m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03319
|
[1], [2] | |||
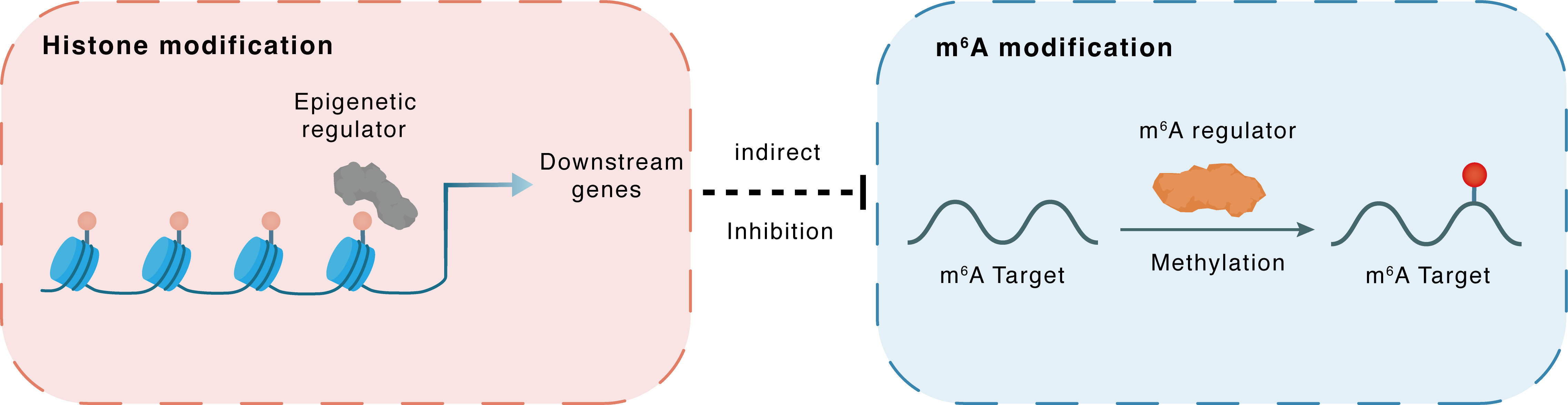 Histone modification
H3K9me1
JMJD1C
hsa-miR-302a
Indirect
Inhibition
m6A modification
CTNNBIP1
CTNNBIP1
METTL3
Methylation
Histone modification
H3K9me1
JMJD1C
hsa-miR-302a
Indirect
Inhibition
m6A modification
CTNNBIP1
CTNNBIP1
METTL3
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Beta-catenin-interacting protein 1 (CTNNBIP1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Probable JmjC domain-containing histone demethylation protein 2C (JMJD1C) | ERASER | View Details | ||
| Regulated Target | Histone H3 lysine 9 monomethylation (H3K9me1) | View Details | |||
| Downstream Gene | hsa-miR-302a | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Inhibition | |||
| Crosstalk Mechanism | histone modification indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | JMJD1C increased MIR302A expression through promoting Histone H3 lysine 9 monomethylation (H3K9me1) demethylation at the miR-302a promoter region. miR-302a was identified to target METTL3, which could inhibit SOCS2 expression via m6A modification. METTL3-mediated m6A modification upregulated circDLC1 expression, and circDLC1 promoted Beta-catenin-interacting protein 1 (CTNNBIP1) transcription by sponging miR-671-5p, thus repressing the malignant proliferation of glioma. | ||||
| Responsed Disease | Brain cancer | ICD-11: 2A00 | |||
| Pathway Response | Transcriptional misregulation in cancer | hsa05202 | |||
| Cell Process | RNA stability | ||||
In-vitro Model |
LN-229 | Glioblastoma | Homo sapiens | CVCL_0393 | |
| LN-18 | Glioblastoma | Homo sapiens | CVCL_0392 | ||
| In-vivo Model | Mice were subcutaneously injected with 2.5 × 106 U251 cells stably infected with OE-NC, OE-JMJD1C, OE-NC and sh-NC, OE-JMJD1C and sh-NC or OE-JMJD1C and sh-SOCS2 (n = 10 in each group) in 0.1 ml PBS. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| 2A00: Brain cancer | 53 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Zinostatin stimalamer | Approved | [3] | ||
| Synonyms |
Smancs (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Motexafin gadolinium | Approved | [4] | ||
| Synonyms |
Xcytrin; Gadolinium texaphyrin; GdT2B2; GD-Tex; Motexafin gadolinium (USAN); PCI-0120; Xcytrin (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Lomustine | Approved | [5] | ||
| Synonyms |
Belustine; CCNU; CINU; Cecenu; CeeNU; Chloroethylcyclohexylnitrosourea; Lomustina; Lomustinum; Bristol Myers Squibb Brand of Lomustine; CCNU [Chloroethyl nitrosoureas]; Cyclohexyl chloroethyl nitrosourea; Lomustine medac Brand; Medac Brand of Lomustine; Rhone Poulenc Rorer Brand of Lomustine; OR5087; RB 1509; SRI 2200; Bristol-Myers Squibb Brand of Lomustine; CeeNU (TN); Lomustina [INN-Spanish]; Lomustinum [INN-Latin]; NPFAPI-06; Rhone-Poulenc Rorer Brand of Lomustine; CeeNU, CCNU, Lomustine; Lomustine (USAN/INN); Lomustine [USAN:BAN:INN]; N-(2-Chloroethyl)-N'-cyclohexyl-N-nitrosourea; (Chloro-2-ethyl)-1-cyclohexyl-3-nitrosourea; (Cloro-2-etil)-1-cicloesil-3-nitrosourea; (Cloro-2-etil)-1-cicloesil-3-nitrosourea [Italian];1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea; 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea [Chloroethyl nitrosoureas]; 1-(2-Chloroethyl)-3-cyclohexylnitrosourea
Click to Show/Hide
|
|||
| External Link | ||||
| Borocaptate sodium B 10 | Approved | [3] | ||
| External Link | ||||
| DTI-015 | Approved | [6] | ||
| Synonyms |
Carmustine; 154-93-8; 1,3-Bis(2-chloroethyl)-1-nitrosourea; BCNU; Carmustin; Nitrumon; Carmubris; Gliadel; BiCNU; Bi CNU; Carmustinum; Bischlorethylnitrosurea; Bischlorethylnitrosourea; Carmustina; Becenun; Becenum; Bischloroethyl nitrosourea; N,N'-BIS(2-CHLOROETHYL)-N-NITROSOUREA; Bis(2-chloroethyl)nitrosourea; Urea, N,N'-bis(2-chloroethyl)-N-nitroso-; Gliadel Wafer; FDA 0345; Bischloroethylnitrosourea; SRI 1720; 1,3-Bis(2-chloroethyl)nitrosourea; BiCNU (TN); Carmustinum [INN-Latin]; Carmustina [INN-Spanish]; DTI 015; NCI-C04773; SK; Injectable carmustine, Direct Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| Prinomastat | Approved | [7] | ||
| Synonyms |
AG-3354; AG-3362; Prinomastat (USAN/INN); (3S)-N-hydroxy-2,2-dimethyl-4-(4-pyridin-4-yloxyphenyl)sulfonylthiomorpholine-3-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| INO-1001 | Phase 3 | [8] | ||
| Synonyms |
Hypoxanthine arabinoside; LT00454797; 9-[(3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-3H-purin-6-one
Click to Show/Hide
|
|||
| External Link | ||||
| GliAtak | Phase 3 | [9] | ||
| Synonyms |
GliAtak (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| SOT-107 | Phase 3 | [10] | ||
| Synonyms |
TransMID
Click to Show/Hide
|
|||
| External Link | ||||
| ICT-107 | Phase 3 | [11] | ||
| External Link | ||||
| Cintredekin besudotox | Phase 3 | [12] | ||
| External Link | ||||
| Rindopepimut | Phase 3 | [13] | ||
| External Link | ||||
| DCVax-Ovarian | Phase 3 | [14] | ||
| Synonyms |
DCVax-L; Dendritic cell-based immunotherapy (ovarian cancer), Northwest Biotherapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| DCVax-Brain | Phase 3 | [15] | ||
| Synonyms |
Dendritic cell-based immunotherapy (brain cancer), Northwest Biotherapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| TVI-Brain-1 | Phase 2/3 | [16] | ||
| External Link | ||||
| NLG8189 | Phase 2/3 | [17] | ||
| Synonyms |
1-Methyl-D-tryptophan; Indoximod; 110117-83-4; D-Tryptophan, 1-methyl-; D-1MT; Indoximod (NLG-8189); D-1-methyltryptophan; UNII-TX5CYN1KMZ; D-(+)-1-Methyltryptophan; TX5CYN1KMZ; (R)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic acid; (2R)-2-amino-3-(1-methylindol-3-yl)propanoic acid; NSC-721782; (2R)-2-amino-3-(1-methyl-3-indolyl)propanoic acid; 1-MT; (2R)-2-azanyl-3-(1-methylindol-3-yl)propanoic acid; (2R)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic acid; D-l-Methyltryptophan; Indoximod [USAN:INN]; NLG-8189; NLG 8189
Click to Show/Hide
|
|||
| External Link | ||||
| Synthetic survivin peptide vaccine | Phase 2 | [18] | ||
| External Link | ||||
| Fresolimumab | Phase 2 | [19] | ||
| Synonyms |
GC-1008
Click to Show/Hide
|
|||
| External Link | ||||
| DNX-2401 | Phase 2 | [20] | ||
| Synonyms |
Tasadenoturev
Click to Show/Hide
|
|||
| External Link | ||||
| TVI-Brain-1 cancer vaccine | Phase 2 | [21] | ||
| External Link | ||||
| PDT with Photofrin | Phase 2 | [17] | ||
| External Link | ||||
| F18-ML-10 | Phase 2 | [22] | ||
| External Link | ||||
| PT2385 | Phase 2 | [23] | ||
| Synonyms |
ONBSHRSJOPSEGS-INIZCTEOSA-N; PT-2385; UNII-6O16716DXP; 1672665-49-4; 6O16716DXP; SCHEMBL16555810; ZINC230453533; AKOS030526641; HY-12867; PT2385,1672665-49-4, PT 2385,PT-2385; Benzonitrile, 3-(((1S)-2,2-difluoro-2,3-dihydro-1-hydroxy-7-(methylsulfonyl)-1H-inden-4-yl)oxy)-5-fluoro-; 3-{[(1s)-2,2-Difluoro-1-Hydroxy-7-(Methylsulfonyl)-2,3-Dihydro-1h-Inden-4-Yl]oxy}-5-Fluorobenzonitrile; 3-(((1S)-2,2-Difluoro-1-hydroxy-7-methanesulfonyl-2,3-dihydro-1hinden-4-yl)oxy)-5-fluorobenzonitrile; 79A
Click to Show/Hide
|
|||
| External Link | ||||
| ABT-414 | Phase 2 | [24] | ||
| External Link | ||||
| CLR1404-I-124 | Phase 1/2 | [25] | ||
| External Link | ||||
| DM-CHOC-PEN | Phase 2 | [26] | ||
| External Link | ||||
| L-alanosine | Phase 2 | [27] | ||
| Synonyms |
SDX-102
Click to Show/Hide
|
|||
| External Link | ||||
| APX005M | Phase 2 | [17] | ||
| External Link | ||||
| WP-1066 | Phase 1/2 | [21] | ||
| Synonyms |
WP1066; 857064-38-1; (S,E)-3-(6-Bromopyridin-2-yl)-2-cyano-N-(1-phenylethyl)acrylamide; WP 1066; UNII-63V8AIE65T; 63V8AIE65T; AK-99218; C17H14BrN3O; (E)-3-(6-bromopyridin-2-yl)-2-cyano-N-[(1S)-1-phenylethyl]prop-2-enamide; MLS006010178; SCHEMBL1315826; QCR-16; SCHEMBL1315831; GTPL7972; CHEMBL1923234; EX-A760; AOB1497; DTXSID50235007; MolPort-044-723-708; MolPort-023-219-149; ZINC13983221; AKOS016007983; WP1066/WP-1066; CS-2736; DB12679; 2-Propenamide, 3-(6-bromo-2-pyridinyl)-2-cyano-N-((1S)-1-phenylethyl)-, (2E)-; HY-15312
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-EGFRvIII CAR transduced PBL | Phase 1/2 | [28] | ||
| External Link | ||||
| RG6156 | Phase 1 | [29] | ||
| External Link | ||||
| rhenium-186 | Phase 1 | [30] | ||
| Synonyms |
(~186~Re)Rhenium; 14998-63-1; 186Re; DTXSID60933825; Q18882928; RHENIUM (186-RE); Rhenium Re-186; Rhenium, isotope of mass 186; Rhenium-186; RHENIUM-186 [WHO-DD]; UNII-ZU7F1ET6TM; ZU7F1ET6TM
Click to Show/Hide
|
|||
| External Link | ||||
| IGV-001 | Phase 1 | [31] | ||
| External Link | ||||
| DA-3607 | Phase 1 | [32] | ||
| Synonyms |
Ad-stTRAIL; TRAIL adenoviral gene therapy (cancer), Dong-A
Click to Show/Hide
|
|||
| External Link | ||||
| DC/I540/KLH vaccine | Phase 1 | [33] | ||
| Synonyms |
HTERT:540-548; Telomerase: 540-548 peptide vaccine; DC/I540/KLH vaccine (cancer); Dendritic cell/hTERT peptide I540/keyhole limpet hemocyanin vaccine, Dana-Farber; DC/I540/KLH vaccine (cancer), Dana-Farber
Click to Show/Hide
|
|||
| External Link | ||||
| KX2-361 | Phase 1 | [34] | ||
| Synonyms |
KX-02
Click to Show/Hide
|
|||
| External Link | ||||
| MR1-1 | Phase 1 | [35] | ||
| Synonyms |
MR1-1KDEL; EGFR-specific immunotoxin, IVAX; Anticancer immunotoxin (EGFR-specific), IVAX
Click to Show/Hide
|
|||
| External Link | ||||
| INdoximod + temozolomide | Phase 1 | [21] | ||
| External Link | ||||
| Anti-CD133-CAR vector-transduced T cells | Phase 1 | [36] | ||
| External Link | ||||
| 8H9 | Phase 1 | [37] | ||
| External Link | ||||
| CC-8490 | Phase 1 | [38] | ||
| External Link | ||||
| Sitimagene ceradenovec | Discontinued in Phase 3 | [39] | ||
| Synonyms |
Cerepro; EG-009; HSV thymidine kinase gene therapy, Ark
Click to Show/Hide
|
|||
| External Link | ||||
| Ranagengliotucel-T | Discontinued in Phase 3 | [40] | ||
| Synonyms |
Glionix; Brain tumor vaccine, NovaRx; Antisense (TGFbeta) brain tumor vaccine, NovaRx
Click to Show/Hide
|
|||
| External Link | ||||
| Oncolysin S | Discontinued in Phase 2 | [41] | ||
| Synonyms |
N901-bR
Click to Show/Hide
|
|||
| External Link | ||||
| Labradimil | Discontinued in Phase 2 | [42] | ||
| Synonyms |
Cereport; Lobradimil; Receptor mediated permeabilizer; RMP 7; DRG-0182; RMP-7; N2-((S)-2-(L-Arginyl-L-prolyl-trans-4-hydroxy-L-prolylglycyl-3-(2-thienyl)-L-alanyl-L-seryl-L-prolinamido)-3-(p-methoxyphenyl)propyl)-L-arginine; (S-(R*,R*))-L-Arginyl-L-prolyl-trans-4-hydroxy-L-prolyl-3-(2-thienyl)-L-alanylglycyl-L-seryl-N-(2-((4-((aminoiminomethyl)amino)-1-carboxybutyl)amino)-1-((4-methoxyphenyl)methyl)ethyl)-L-prolinamide
Click to Show/Hide
|
|||
| External Link | ||||
| Brain tumor vaccine | Discontinued in Phase 1 | [43] | ||
| Synonyms |
Brain cancer vaccine, IRC; Brain tumor vaccine, IRC; IR-850; Established cancer cell line therapy (brain cancer), IRC; GM-CSF vaccine (brain cancer), IRC
Click to Show/Hide
|
|||
| External Link | ||||
| PCNU | Terminated | [44] | ||
| Synonyms |
NSC-95466
Click to Show/Hide
|
|||
| External Link | ||||
| 131I-81C6 | Terminated | [45] | ||
| Synonyms |
Neuradiab; MAb-81C6; Iodine-131-81C6; Astatine-211-MAb-81C6; Iodine-131-MAb-81C6; Iodine-131-ch-81C6; Iodine-131-ch-81C6-F(ab)2; 211At-MAb-81C6
Click to Show/Hide
|
|||
| External Link | ||||
| NSD-551 | Terminated | [46] | ||
| Synonyms |
BK channel activator (cancer), NeuroSearch/TopoTarget
Click to Show/Hide
|
|||
| External Link | ||||
| AGT-2000 | Investigative | [47] | ||
| Synonyms |
Gene therapy (intravenous, brain cancer), ArmaGen
Click to Show/Hide
|
|||
| External Link | ||||
| NV.XOD.09 | Investigative | [48] | ||
| External Link | ||||
| EDP-19 | Investigative | [48] | ||
| Synonyms |
SiRNA (convection-enhanced delivery, brain tumor), Sheba Medical Center/BioLineRx
Click to Show/Hide
|
|||
| External Link | ||||
| MIQ-004 | Investigative | [48] | ||
| Synonyms |
M-IQ-004
Click to Show/Hide
|
|||
| External Link | ||||
References