m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03224
|
[1] | |||
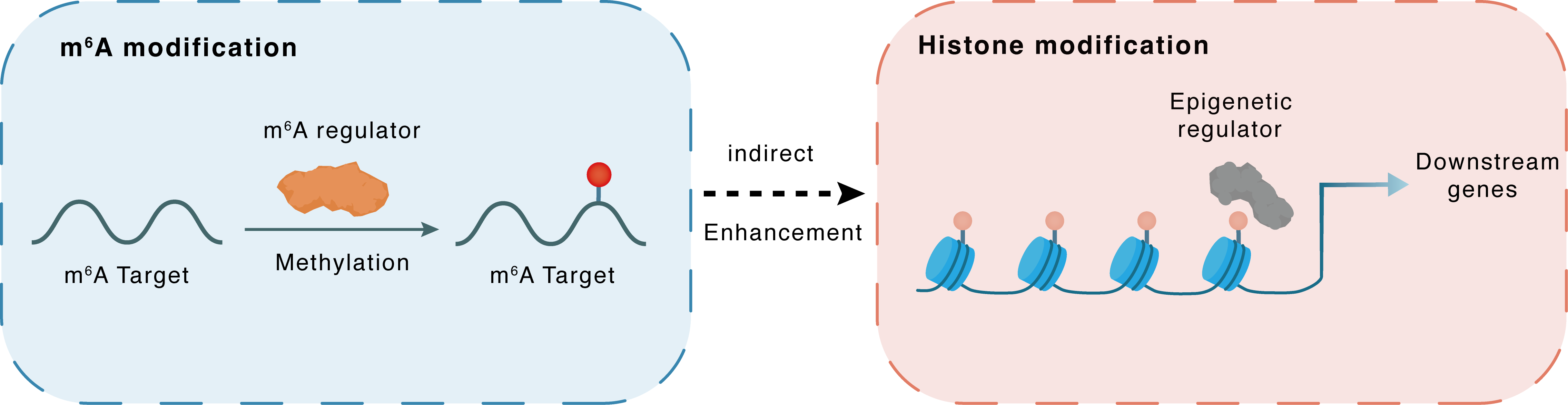 m6A modification
PHF19
PHF19
WTAP
Methylation
m6A modification
PHF19
PHF19
WTAP
Methylation
 : m6A sites
Indirect
Enhancement
Histone modification
H3K27me3
PRC2
Downstream Gene : m6A sites
Indirect
Enhancement
Histone modification
H3K27me3
PRC2
Downstream Gene
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Wilms tumor 1-associating protein (WTAP) | WRITER | |||
| m6A Target | PHD finger protein 19 (PHF19) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Polycomb Repressive Complex 2 (PRC2) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 27 trimethylation (H3K27me3) | View Details | |||
| Crosstalk Relationship | m6A → Histone modification | Enhancement | |||
| Crosstalk Mechanism | m6A modification indirectly regulates histone modification through downstream signaling pathways | ||||
| Crosstalk Summary | Knockdown of PHD finger protein 19 (PHF19) precipitates loss of Histone H3 lysine 27 trimethylation (H3K27me3) modulated by PRC2 and enhanced chromatin accessibility, ultimately resulting in upregulated expression of genes involved in the cell cycle and DNA damage checkpoints. Therefore, WTAP/m6A-dependent PHF19 upregulation accelerates leukemia progression by coordinating m6A modification and histone methylation, establishing its status as a novel therapeutic target for t(8; 21) AML. | ||||
| Responsed Disease | T(8; 21) acute myeloid leukemia | ICD-11: XH3CX5 | |||
In-vitro Model |
Kasumi-1 | Myeloid leukemia with maturation | Homo sapiens | CVCL_0589 | |
| SKNO-1 | Myeloid leukemia with maturation | Homo sapiens | CVCL_2196 | ||
| U-937 | Adult acute monocytic leukemia | Homo sapiens | CVCL_0007 | ||
| U-937 | Adult acute monocytic leukemia | Homo sapiens | CVCL_0007 | ||