m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03207
|
[1] | |||
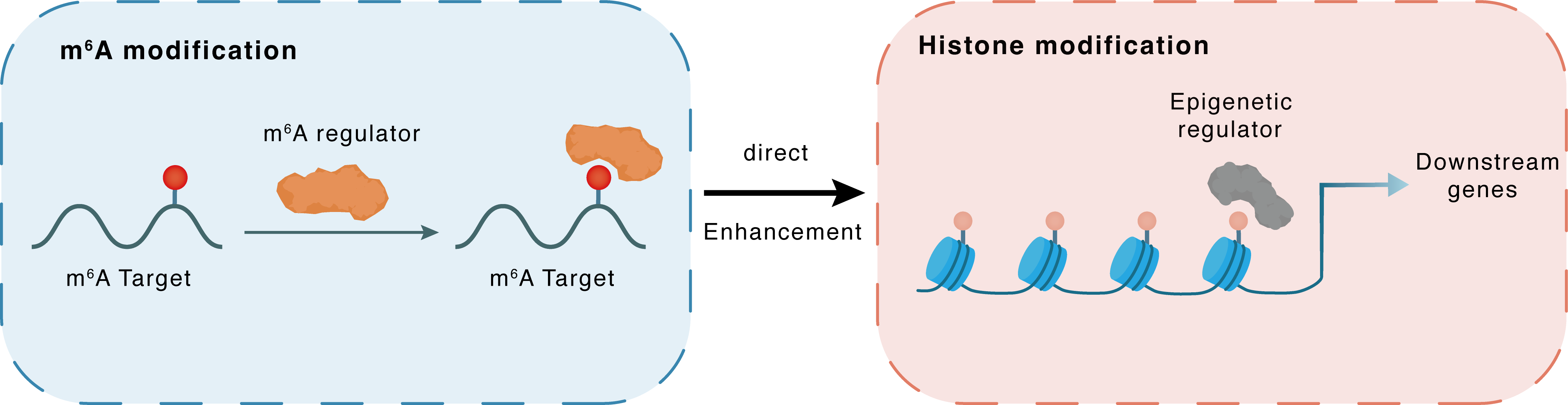 m6A modification
HDAC3
HDAC3
IGF2BP3
m6A modification
HDAC3
HDAC3
IGF2BP3
 : m6A sites
Direct
Enhancement
Histone modification
Regulated Target
HDAC3
Downstream Gene : m6A sites
Direct
Enhancement
Histone modification
Regulated Target
HDAC3
Downstream Gene
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3) | READER | |||
| m6A Target | Histone deacetylase 3 (HDAC3) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone deacetylase 3 (HDAC3) | ERASER | View Details | ||
| Crosstalk Relationship | m6A → Histone modification | Enhancement | |||
| Crosstalk Mechanism | m6A modification impacts directly histone modification through modulating the expression level of histone-associated enzymes | ||||
| Crosstalk Summary | METTL14 induced Histone deacetylase 3 (HDAC3) m6A modification in an IGF2BP3-dependent manner, and could activate cGAS-STING pathway through HDAC3. | ||||
| Responsed Disease | Acute ischemic stroke | ICD-11: 8B11 | |||
| Pathway Response | cGAS-STING signaling pathway | hsa:00395 | |||
| Cell Process | Pyroptosis | ||||
In-vitro Model |
BV-2 | Normal | Mus musculus | CVCL_0182 | |
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Histone deacetylase 3 (HDAC3) | 19 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| CHR-3996 | Phase 1/2 | [2] | ||
| Synonyms |
CCT-075453; CHR-2504; HDAC inhibitors, Chroma Therapeutics; Histone deacetylase inhibitors, Chroma
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7 nM | |||
| External Link | ||||
| PMID29671355-Compound-57 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| Diaryl amine derivative 4 | Patented | [4] | ||
| Synonyms |
PMID28092474-Compound-9
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 76 nM | |||
| External Link | ||||
| PMID29671355-Compound-59 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 43 nM | |||
| External Link | ||||
| PMID29671355-Compound-55 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID29671355-Compound-11 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 546 nM | |||
| External Link | ||||
| PMID29671355-Compound-9 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 158 nM | |||
| External Link | ||||
| PMID29671355-Compound-8 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 22800 nM | |||
| External Link | ||||
| PMID29671355-Compound-61 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 839.3 nM | |||
| External Link | ||||
| PMID29671355-Compound-44 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 150 nM | |||
| External Link | ||||
| PMID29671355-Compound-56 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 28.7 nM | |||
| External Link | ||||
| PMID29671355-Compound-67 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 10000 nM | |||
| External Link | ||||
| PMID29671355-Compound-31 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.6 nM | |||
| External Link | ||||
| PMID29671355-Compound-21 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 10300 nM | |||
| External Link | ||||
| PMID29671355-Compound-62 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 500 nM | |||
| External Link | ||||
| PMID29671355-Compound-43 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 140 nM | |||
| External Link | ||||
| PMID29671355-Compound-25 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1310 nM | |||
| External Link | ||||
| RGFP966 | Investigative | [5] | ||
| Synonyms |
RGFP-966
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 80 nM | |||
| External Link | ||||
| droxinostat | Investigative | [6] | ||
| Synonyms |
NS-41080; NS41080
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8B11: Acute ischemic stroke | 7 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| PEG-bHb-CO | Phase 2 | [7] | ||
| Synonyms |
Sanguinate; Oxygen transfer agent (trauma/cardiovascular disease), Prolong Pharmaceuticals; PEG-bHb-CO (trauma/cardiovascular disease); PEGylated bovine hemoglobin-carbon monoxide (trauma/cardiovascular disease), Prolong Pharmaceuticals; PEG-bHb-CO (trauma/cardiovascular disease), Prolong Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| DM199 | Phase 2 | [8] | ||
| External Link | ||||
| BIIB131 | Phase 2 | [9] | ||
| Synonyms |
(2S)-2,5-bis[(2S,3S)-2-[(3E)-4,8-dimethylnona-3,7-dienyl]-3,5-dihydroxy-2-methyl-7-oxo-4,9-dihydro-3H-pyrano[2,3-e]isoindol-8-yl]pentanoic acid; 733805-92-0; BCP33210; BIIB131; CS-0083560; GTPL12300; HY-122311; Orniplabin; SCHEMBL2624852; SMTP 7; SMTP7; SMTP7; SMTP-7; Stachybotrys microspora triprenyl phenol 7; TMS-007
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-986177 | Phase 2 | [10] | ||
| Synonyms |
Milvexian; UNII-0W79NDQ608; Milvexian (USAN); Milvexian [USAN]; BMS 986177; JNJ-70033093; 1802425-99-5; CHEMBL4112929; SCHEMBL16982989; WHO 11401; D11802; (5R,9S)-9-(4-(5-Chloro-2-(4-chloro-1H-1,2,3-triazol-1-yl)phenyl)-6-oxopyrimidin-1(6H)-yl)-21-(difluoromethyl)-5-methyl-21H-3-aza-1(4,2)-pyridina-2(5,4)-pyrazolacyclonaphan-4-one; (9R,13S)-13-{4-[5-chloro-2-(4-chloro-1H-1,2,3-triazol-1-yl)phenyl]-6-oxo-1,6-dihydropyrimidin-1-yl}-3-(difluoromethyl)-9-methyl-3,4,7,15-tetraazatricyclo[12.3.1.02,6]octadeca-1(18),2(6),4,14,16-pentaen-8-one; 11,15-Metheno-15H-pyrazolo(4,3-b)(1,7)diazacyclotetradecin-5(6H)-one, 10-(4-(5-chloro-2-(4-chloro-1H-1,2,3-triazol-1-yl)phenyl)-6-oxo-1(6H)-pyrimidinyl)-1-(difluoromethyl)-1,4,7,8,9,10-hexahydro-6-methyl-, (6R,10S)-2
Click to Show/Hide
|
|||
| External Link | ||||
| SonoLysis Prolyse | Phase 2 | [11] | ||
| External Link | ||||
| ACT017 | Phase 1/2 | [12] | ||
| External Link | ||||
| Dimethoxybenzylidene-2-thio-imidazole-4-one derivative 1 | Patented | [13] | ||
| Synonyms |
PMID27998201-Compound-16
Click to Show/Hide
|
|||
| External Link | ||||
References