
| Synonyms |
2326521-71-3; MRTX-849; UNII-8EOO6HQF8Y; 8EOO6HQF8Y; 2-((S)-4-(7-(8-Chloronaphthalen-1-yl)-2-(((S)-1-methylpyrrolidin-2-yl)methoxy)-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4-yl)-1-(2-fluoroacryloyl)piperazin-2-yl)acetonitrile; CHEMBL4594350; SCHEMBL20974691; GTPL10888; Kras G12C inhibitor MRTX849; BCP31538; EX-A3258; MRTX 849; MFCD32263433; s8884; compound 20 [PMID: 32250617]; BS-16211; HY-130149; CS-0105265; 2-Piperazineacetonitrile, 4-(7-(8-chloro-1-naphthalenyl)-5,6,7,8-tetrahydro-2-(((2S)-1-methyl-2-pyrrolidinyl)methoxy)pyrido(3,4-d)pyrimidin-4-yl)-1-(2-fluoro-1-oxo-2-propen-1-yl)-, (2S)-
Click to Show/Hide
|
|||
| External Link | ||||
| Amivantamab | Approved | [4] | ||
| External Link | ||||
| Mobocertinib | Approved | [5] | ||
| Synonyms |
1847461-43-1; TAK-788; TAK788; AP32788; UNII-39HBQ4A67L; 39HBQ4A67L; propan-2-yl 2-[4-{[2-(dimethylamino)ethyl](methyl)amino}-2-methoxy-5-(prop-2-enamido)anilino]-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Mobocertinib [INN]; Mobocertinib [USAN]; AP-32788; Mobocertinib (TAK788); Mobocertinib(TAK-788); SCHEMBL17373133; GTPL10468; BDBM368374; BCP31045; EX-A3392; US10227342, Example 10; MFCD32669806; NSC825519; s6813; TAK-788;AP32788; WHO 11183; NSC-825519; example 94 [WO2015195228A1]; HY-135815; CS-0114256; TAK-788;TAK 788; AP 32788; 5-Pyrimidinecarboxylic acid, 2-((4-((2-(dimethylamino)ethyl)methylamino)-2-methoxy-5-((1-oxo-2-propen-1-yl)amino)phenyl)amino)-4-(1-methyl-1H-indol-3-yl)-, 1-methylethyl ester; C(C=C)(=O)NC=1C(=CC(=C(C=1)NC1=NC=C(C(=N1)C1=CN(C2=CC=CC=C12)C)C(=O)OC(C)C)OC)N(C)CCN(C)C; Isopropyl 2-((5-acrylamido-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenyl)amino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Isopropyl 2-(5-acrylamido-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenylamino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Propan-2-yl 2-(5-(acryloylamino)-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyanilino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; propan-2-yl 2-[4-[2-(dimethylamino)ethyl-methylamino]-2-methoxy-5-(prop-2-enoylamino)anilino]-4-(1-methylindol-3-yl)pyrimidine-5-carboxylate
Click to Show/Hide
|
|||
| External Link | ||||
| Sugemalimab | Approved in China | [6] | ||
| External Link | ||||
| Sacituzumab govitecan | Approved | [7] | ||
| Synonyms |
1491917-83-9; 1535963-91-7; 1796566-95-4; CYSTEINYL CL2A-SN-38; DA64T2C2IO; DTXSID401335985; EX-A4354; F82944; GOVITECAN CYSTEINYL CONJUGATE; hRS 7SN38; hRS7-SN38; IMMU 132; IMMU-132; M9BYU8XDQ6; Sacituzumab govitecan; Sacituzumab govitecan [USAN]; sacituzumab-govitecan; Satralizumab linker; SN-38 CYSTEINYL CONJUGATE; UNII-M9BYU8XDQ6
Click to Show/Hide
|
|||
| External Link | ||||
| Atezolizumab | Approved | [8] | ||
| External Link | ||||
| Tepotinib | Approved | [9] | ||
| Synonyms |
1100598-32-0; EMD 1214063; UNII-1IJV77EI07; Tepotinib (EMD 1214063); EMD1214063; 1IJV77EI07; MSC-2156119J; Benzonitrile, 3-[1,6-dihydro-1-[[3-[5-[(1-methyl-4-piperidinyl)methoxy]-2-pyrimidinyl]phenyl]methyl]-6-oxo-3-pyridazinyl]-; C29H28N6O2; Tepotinib [INN]; Benzonitrile, 3-[1,6-Dihydro-1-[[3-[5-[(1-Methyl-4-Piperidinyl)Methoxy]-2-PyriMidinyl]Phenyl]Methyl]-6-Oxo-3-Pyridazinyl]; Benzonitrile, 3-(1,6-dihydro-1-((3-(5-((1-methyl-4-piperidinyl)methoxy)-2-pyrimidinyl)phenyl)methyl)-6-oxo
Click to Show/Hide
|
|||
| External Link | ||||
| Sotorasib | Approved | [10] | ||
| Synonyms |
AMG-510; AMG510; AMG-510 racemate; 2252403-56-6; AMG 510; Kras G12C inhibitor 9; 2296729-00-3; UNII-2B2VM6UC8G; 2B2VM6UC8G; CHEMBL4535757; 2296729-00-3 (racemate); 4-((S)-4-Acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(2-isopropyl-4-methylpyridin-3-yl)pyrido[2,3-d]pyrimidin-2(1H)-one; 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-[(2S)-2-methyl-4-prop-2-enoylpiperazin-1-yl]pyrido[2,3-d]pyrimidin-2-one; Sotorasib [INN]; 6-Fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-((2S)-2-methyl-4-prop-2-enoylpiperazin-1-yl)pyrido(2,3-d)pyrimidin-2-one; AMG510 racemate; Sotorasib [USAN]; AMG-510(racemate); Kras mutant-targeting AMG 510; SCHEMBL20560375; GTPL10678; AMG 510 pound>>AMG-510; AMY16918; BCP30452; BCP33368; EX-A3538; BDBM50514402; NSC818433; s8830; WHO 11370; DB15569; NSC-818433; BS-16684; HY-114277; CS-0081316; compound (R)-38 [PMID: 31820981]; (1m)-6-Fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-(propan-2-yl)pyridin-3-yl)-4-((2S)-2-methyl-4-(prop-2-enoyl)piperazin-1-yl)pyrido(2,3-d)pyrimidin-2(1H)-one; (1S)-4-((S)-4-Acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(2-isopropyl-4-methylpyridin-3-yl)pyrido[2,3-d]pyrimidin-2(1H)-one; 2296729-66-1; Pyrido(2,3-d)pyrimidin-2(1H)-one, 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-(1-methylethyl)-3-pyridinyl)-4-((2S)-2-methyl-4-(1-oxo-2-propen-1-yl)-1-piperazinyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| Entrectinib | Approved | [11] | ||
| Synonyms |
1108743-60-7; RXDX-101; UNII-L5ORF0AN1I; Entrectinib (RXDX-101); L5ORF0AN1I; Benzamide, N-[5-[(3,5-difluorophenyl)methyl]-1H-indazol-3-yl]-4-(4-methyl-1-piperazinyl)-2-[(tetrahydro-2H-pyran-4-yl)amino]-; Benzamide, N-(5-((3,5-difluorophenyl)methyl)-1H-indazol-3-yl)-4-(4-methyl-1-piperazinyl)-2-((tetrahydro-2H-pyran-4-yl)amino)-; Entrectinib [USAN:INN]; YMX; Kinome_2659; Entrectinib(rxdx-101); Entrectinib (USAN/INN); SCHEMBL3512601; GTPL8290; CHEMBL1983268; KS-00000TSK
Click to Show/Hide
|
|||
| External Link | ||||
| Repotrectinib | Approved | [12] | ||
| Synonyms |
FIKPXCOQUIZNHB-RRKGBCIJSA-N; SCHEMBL20438940; TPX 0005; BCP19778
Click to Show/Hide
|
|||
| External Link | ||||
| MYL-1402O | Phase 3 | [13] | ||
| Synonyms |
bevacizumab biosimilar
Click to Show/Hide
|
|||
| External Link | ||||
| AB154 | Phase 3 | [14] | ||
| Synonyms |
Domvanalimab
Click to Show/Hide
|
|||
| External Link | ||||
| Datopotamab deruxtecan | Phase 3 | [15] | ||
| External Link | ||||
| CS1001 | Phase 3 | [16] | ||
| External Link | ||||
| JDQ443 | Phase 3 | [17] | ||
| Synonyms |
(S)-JDQ-443; 1-(6-((4S)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl)-2-azaspiro[3.3]heptan-2-yl)prop-2-en-1-one; 1-[6-[(4R)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-inda zol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]- 2-Propen-1-one; 1-[6-[(4R)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]-2-propen-1-one; 1-[6-[4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methylindazol-5-yl)pyrazol-1-yl]-2-azaspiro[3.3]heptan-2-yl]prop-2-en-1-one; 1-{6-[(4M)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5- methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2- azaspiro[3.3]heptan-2-yl}prop-2-en-1-one; 1-{6-[(4M)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]heptan-2-yl}prop-2-en-1-one; 2653994-08-0; 2653994-10-4; 2-Propen-1-one, 1-[6-[(4R)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]-; AKOS040757949; AT36708; BDBM50579985; CHEMBL5077861; compound 5 [PMID: 35404998]; CS-0226220; CS-0311034; EX-A5693; example 1a [WO2021120890A1]; GLXC-25533; GTPL11715; HY-139612A; JDQ 443; JDQ 443 [WHO-DD]; JDQ443; JDQ-443; MS-29737; NSC846146; NSC-846146; NVP-JDQ443; NVP-JDQ-443; Opnurasib; opnurasib [INN]; -PROPEN-1-ONE, 1-(6-((4R)-4-(5-CHLORO-6-METHYL-1H-INDAZOL-4-YL)-5-METHYL-3-(1-METHYL-1H-INDAZOL-5-YL)-1H-PYRAZOL-1-YL)-2-AZASPIRO(3.3)HEPT-2-YL)-; Q3W0H3V1LQ; SCHEMBL23533580
Click to Show/Hide
|
|||
| External Link | ||||
| TRS003 | Phase 3 | [18] | ||
| External Link | ||||
| Tusamitamab ravtansine | Phase 3 | [19] | ||
| Synonyms |
SAR408701
Click to Show/Hide
|
|||
| External Link | ||||
| RG6058 | Phase 3 | [20] | ||
| Synonyms |
Tiragolumab
Click to Show/Hide
|
|||
| External Link | ||||
| GSK4069889 | Phase 2 | [21] | ||
| Synonyms |
TSR-022
Click to Show/Hide
|
|||
| External Link | ||||
| APL-101 | Phase 2 | [22] | ||
| Synonyms |
Bozitinib; PLB-1001; 1440964-89-5; Vebreltinib; Vebreltinib [USAN]; UNII-2WZP8A9VFN; 2WZP8A9VFN; Bozitinib (PLB-1001); SCHEMBL15594471; BDBM107096; CBI-3103; s6762; WHO 11677; HY-125017; CS-0088607; US9695175, 44; 1,2,4-Triazolo(4,3-b)pyridazine, 6-(1-cyclopropyl-1H-pyrazol-4-yl)-3-(difluoro(6- fluoro-2-methyl-2H-indazol-5-yl)methyl)-; 6-(1-Cyclopropyl-1H-pyrazol-4-yl)-3-(difluoro(6-fluoro-2-methyl-2H-indazol-5- yl)methyl)-1,2,4-triazolo(4,3-b)pyridazine
Click to Show/Hide
|
|||
| External Link | ||||
| SGN-LIV1A | Phase 2 | [23] | ||
| Synonyms |
Ladiratuzumab Vedotin
Click to Show/Hide
|
|||
| External Link | ||||
| BNT116 | Phase 2 | [24] | ||
| External Link | ||||
| AZD7789 | Phase 2 | [25] | ||
| External Link | ||||
| L-DOS47 | Phase 1/2 | [26] | ||
| External Link | ||||
| NC318 | Phase 2 | [27] | ||
| External Link | ||||
| Vorolanib | Phase 2 | [28] | ||
| Synonyms |
UNII-YP8G3I74EL; YP8G3I74EL; 1013920-15-4; (S,Z)-N-(1-(Dimethylcarbamoyl)pyrrolidin-3-yl)-5-((5-fluoro-2-oxoindolin-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide; Vorolanib [INN]; SCHEMBL2439528; CHEMBL4297587; N-((3S)-1-(dimethylcarbamoyl)pyrrolidin-3-yl)-5-((Z)-(5-fluoro-2-oxo-1H-indol-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide; s6843; DB15247; HY-109019; CS-0030517; Q27294638; 1H-Pyrrole-3-carboxamide, N-((3S)-1-((dimethylamino)carbonyl)-3-pyrrolidinyl)-5-((Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidene)methyl)-2,4-dimethyl-
Click to Show/Hide
|
|||
| External Link | ||||
| Xofigo | Phase 2 | [29] | ||
| External Link | ||||
| Bintrafusp alfa | Phase 2 | [30] | ||
| External Link | ||||
| Voyager-V1 | Phase 2 | [31] | ||
| Synonyms |
VSV-IFNBeta-NIS
Click to Show/Hide
|
|||
| External Link | ||||
| AB-106 | Phase 2 | [32] | ||
| Synonyms |
DS6051b; GTPL11198; AB106
Click to Show/Hide
|
|||
| External Link | ||||
| RO-5126766 | Phase 2 | [33] | ||
| Synonyms |
VS-6766; CH-5126766; Dual Raf/MEK protein kinase inhibitor (cancer), Roche
Click to Show/Hide
|
|||
| External Link | ||||
| TC-210 | Phase 1/2 | [34] | ||
| External Link | ||||
| EMB-01 | Phase 1/2 | [35] | ||
| External Link | ||||
| NM21-1480 | Phase 1/2 | [36] | ||
| External Link | ||||
| MRx0518 | Phase 1/2 | [37] | ||
| External Link | ||||
| DZD9008 | Phase 1/2 | [38] | ||
| External Link | ||||
| BGB-A425 | Phase 1/2 | [39] | ||
| External Link | ||||
| Rilvegostomig | Phase 1/2 | [40] | ||
| Synonyms |
AZD2936
Click to Show/Hide
|
|||
| External Link | ||||
| IK-007 | Phase 1/2 | [41] | ||
| Synonyms |
grapiprant
Click to Show/Hide
|
|||
| External Link | ||||
| IBI318 | Phase 1 | [42] | ||
| External Link | ||||
| GEN-011 | Phase 1 | [43] | ||
| External Link | ||||
| ENV-105 | Phase 1 | [44] | ||
| Synonyms |
Carotuximab
Click to Show/Hide
|
|||
| External Link | ||||
| AMG 160 | Phase 1 | [45] | ||
| External Link | ||||
| ADP-A2M10 | Phase 1 | [46] | ||
| External Link | ||||
| MEDI5752 | Phase 1 | [47] | ||
| External Link | ||||
| PF-07104091 | Phase 1 | [48] | ||
| External Link | ||||
| PF-06936308 | Phase 1 | [49] | ||
| External Link | ||||
| GEM3PSCA | Phase 1 | [50] | ||
| External Link | ||||
| PF-07257876 | Phase 1 | [51] | ||
| External Link | ||||
| Cosibelimab | Phase 1 | [52] | ||
| Synonyms |
CK-301/TG-1501
Click to Show/Hide
|
|||
| External Link | ||||
| Gemcitabine | Approved | [53] | ||
| External Link | ||||
| SMI-4a | Investigative | [54] | ||
| Synonyms |
438190-29-5; SMI 4a; TCS PIM-1 4a; (Z)-SMI-4a; (Z)-5-(3-(trifluoromethyl)benzylidene)thiazolidine-2,4-dione; (5Z)-5-[3-(trifluoromethyl)benzylidene]-1,3-thiazolidine-2,4-dione; CHEMBL183906; (5Z)-5-[[3-(TRIFLUOROMETHYL)PHENYL]METHYLENE]-2,4-THIAZOLIDINEDIONE; (5Z)-5-[[3-(trifluoromethyl)phenyl]methylidene]-1,3-thiazolidine-2,4-dione; 327033-36-3; C11H6F3NO2S; (Z)-5-(3-(Trifluoromethyl)benzylidene)-thiazolidine-2,4-dione; (5Z)-5-{[3-(trifluoromethyl)phenyl]methylidene}-1,3-thiazolidine-2,4-dione; Pim inhibitor 4a; 3vc4; SMI-4q; TCS PIM-1-4a; 5-(3-(Trifluoromethyl)benzylidene)thiazolidine-2,4-dione; 5-[3-(Trifluoromethyl)benzylidene]thiazolidine-2,4-dione; cc-717; thiazolidine-2,4-dione, 4a; SCHEMBL2541382; SCHEMBL2541388; BDBM26626; AOB6260; EX-A111; SYN1113; BDBM138364; HMS3229J21; 2720AH; HY-16576A; MFCD01152003; s8005; ZINC12576047; AKOS001314163; SMI-4a, >=98% (HPLC); CCG-265027; NCGC00345836-02; NCGC00345836-14; AC-32861; HY-15474; AB0165836; EC-000.2291; J3.561.866J; A11945; W-5256; US8877795, 12; Q27451064; 5-[[3-(trifluoromethyl)phenyl]methylene]-2,4-thiazolidinedione
Click to Show/Hide
|
|||
| External Link | ||||
References