m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03366
|
[1], [2] | |||
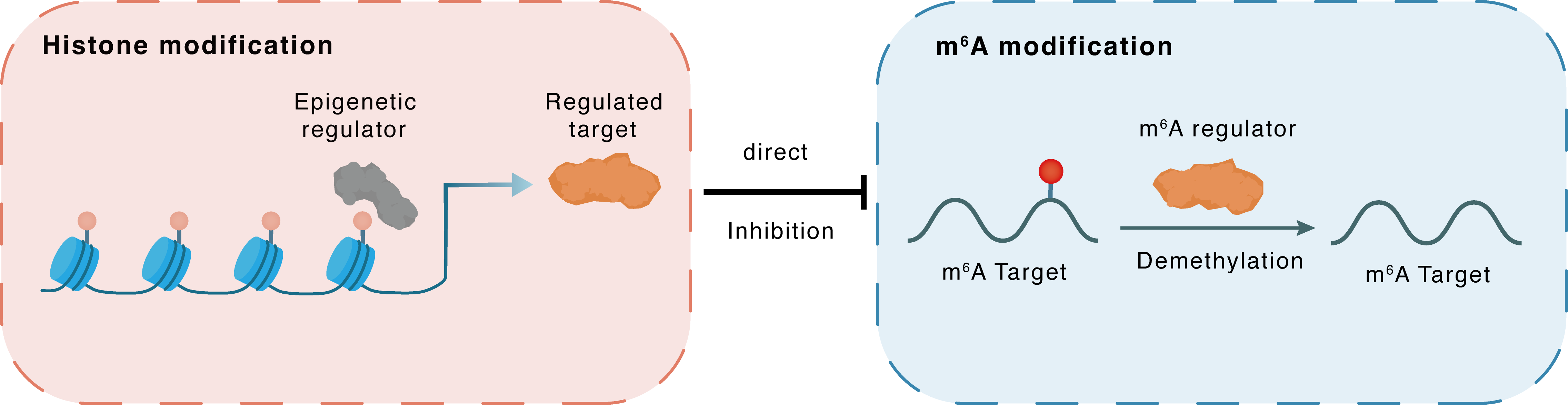 Histone modification
H3K27ac
HDAC2
ALKBH5
Direct
Inhibition
m6A modification
TP53
TP53
ALKBH5
Demethylation
Histone modification
H3K27ac
HDAC2
ALKBH5
Direct
Inhibition
m6A modification
TP53
TP53
ALKBH5
Demethylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | RNA demethylase ALKBH5 (ALKBH5) | ERASER | |||
| m6A Target | Cellular tumor antigen p53 (TP53/p53) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone deacetylase 2 (HDAC2) | ERASER | View Details | ||
| Regulated Target | Histone H3 lysine 27 acetylation (H3K27ac) | View Details | |||
| Downstream Gene | ALKBH5 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Inhibition | |||
| Crosstalk Mechanism | histone modification directly impacts m6A modification through modulating the level of m6A regulator | ||||
| Crosstalk Summary | Mechanically, HDAC2-reduced Histone H3 lysine 27 acetylation (H3K27ac) inhibits ALKBH5 transcription in CRC, whereas ectopic ALKBH5 expression decreases tumorigenesis of CRC cells and protects mice from colitis-associated tumor development.METTL14/ALKBH5/IGF2BPs combine to modulate JMJD8 stability in an m6A-dependent manner, which increases glycolysis and accelerates the development of CRC by enhancing the enzymatic activity of PKM2. The interplay between CARMN and ALKBH5 promoted tumourigenesis in colorectal cancer patients via the Cellular tumor antigen p53 (TP53/p53)/ALKBH5/CARMN/miR-5683 pathway. These findings illuminate the role of m6A methylation in colorectal cancer patients with p53R273H mutation. | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
In-vitro Model |
HIEC-6 | Normal | Homo sapiens | CVCL_6C21 | |
| FHC | Normal | Homo sapiens | CVCL_3688 | ||
| HCT 116 | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| SW480 | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| HT29 | Colon cancer | Mus musculus | CVCL_A8EZ | ||
| In-vivo Model | Colorectal cancer cells were seeded in culture plates for 24 h prior to cotransfection with GFP-CARMN, and a vector using Lipofectamine 2000. After 48 h, RNA immunoprecipitation was performed using/Colorectal cancer cells were plated in 24-well plates and incubated for 24 h before cotransfection with the luciferase reporter vector, and the Renilla vector. antibodies against FTO, METTL3 and ALKBH5 from the EZ-Magna RIP Kit (Millipore). | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Cellular tumor antigen p53 (TP53/p53) | 27 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Contusugene ladenovec | Phase 3 | [3] | ||
| Synonyms |
Advexin; Ad5CMV-p53; INGN-004; INGN-201; Ad-p53, Introgen; Gene therapy (p53/adenovirus), University of Texas; Gene therapy (p53/adenoviral), Introgen/Aventis; Gene therapy (p53/adenoviral), Introgen/RPR
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| QPI-1002 | Phase 3 | [4] | ||
| MOA | Modulator | |||
| External Link | ||||
| Thymoquinone | Phase 2/3 | [5] | ||
| Synonyms |
490-91-5; Thymoquinon; p-Cymene-2,5-dione; 2-Isopropyl-5-methyl-1,4-benzoquinone; 2,5-CYCLOHEXADIENE-1,4-DIONE, 2-METHYL-5-(1-METHYLETHYL)-; 2-Isopropyl-5-methyl-p-benzoquinone; 2-Isopropyl-5-methylbenzoquinone; Polythymoquinone; 5-Isopropyl-2-methyl-1,4-benzoquinone; 2-Isopropyl-5-methylbenzo-1,4-quinone; p-Mentha-3,6-diene-2,5-dione; NSC 2228; 2-Isopropyl-5-methylcyclohexa-2,5-diene-1,4-dione; 2-Methyl-5-isopropyl-p-benzoquinone; 2-methyl-5-propan-2-ylcyclohexa-2,5-diene-1,4-dione; NSC2228; 2-methyl-5-(propan-2-yl)cyclohexa-2,5-diene-1,4-dione; UNII-O60IE26NUF; 2-Methyl-5-isopropyl-1,4-benzoquinone; O60IE26NUF; 2,5-Cyclohexadiene-1,4-dione, 5-isopropyl-2-methyl-; NSC-2228; 5-Isopropyl-2-methyl-p-benzoquinone; MFCD00001602; 2-Methyl-5-(1-methylethyl)-2,5-cyclohexadiene-1,4-dione; p-Mentha-3,6-diene-2,5-dione (8CI); 5-Isopropyl-2-methyl-2,5-Cyclohexadiene-1,4-dione; CCRIS 7152; EINECS 207-721-1; 2-methyl-5-(methylethyl)cyclohexa-2,5-diene-1,4-dione; BRN 1939047; thymolquinone; Thymoil; AI3-17758; 4hco; p-Mentha-3,5-dione; Spectrum_001237; SpecPlus_000457; Thymoquinone, >=98%; Spectrum2_000700; Spectrum3_001345; Spectrum4_001895; Spectrum5_000550; BSPBio_003129; KBioGR_002455; KBioSS_001717; DivK1c_006553; SCHEMBL542535; SPBio_000859; CHEMBL1672002; DTXSID9060079; KBio1_001497; KBio2_001717; KBio2_004285; KBio2_006853; KBio3_002349; Thymoquinone, analytical standard; CHEBI:113532; 2-Methyl-5-iso-propylbenzoquinone; BDBM166686; ZINC164367; BCP16946; HY-D0803; WLN: L6V DVJ B1 EY1&1; 2,4-dione, 5-isopropyl-2-methyl-; ANW-41600; CCG-40027; s4761; SBB008296; AKOS003368628; MCULE-9899033250; NCGC00178250-01; NCGC00178250-05; 73940-92-8; AK101679; AS-11327; 2-Isopropyl-5-methylbenzo-1,4-quinone #; 2,4-dione, 2-methyl-5-(1-methylethyl)-; CS-0012226; FT-0612708; ST45023960; K-9199; SR-05000002192; Q7799650; SR-05000002192-2; W-202869; BRD-K97566842-001-03-5; 2-methyl-5-(propan-2-yl)cyclohexa-2,5-diene-1,4-dione (F8); 2-Methyl-5-(1-methylethyl)-2,5-cyclohexadiene-1,4-dione, 9CI
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SGT-53 | Phase 2 | [6] | ||
| Synonyms |
P53 gene stimulator (solid tumor), Synergene Therapeutics
Click to Show/Hide
|
|||
| MOA | Stimulator | |||
| External Link | ||||
| APR-246 | Phase 2 | [7] | ||
| Synonyms |
Eprenetapopt
Click to Show/Hide
|
|||
| MOA | Stimulator | |||
| External Link | ||||
| Ad-p53 | Phase 2 | [8] | ||
| Synonyms |
P53 gene therapy, Transgene/Schering-Plough; Ad-p53, Transgene/Schering-Plough
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Kevetrin | Phase 2 | [9] | ||
| MOA | Stimulator | |||
| External Link | ||||
| INGN-225 | Phase 2 | [10] | ||
| Synonyms |
Cancer vaccine (p53), Introgen
Click to Show/Hide
|
|||
| External Link | ||||
| ALT-801 | Phase 2 | [11] | ||
| Synonyms |
ALT-801 (donor lymphocyte infusion, cancer); ALT-801 (donor lymphocyte infusion, cancer), Altor; STAR IL-2 conjugate (donor lymphocyte infusion, cancer), Altor; STAR-Ck (donor lymphocyte infusion, cancer), Altor; Soluble T-cell Antigen Receptor IL-2 conjugate (donor lymphocyte infusion, cancer), Altor
Click to Show/Hide
|
|||
| MOA | Immunomodulator (Immunostimulant) | |||
| External Link | ||||
| APG-115 | Phase 2 | [9] | ||
| Synonyms |
15Qau0SI9J; UNII-15QAU0SI9J; 1818393-16-6; APG 115 [WHO-DD]; SCHEMBL17189805; Bicyclo(2.2.2)octane-1-carboxylic acid, 4-((((3'R,4'S,5'R)-6''-chloro-4'-(3-chloro-2-fluorophenyl)-1'-ethyl-1'',2''-dihydro-2''-oxodispiro(cyclohexane-1,2'-pyrrolidine-3',3''-(3H)indol)-5'-yl)carbonyl)amino)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ISA-P53-01 | Phase 1/2 | [12] | ||
| Synonyms |
P53-SLP; P53 vaccine (colorectal/ovarian cancer), ISA Pharmaceuticals; P53 vaccine (Montanide ISA-51 adjuvanted, colorectal/ovarian cancer), ISA Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| SAR-405838 | Phase 1 | [13] | ||
| Synonyms |
AT-219; MI-147; MI-219; MI-319; MI-43; MI-5; MI-63; MI-772; MI-773; MI-519-64; P53-HDM2 protein interaction inhibitors (cancer); P53-HDM2 protein interaction inhibitors (cancer), Ascenta/Sanofi; P53-HDM2 protein interaction inhibitors (cancer), Ascenta/sanofi-aventis
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Dendritic cell vaccine | Phase 1 | [14] | ||
| Synonyms |
Dendritic cell vaccine (injectable, head and neck cancer); Dendritic cell vaccine (injectable, head and neck cancer), National Cancer Institute; Mutant p53 peptide pulsed dendritic cell vaccine (injectable, head and neck cancer), National Cancer Institute
Click to Show/Hide
|
|||
| External Link | ||||
| ONYX-015 | Phase 1 | [15] | ||
| Synonyms |
Dl1520; E1B-deleted adenovirus (cancer), ONYX
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| COTI-2 | Phase 1 | [9] | ||
| Synonyms |
UNII-2BTA1O65BR; 2BTA1O65BR; 1039455-84-9; ZINC114475331; CS-8156; HY-19896; 1-Piperazinecarbothioic acid, 4-(2-pyridinyl)-, 2-(6,7-dihydro-8(5H)-quinolinylidene)hydrazide
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| CGM097 | Phase 1 | [4] | ||
| MOA | Modulator | |||
| External Link | ||||
| HDM201 | Phase 1 | [16] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| INGN-234 | Discontinued in Phase 2 | [17] | ||
| Synonyms |
P53 tumor suppressor (topical formulation), Introgen
Click to Show/Hide
|
|||
| MOA | Suppressor | |||
| External Link | ||||
| Pifithrin-alpha | Terminated | [18] | ||
| Synonyms |
P53 inhibitor, Univ of Illinois; PFT-alpha; PFT-beta; Pifithrin compounds, Quark; Pifithrin-beta
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| TAR-1 | Terminated | [19] | ||
| Synonyms |
P53 protein modulator (single-chain antibody fragment, cancer), Ramot/Champions
Click to Show/Hide
|
|||
| External Link | ||||
| 1-(9-ethyl-9H-carbazol-3-yl)-N-methylmethanamine | Investigative | [20] | ||
| Synonyms |
PhiKan 083; [(9-ethyl-9H-carbazol-3-yl)methyl](methyl)amine; EN300-43214; 880813-36-5; AC1NGDXR; PhiKan-083; BAS 13152361; PhiKan-083 Hydrochloride; CHEMBL1235116; SCHEMBL20181195; AC1Q3123; ZINC3888893; STK511393; IMED102848735; AKOS000284549; MCULE-1841863738; DB08363; NCGC00379107-01; NCGC00379107-02; ST072505; [(9-ethylcarbazol-3-yl)methyl]methylamine; HY-108637; CS-0029368; [(9-ethylcarbazol-3-yl)methyl](methyl)amine; 1-(9-ethylcarbazol-3-yl)-N-methylmethanamine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NUTLIN-3 | Investigative | [21] | ||
| Synonyms |
548472-68-0; 890090-75-2; nutlin-3A; nutlin 3; (+/-)-Nutlin3; CHEMBL211045; Nutlin 3(Random Configuration); MDM2 Antagonist, Nutlin-3, Racemic; 4-(4,5-bis(4-chlorophenyl)-2-(2-isopropoxy-4-methoxyphenyl)-4,5-dihydro-1H-imidazole-1-carbonyl)piperazin-2-one; 4-({4,5-bis(4-chlorophenyl)-2-[4-methoxy-2-(propan-2-yloxy)phenyl]-4,5-dihydro-1H-imidazol-1-yl}carbonyl)piperazin-2-one; 4-[4,5-bis(4-chlorophenyl)-2-(4-methoxy-2-propan-2-yloxyphenyl)-4,5-dihydroimidazole-1-carbonyl]piperazin-2-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NU-8231 | Investigative | [22] | ||
| Synonyms |
SCHEMBL2454464; CHEMBL360944
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| OPI-1002 | Phase 2 | [23] | ||
| External Link | ||||
| Cenersen | Phase 2 | [23] | ||
| External Link | ||||
| AHL | Investigative | [23] | ||
| Synonyms |
AHLi-11; SiRNA therapeutics (hearing loss), Quark; P53 gene-silencing siRNA (hearing loss), Quark
Click to Show/Hide
|
|||
| External Link | ||||
| PC14586 | Phase 1/2 | [23] | ||
| External Link | ||||
| Histone deacetylase 2 (HDAC2) | 113 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| CHR-3996 | Phase 1/2 | [24] | ||
| Synonyms |
CCT-075453; CHR-2504; HDAC inhibitors, Chroma Therapeutics; Histone deacetylase inhibitors, Chroma
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4 nM | |||
| External Link | ||||
| PMID29671355-Compound-74 | Patented | [25] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 119 nM | |||
| External Link | ||||
| PMID29671355-Compound-59 | Patented | [25] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| PMID29671355-Compound-55 | Patented | [25] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID29671355-Compound-11 | Patented | [25] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 24 nM | |||
| External Link | ||||
| PMID29671355-Compound-9 | Patented | [25] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 128 nM | |||
| External Link | ||||
| PMID29671355-Compound-8 | Patented | [25] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 123000 nM | |||
| External Link | ||||
| PMID29671355-Compound-61 | Patented | [25] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 45.9 nM | |||
| External Link | ||||
| PMID29671355-Compound-23 | Patented | [25] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 7450 nM | |||
| External Link | ||||
| PMID29671355-Compound-44 | Patented | [25] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 150 nM | |||
| External Link | ||||
| PMID29671355-Compound-56 | Patented | [25] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 54.4 nM | |||
| External Link | ||||
| PMID29671355-Compound-67 | Patented | [25] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 50000 nM | |||
| External Link | ||||
| PMID29671355-Compound-31 | Patented | [25] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2 nM | |||
| External Link | ||||
| PMID29671355-Compound-21 | Patented | [25] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 4880 nM | |||
| External Link | ||||
| PMID29671355-Compound-62 | Patented | [25] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 500 nM | |||
| External Link | ||||
| PMID29671355-Compound-43 | Patented | [25] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 124 nM | |||
| External Link | ||||
| PMID29671355-Compound-25 | Patented | [25] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5400 nM | |||
| External Link | ||||
| N8-hydroxy-2-methoxy-N1-phenyloctanediamide | Investigative | [26] | ||
| Synonyms |
CHEMBL251010; SCHEMBL8158442
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| N-(4'-acetyl-4-aminobiphenyl-3-yl)benzamide | Investigative | [27] | ||
| Synonyms |
CHEMBL1099078; BDBM50317997
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3800 nM | |||
| External Link | ||||
| N-Hydroxy-E-3-(4'-chlorobiphenyl-4-yl)-acrylamide | Investigative | [28] | ||
| Synonyms |
CHEMBL557066; SCHEMBL3292989; SCHEMBL3292983; BDBM50293365
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1860 nM | |||
| External Link | ||||
| N7-hydroxy-2-methoxy-N1-phenylheptanediamide | Investigative | [26] | ||
| Synonyms |
CHEMBL251206; SCHEMBL8143763
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| N7-hydroxy-N1-phenyl-2-propoxyheptanediamide | Investigative | [26] | ||
| Synonyms |
CHEMBL251406; SCHEMBL8144856
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| N-(4-amino-4'-vinylbiphenyl-3-yl)benzamide | Investigative | [27] | ||
| Synonyms |
CHEMBL1096397; BDBM50318002
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 800 nM | |||
| External Link | ||||
| N-(4-amino-3'-methoxybiphenyl-3-yl)benzamide | Investigative | [27] | ||
| Synonyms |
CHEMBL1097747; BDBM50317991
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1300 nM | |||
| External Link | ||||
| KAR-1880 | Investigative | [29] | ||
| Synonyms |
Anti-inflammatory OS-HDI; OS-HDI-2; HDAC 2 inhibitors (topical, dermatitis/psoriasis), Karus
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(2-amino-5-(pyridin-4-yl)phenyl)benzamide | Investigative | [27] | ||
| Synonyms |
CHEMBL1095096
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3900 nM | |||
| External Link | ||||
| N-(4-amino-3'-methylbiphenyl-3-yl)benzamide | Investigative | [27] | ||
| Synonyms |
CHEMBL1094108; BDBM50317995
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3100 nM | |||
| External Link | ||||
| N-(3'-acetyl-4-aminobiphenyl-3-yl)benzamide | Investigative | [27] | ||
| Synonyms |
CHEMBL1097746; BDBM50317992
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 13000 nM | |||
| External Link | ||||
| N-(4-amino-4'-fluorobiphenyl-3-yl)benzamide | Investigative | [27] | ||
| Synonyms |
CHEMBL1098337; SCHEMBL15398027; BDBM50317988
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1400 nM | |||
| External Link | ||||
| N8,2-dihydroxy-N1-phenyloctanediamide | Investigative | [26] | ||
| Synonyms |
CHEMBL251009; SCHEMBL8144564
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| N-(2-amino-5-(furan-3-yl)phenyl)benzamide | Investigative | [27] | ||
| Synonyms |
CHEMBL1095095; Benzamide, N-[2-amino-5-(3-furanyl)phenyl]-; BDBM50318000
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 39 nM | |||
| External Link | ||||
| N-Hydroxy-E-3-(biphenyl-4-yl)-acrylamide | Investigative | [28] | ||
| Synonyms |
CHEMBL556532; SCHEMBL3292226; SCHEMBL3290139
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 820 nM | |||
| External Link | ||||
| 2-(allyloxy)-N8-hydroxy-N1-phenyloctanediamide | Investigative | [26] | ||
| Synonyms |
CHEMBL402719; SCHEMBL8150833
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| 2-(benzyloxy)-N7-hydroxy-N1-phenylheptanediamide | Investigative | [26] | ||
| Synonyms |
CHEMBL402718; SCHEMBL8152458
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| N-(4-amino-4'-bromobiphenyl-3-yl)benzamide | Investigative | [27] | ||
| Synonyms |
CHEMBL1097748; BDBM50317990
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 600 nM | |||
| External Link | ||||
| N-(2-amino-5-(furan-2-yl)phenyl)benzamide | Investigative | [27] | ||
| Synonyms |
CHEMBL1097651
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 43 nM | |||
| External Link | ||||
| 5-(Biphenyl-4-yl)-pentanoic acid N-hydroxyamide | Investigative | [28] | ||
| Synonyms |
CHEMBL541239; SCHEMBL7045815
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 720 nM | |||
| External Link | ||||
| N-(4-aminobiphenyl-3-yl)benzamide | Investigative | [27] | ||
| Synonyms |
CHEMBL271741; 3max; SCHEMBL16380794; BDBM50232053; N-(4-amino-biphenyl-3-yl)-benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 27 nM | |||
| External Link | ||||
| N-(2-amino-5-(benzofuran-2-yl)phenyl)benzamide | Investigative | [27] | ||
| Synonyms |
CHEMBL1097063
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10000 nM | |||
| External Link | ||||
| N-(2-aminophenyl)benzamide | Investigative | [27] | ||
| Synonyms |
2'-AMINOBENZANILIDE; 721-47-1; CHEMBL405072; AC1LFSYX; AC1Q5NOK; ACMC-1AE0C; Oprea1_478192; MLS000084661; SCHEMBL407834; N-(2-amino-phenyl)-benzamide; AC1Q514U; Benzamide, N-(2-aminophenyl)-; CTK2H2825; DTXSID60353948; RFDVMOUXHKTCDO-UHFFFAOYSA-N; MolPort-001-783-352; ZINC225957; HMS2355D18; STL497474; BDBM50232046; AKOS000133162; MCULE-5545982713; NE17312; SMR000019009; TC-170926; KB-298435; ST51030142; EN300-31745; SR-01000389415; AE-641/00785046
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3100 nM | |||
| External Link | ||||
| N-(4-amino-4'-methoxybiphenyl-3-yl)benzamide | Investigative | [27] | ||
| Synonyms |
CHEMBL1095412; BDBM50317998
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 140 nM | |||
| External Link | ||||
| N-Hydroxy-E-3-(4'-cyanobiphenyl-4-yl)-acrylamide | Investigative | [28] | ||
| Synonyms |
CHEMBL538710; SCHEMBL3292721; SCHEMBL3292715; BDBM50293355
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 330 nM | |||
| External Link | ||||
| N-(2-amino-5-(thiazol-2-yl)phenyl)benzamide | Investigative | [27] | ||
| Synonyms |
CHEMBL1097278
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 390 nM | |||
| External Link | ||||
| N-(2-aminophenyl)quinoxaline-6-carboxamide | Investigative | [30] | ||
| Synonyms |
benzamide-type inhibitor, 20; CHEMBL236060; BDBM19424
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2000 nM | |||
| External Link | ||||
| 7-Biphenyl-4-yl-heptanoic acid hydroxyamide | Investigative | [31] | ||
| Synonyms |
CHEMBL125098; BDBM50222335; 7-(4-Biphenylyl)heptanehydroximic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-(Biphenyl-4-yloxy)-2-oxo-octanoic acid | Investigative | [32] | ||
| Synonyms |
CHEMBL115049; 436150-72-0; SCHEMBL7368556; CTK1D2674; DTXSID40658342; BDBM50221807; 8-[([1,1'-Biphenyl]-4-yl)oxy]-2-oxooctanoic acid; Octanoic acid, 8-([1,1'-biphenyl]-4-yloxy)-2-oxo-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1,1,1-Trifluoro-8-phenoxy-octan-2-one | Investigative | [33] | ||
| Synonyms |
CHEMBL114796; BDBM50217940
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Biphenyl-4-yloxy)-heptanoic acid hydroxyamide | Investigative | [32] | ||
| Synonyms |
CHEMBL114184; SCHEMBL3383144
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Phenoxy-heptanoic acid hydroxyamide | Investigative | [31] | ||
| Synonyms |
CHEMBL124322; N-hydroxy-7-phenoxyheptanamide; 7-Phenoxyheptanehydroximic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1,1,1-Trifluoro-8-(4-phenoxy-phenoxy)-octan-2-one | Investigative | [33] | ||
| Synonyms |
CHEMBL117916; SCHEMBL7366611; BDBM50217945
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(4-hydroxybiphenyl-3-yl)benzamide | Investigative | [34] | ||
| Synonyms |
CHEMBL269935; SCHEMBL5724398; BDBM50232005
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 200 nM | |||
| External Link | ||||
| 8-Phenyl-octanoic acid hydroxyamide | Investigative | [31] | ||
| Synonyms |
CHEMBL123624; N-Hydroxy-8-phenyloctanamide; SCHEMBL5807174
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-(Biphenyl-3-yloxy)-1,1,1-trifluoro-octan-2-one | Investigative | [33] | ||
| Synonyms |
CHEMBL116023; SCHEMBL7368359; BDBM50218558
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(2-aminophenyl)nicotinamide | Investigative | [34] | ||
| Synonyms |
N-(2-Amino-phenyl)-nicotinamide; 436089-31-5; N-(2-aminophenyl)pyridine-3-carboxamide; CHEMBL236678; AC1LMN6K; SCHEMBL18086514; CTK4I7538; DTXSID50360661; CHEBI:125506; ZINC873967; BDBM50220259; 3463AE; AKOS000129725; RTR-042156; MCULE-7933541910; N-(2-aminophenyl)-3-pyridylcarboxamide; ZB014940; ACM436089315; ST086607; ASN 01337807; KB-298440; TR-042156; BC4148434; SR-01000329900; SR-01000329900-1; BRD-K20880473-001-04-6
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 6980 nM | |||
| External Link | ||||
| santacruzamate A | Investigative | [35] | ||
| Synonyms |
CAY10683
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.112 nM | |||
| External Link | ||||
| N-(4-aminobiphenyl-3-yl)nicotinamide | Investigative | [34] | ||
| Synonyms |
CHEMBL255805; BDBM50232035
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 900 nM | |||
| External Link | ||||
| N-(2-amino-5-(thiophen-2-yl)phenyl)nicotinamide | Investigative | [34] | ||
| Synonyms |
CHEMBL256440; SCHEMBL1066609
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 390 nM | |||
| External Link | ||||
| N-(2-aminophenyl)-4-methoxybenzamide | Investigative | [30] | ||
| Synonyms |
AC1LFX2W; Cambridge id 5129152; Oprea1_722128; benzamide-type inhibitor, 22; CHEMBL236061; SCHEMBL5226034; BDBM19426; CTK7A1998; MolPort-001-019-504; BDYVCYUXCNZYRW-UHFFFAOYSA-N; ZINC281656; STK156256; AKOS000130378; MCULE-9183453747; N-(2-Amino-phenyl)4-methoxy-benzamide; N-(2-amino-phenyl)-4-methoxy-benzamide; NCGC00240897-01; N1-(4-methoxybenzoyl)-1,2-benzenediamine; N1-(4-methoxy-benzoyl)-1,2-benzenediamine; ST50908739; N-(2-aminophenyl)(4-methoxyphenyl)carboxamide; SR-01000196394
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 900 nM | |||
| External Link | ||||
| 4-Phenylbutyrohydroxamic acid | Investigative | [36] | ||
| Synonyms |
N-Hydroxy-4-phenylbutanamide; 32153-46-1; NSC131300; UNII-QX182FOM5S; QX182FOM5S; 4-phenylbutanehydroxamic acid; CHEMBL55895; Benzenebutanamide, N-hydroxy-; NSC 131300; AC1Q7DIW; AC1L5RDX; Phenylbutyrylhydroxamic Acid; AC1Q5QD1; N-Hydroxy-4-phenyl-butyramide; 4-Phenylbutyryl hydroxamic acid; SCHEMBL1350853; CTK4G8310; DTXSID60185943; MolPort-011-492-164; UPHXPXYRKPCXHK-UHFFFAOYSA-N; ZINC4962622; STL301752; BDBM50015142; AKOS009266186; MCULE-9765156954; NSC-131300; NE28489; BCB03_000829; EN300-68596
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 430 nM | |||
| External Link | ||||
| 8-Oxo-8-phenyl-octanoic acid hydroxyamide | Investigative | [37] | ||
| Synonyms |
CHEMBL95959; SCHEMBL3383197; N-hydroxy-8-oxo-8-phenyloctanamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Octanedioic acid bis-hydroxyamide | Investigative | [38] | ||
| Synonyms |
Suberohydroxamic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 29 nM | |||
| External Link | ||||
| ST-2986 | Investigative | [39] | ||
| Synonyms |
CHEMBL471041; SCHEMBL3444455; BDBM50278219
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 11000 nM | |||
| External Link | ||||
| 9,9,9-Trifluoro-8-oxo-nonanoic acid phenylamide | Investigative | [33] | ||
| Synonyms |
9,9,9-Trifluoro-8-Oxo-N-Phenylnonanamide; CHEMBL113537; 2gh6; SCHEMBL2702892; KRCXZGYVOZSCSF-UHFFFAOYSA-N; BDBM50121062; DB07553
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1400 nM | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid phenylamide | Investigative | [40] | ||
| Synonyms |
Thiol-SAHA (t-SAHA); CHEMBL325676; SCHEMBL14821761; BDBM152692
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1300 nM | |||
| External Link | ||||
| 6-benzenesulfinylhexanoic acid hydroxamide | Investigative | [41] | ||
| Synonyms |
6-(benzenesulfinyl)hexanoic acid hydroxyamide; 875737-03-4
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(2-Mercapto-ethyl)-N'-phenyl-succinamide | Investigative | [42] | ||
| Synonyms |
CHEMBL193959
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid biphenyl-4-ylamide | Investigative | [40] | ||
| Synonyms |
CHEMBL112311
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-phenylacetylamino-benzamide | Investigative | [43] | ||
| Synonyms |
CHEMBL356824; 656261-23-3; SCHEMBL675578; CTK1J6158; DTXSID40458440; ZINC13533297; AKOS030583151; Benzeneacetamide, N-[4-[(hydroxyamino)carbonyl]phenyl]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-(2-Bromo-acetylamino)-hexanoic acid phenylamide | Investigative | [40] | ||
| Synonyms |
CHEMBL344920; 651767-99-6; SCHEMBL3736839; CTK1J8444; DTXSID50432973; HWYLREOMBVUGJQ-UHFFFAOYSA-N; BDBM50222416; ZINC13587789; AKOS030603042; N-Phenyl-6-(bromoacetylamino)hexanamide; Hexanamide, 6-[(bromoacetyl)amino]-N-phenyl-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(phenylacetylamino-methyl)-benzamide | Investigative | [44] | ||
| Synonyms |
CHEMBL143674; SCHEMBL673760
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Biphenyl-4-yloxy)-1-oxazol-2-yl-heptan-1-one | Investigative | [45] | ||
| Synonyms |
CHEMBL126355; BDBM50222394
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Octanedioic acid hydroxyamide pyridin-4-ylamide | Investigative | [46] | ||
| Synonyms |
SCHEMBL8082656; CHEMBL165162; ZINC13472304
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(6-Mercapto-hexyl)-benzamide | Investigative | [40] | ||
| Synonyms |
CHEMBL112364; BDBM50223650
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Hydroxy-N-(5-hydroxycarbamoyl-pentyl)-benzamide | Investigative | [46] | ||
| Synonyms |
CHEMBL167455
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-((R)-2-phenyl-butyrylamino)-benzamide | Investigative | [43] | ||
| Synonyms |
SCHEMBL675474
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-benzenesulfonylhexanoic acid hydroxamide | Investigative | [41] | ||
| Synonyms |
CHEMBL203207
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 9-(Biphenyl-4-yloxy)-1,1,1-trifluoro-nonan-2-one | Investigative | [33] | ||
| Synonyms |
SCHEMBL7373122; CHEMBL116578
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Thioacetic acid S-(6-phenylcarbamoyl-hexyl) ester | Investigative | [40] | ||
| Synonyms |
CHEMBL111806; SCHEMBL14812153
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Butyrylamino-N-hydroxy-benzamide | Investigative | [44] | ||
| Synonyms |
CHEMBL142254; 656261-22-2; Benzamide, N-hydroxy-4-[(1-oxobutyl)amino]-; SCHEMBL675234; CTK1J6159; DTXSID90461262
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Naphthalen-2-yloxy)-1-oxazol-2-yl-heptan-1-one | Investigative | [45] | ||
| Synonyms |
CHEMBL127328
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid biphenyl-3-ylamide | Investigative | [40] | ||
| Synonyms |
CHEMBL320323
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione | Investigative | [47] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(2-phenyl-butyrylamino)-benzamide | Investigative | [43] | ||
| Synonyms |
SCHEMBL676079
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Dimethylamino-N-(6-mercapto-hexyl)-benzamide | Investigative | [40] | ||
| Synonyms |
CHEMBL324126
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid pyridin-3-ylamide | Investigative | [40] | ||
| Synonyms |
CHEMBL332246; Heptanamide, 7-mercapto-N-3-pyridinyl-; BDBM50223653
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-Phenoxy-hexane-1-thiol | Investigative | [40] | ||
| Synonyms |
CHEMBL109796; 6-phenoxyhexane-1-thiol; 1-Hexanethiol, 6-phenoxy-; SCHEMBL5679745; MolPort-020-180-823; BDBM50223652; AKOS018584222; MCULE-9521857089
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Benzoylamino-N-hydroxy-benzamide | Investigative | [43] | ||
| Synonyms |
SCHEMBL673678; CHEMBL191227
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Chloro-N-(5-hydroxycarbamoyl-pentyl)-benzamide | Investigative | [37] | ||
| Synonyms |
CHEMBL143734; NSC718168; AC1L8L82; SCHEMBL13039735; ZINC5579677; BDBM50082664; NSC-718168; NCI60_040737; 6-(4-Chlorobenzoylamino)hexanehydroxamic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-(Biphenyl-4-yloxy)-1,1,1-trifluoro-octan-2-one | Investigative | [33] | ||
| Synonyms |
CHEMBL112148; SCHEMBL7364383; BDBM50218532
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Biphenyl-3-yloxy)-1-oxazol-2-yl-heptan-1-one | Investigative | [45] | ||
| Synonyms |
CHEMBL127351; SCHEMBL7365180; HWZHDGRMABBYOV-UHFFFAOYSA-N; BDBM50222367; 7-((1,1'-biphenyl)-3-yloxy)-1-(1 ,3-oxazol-2-yl)-1-heptanone
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-Mercapto-hexanoic acid phenylamide | Investigative | [40] | ||
| Synonyms |
CHEMBL109654; Hexanamide, 6-mercapto-N-phenyl-; SCHEMBL14254925; BDBM50027600
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2220 nM | |||
| External Link | ||||
| Cyclostellettamine derivative | Investigative | [48] | ||
| Synonyms |
CHEMBL88332
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(5-Hydroxycarbamoyl-pentyl)-4-nitro-benzamide | Investigative | [37] | ||
| Synonyms |
CHEMBL139999; SCHEMBL1232700; BDBM50082661; ZINC13472309
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-Mercapto-pentanoic acid phenylamide | Investigative | [40] | ||
| Synonyms |
N-Phenyl-5-mercaptovaleramide; CHEMBL114344; Pentanamide, 5-mercapto-N-phenyl-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Octanedioic acid hydroxyamide pyridin-2-ylamide | Investigative | [46] | ||
| Synonyms |
SCHEMBL8090513; CHEMBL164872; ZINC13472303
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(2-Mercapto-ethyl)-N'-phenyl-oxalamide | Investigative | [42] | ||
| Synonyms |
CHEMBL193979
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(methylsulfonylthio)ethyl 2-propylpentanoate | Investigative | [47] | ||
| Synonyms |
CHEMBL271677; SCHEMBL4156413
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (E)-8-Biphenyl-4-yl-1-oxazol-2-yl-oct-7-en-1-one | Investigative | [45] | ||
| Synonyms |
CHEMBL126465; SCHEMBL7368197; SCHEMBL7368201
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-((S)-2-phenyl-butyrylamino)-benzamide | Investigative | [43] | ||
| Synonyms |
SCHEMBL676080
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(3-phenyl-propionylamino)-benzamide | Investigative | [49] | ||
| Synonyms |
N-hydroxy-4-(3-phenylpropanamido)benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(5-phenyl-pentanoylamino)-benzamide | Investigative | [43] | ||
| Synonyms |
SCHEMBL7311087
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-Oxo-8-phenyl-octanoic acid | Investigative | [46] | ||
| Synonyms |
8-Oxo-8-phenyloctanoic acid; 7-Benzoylheptanoic acid; 24314-23-6; Benzeneoctanoic acid, h-oxo-; 7-BENZOYL HEPTANOIC ACID; AC1L6TSB; SCHEMBL3381106; 8-keto-8-phenyl-caprylic acid; CHEMBL162423; 8-Oxo-8-phenyloctanoic acid #; CTK4F3363; DTXSID40305602; UMCSRRHQLAVYRS-UHFFFAOYSA-N; ZINC2168376; 7009f; NSC171230; AKOS016022495; NSC-171230; MCULE-7202530747; ACM24314236; ST50825837
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(4-phenyl-butyrylamino)-benzamide | Investigative | [43] | ||
| Synonyms |
CHEMBL143336; 656261-24-4; SCHEMBL674421; CTK1J6157; DTXSID30433908; ZINC13533300; AKOS030583673; n-hydroxy-4-(4-phenylbutyryl-amino)benzamide; Benzenebutanamide, N-[4-[(hydroxyamino)carbonyl]phenyl]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-phenylsulfanylhexanoic acid hydroxamide | Investigative | [41] | ||
| Synonyms |
Hexanamide, N-hydroxy-6-(phenylthio)-; CHEMBL203028; SCHEMBL7317658
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ST-2987 | Investigative | [39] | ||
| Synonyms |
CHEMBL471042; SCHEMBL3444989; SCHEMBL3444984; BDBM50278220
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2670 nM | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid quinolin-3-ylamide | Investigative | [40] | ||
| Synonyms |
CHEMBL112234
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-(4-Chloro-phenyl)-pentanoic acid hydroxyamide | Investigative | [50] | ||
| Synonyms |
CHEMBL84288
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-Mercapto-octanoic acid phenylamide | Investigative | [40] | ||
| Synonyms |
8-mercapto-N-phenyloctanamide; CHEMBL326433; ZINC13609343
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 754 nM | |||
| External Link | ||||
| N-(6-Hydroxycarbamoyl-hexyl)-benzamide | Investigative | [46] | ||
| Synonyms |
CHEMBL57107; 174664-71-2; SCHEMBL573254; CTK0A7470; DTXSID00433435; BDBM50220823; ZINC13490043; 7-(Benzoylamino)heptanehydroxamic acid; AKOS030580013; Benzamide, N-[7-(hydroxyamino)-7-oxoheptyl]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Biphenyl-4-yloxy)-1,1,1-trifluoro-heptan-2-one | Investigative | [33] | ||
| Synonyms |
CHEMBL326529; SCHEMBL7365237; BDBM50217957
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid benzothiazol-2-ylamide | Investigative | [40] | ||
| Synonyms |
CHEMBL178779
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(pentanoylamino-methyl)-benzamide | Investigative | [44] | ||
| Synonyms |
CHEMBL143102
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PSAMMAPLIN A | Investigative | [37] | ||
| Synonyms |
110659-91-1; Bisprasin; NSC614495; AC1O46WI; SCHEMBL364511; ZINC150352860; NSC-614495; B723735K022; J-002461; Benzenepropanamide, N,N'-(dithiodi-2,1-ethanediyl)bis(3-bromo-4-hydroxy-alpha-(hydroxyimino)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2B91: Colorectal cancer | 25 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Retifanlimab | Approved | [51] | ||
| Synonyms |
INCMGA0012; Retifanlimab
Click to Show/Hide
|
|||
| External Link | ||||
| Aflibercept | Approved | [52] | ||
| Synonyms |
Ziv-Aflibercept; Zaltrap (TN); VEGF Trap; VEGF Trap-Eye
Click to Show/Hide
|
|||
| External Link | ||||
| Regorafenib | Approved | [53] | ||
| Synonyms |
755037-03-7; BAY 73-4506; Regorafenibum; Stivarga; 4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide; BAY73-4506; Regorafenib (BAY 73-4506); UNII-24T2A1DOYB; 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide; BAY-73-4506; 24T2A1DOYB; CHEMBL1946170; CHEBI:68647; Stivarga (TN); BAY73-4506 hydrochloride; Regorafenib [USAN:INN]
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [9] | ||
| Synonyms |
Bevacizumab (ophthalmic slow-release tissue tablet)
Click to Show/Hide
|
|||
| External Link | ||||
| SYM-004 | Phase 3 | [9] | ||
| Synonyms |
Chimeric IgG1 antibody 1024 (cancer), Symphogen; Chimeric IgG1 antibody 992 (cancer), Symphogen; Chimeric IgG1 antibodies992 + 1024 (cancer), Symphogen
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab + Erlotinib | Phase 3 | [54] | ||
| External Link | ||||
| CPI-613 | Phase 3 | [9] | ||
| Synonyms |
95809-78-2; 6,8-bis(benzylthio)octanoic acid; CPI 613; MLS006010202; SCHEMBL1062218; 6,8-Bis(benzylsulfanyl)octanoic acid; Octanoic acid, 6,8-bis((phenylmethyl)thio)-; Octanoic acid, 6,8-bis[(phenylMethyl)thio]-; 6,8-Bis[(phenylmethyl)thio]octanoic acid; CPI613; CHEMBL3186849; QCR-193; AOB1058; MolPort-023-219-128; HMS3656L06; C22H28O2S2; BCP04663; EX-A2043; s2776; AKOS025142095; BCP9000552; DB12109; RL06062; CS-0961; NCGC00344764-01; SMR004701300; AS-16613; BC261916; AK174899; HY-15453; BCP0726000030; KB-293127; AB0035874
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [52] | ||
| External Link | ||||
| AlloStim | Phase 2/3 | [55] | ||
| Synonyms |
AlloStim (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Sibrotuzumab | Phase 2 | [56] | ||
| External Link | ||||
| CV301 | Phase 2 | [57] | ||
| External Link | ||||
| Efatutazone | Phase 2 | [58] | ||
| Synonyms |
Inolitazone; 223132-37-4; 5-[[4-[[6-(4-amino-3,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-yl]methoxy]phenyl]methyl]-2,4-Thiazolidinedione; Efatutazone [INN]; RS5444; CS-7017; SCHEMBL3246054; CHEMBL3545280; JCYNMRJCUYVDBC-UHFFFAOYSA-N; Efatutazone;CS-7017;RS5444; BCP07478; AKOS030526729; DB11894; CS-0778; KB-77905; DA-07988; HY-14792; QC-10456; 4CA-1384; FT-0737589; 5-[4-[6-(4-amino-3 ,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-ylmethoxy]benzyl]thiazolidine-2,4-dione
Click to Show/Hide
|
|||
| External Link | ||||
| LOR-2040 | Phase 2 | [59] | ||
| External Link | ||||
| RG7221 | Phase 2 | [60] | ||
| External Link | ||||
| PEG-SN38 | Phase 2 | [61] | ||
| Synonyms |
EZN-2208
Click to Show/Hide
|
|||
| External Link | ||||
| MEGF0444A | Phase 2 | [62] | ||
| External Link | ||||
| Encapsulated cell therapy | Phase 1/2 | [63] | ||
| External Link | ||||
| AB928 | Phase 1/2 | [64] | ||
| External Link | ||||
| MGD007 | Phase 1 | [60] | ||
| External Link | ||||
| BNC-101 | Phase 1 | [65] | ||
| External Link | ||||
| Navicixizumab | Phase 1 | [9] | ||
| External Link | ||||
| RG7160 | Discontinued in Phase 2 | [66] | ||
| External Link | ||||
| Nimesulide | Terminated | [67] | ||
| Synonyms |
51803-78-2; N-(4-Nitro-2-phenoxyphenyl)methanesulfonamide; Mesulid; Flogovital; Sulidene; Nimed; R-805; 4-NITRO-2-PHENOXYMETHANESULFONANILIDE; Nisulid; Nimesulidum [INN-Latin]; Nimesulida [INN-Spanish]; R 805; UNII-V4TKW1454M; 4-Nitro-2-phenoxy-methanesulfonanilide; 4'-Nitro-2'-phenoxymethanesulfonanilide; Methanesulfonamide, N-(4-nitro-2-phenoxyphenyl)-; EINECS 257-431-4; 4'-Nitro-2'-phenoxymethansulfonanilid; BRN 2421175; CHEMBL56367; MLS000069680; V4TKW1454M; Methanesulfonanilide, 4'-nitro-2'-phenoxy-; CHEBI:44445; Dulanermin
Click to Show/Hide
|
|||
| External Link | ||||
| Saracatinib | Phase 2 | [68] | ||
| External Link | ||||
| G3139 + Irinotecan | Investigative | [69] | ||
| External Link | ||||
References