m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03177
|
[1] | |||
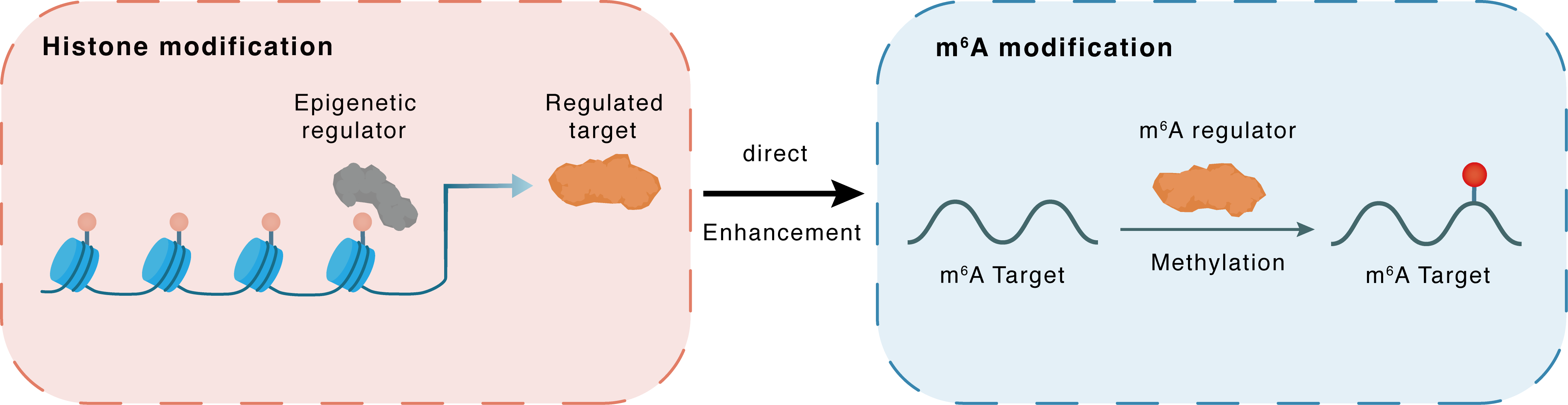 Histone modification
H3K9me3
KDM1A
METTL3
Direct
Enhancement
m6A modification
STUB1
STUB1
METTL3
Methylation
Histone modification
H3K9me3
KDM1A
METTL3
Direct
Enhancement
m6A modification
STUB1
STUB1
METTL3
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | E3 ubiquitin-protein ligase CHIP (STUB1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Lysine-specific histone demethylase 1A (KDM1A) | ERASER | View Details | ||
| Regulated Target | Histone H3 lysine 9 trimethylation (H3K9me3) | View Details | |||
| Downstream Gene | METTL3 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification directly impacts m6A modification through modulating the level of m6A regulator | ||||
| Crosstalk Summary | KDM1A-mediated upregulation of METTL3, which is caused by the abundant Histone H3 lysine 9 trimethylation (H3K9me3), ameliorates Alzheimer's disease via enhancing autophagic clearance of p-Tau through m6A-dependent regulation of E3 ubiquitin-protein ligase CHIP (STUB1). | ||||
| Responsed Disease | Alzheimer disease | ICD-11: 8A20 | |||
In-vitro Model |
SH-SY5Y | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| HT22 | Normal | Mus musculus | CVCL_0321 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Lysine-specific histone demethylase 1A (KDM1A) | 81 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| IMG-7289 | Phase 2 | [2] | ||
| Synonyms |
KQKBMHGOHXOHTD-KKUQBAQOSA-N; UNII-Y2T4ALDEAT; Y2T4ALDEAT; SCHEMBL17984236; Benzamide, N-((1S)-4-(((1R,2S)-2-(4-fluorophenyl)cyclopropyl)amino)-1-((4-methyl-1-piperazinyl)carbonyl)butyl)-4-(1H-1,2,3-triazol-1-yl)-; 1990504-34-1; N-[(2S)-1-(4-(methyl)piperazin-1-yl)-5-[[(1R,2S)-2-(4-fluorophenyl)-cyclopropyl]amino]-1-oxopentan-2-yl]-4-(1H-1,2,3-triazol-1-yl)benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Vafidemstat | Phase 2 | [3] | ||
| Synonyms |
(1R,2S)-2-(4-(Benzyloxy)phenyl)-N-((5-imino-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)cyclopropanamine; 1,3,4-Oxadiazole-2-methanamine, 4,5-dihydro-5-imino-N-((1R,2S)-2-(4-(phenylmethoxy)phenyl)cyclopropyl)-; 1,3,4-Oxadiazole-2-methanamine, 5-amino-N-((1R,2S)-2-(4-(phenylmethoxy)phenyl)cyclopropyl)-; 1357362-02-7; 5-((((1R,2S)-2-(4-(benzyloxy)phenyl)cyclopropyl)amino)methyl)-1,3,4-oxadiazol-2-amine; 5-(((trans)-2-(4-(benzyloxy)phenyl)cyclopropylamino)methyl)-1,3,4-oxadiazol-2-amine; 5-[[[(1R,2S)-2-(4-phenylmethoxyphenyl)cyclopropyl]amino]methyl]-1,3,4-oxadiazol-2-amine; A930244; AKOS040742807; BCP29383; BDBM50594947; CHEMBL4802155; CS-0058593; HY-112623; LZ82JLT4UP; MS-25100; ORY 2001; ORY2001; ORY-2001; ORY-2001; SCHEMBL528204; UNII-LZ82JLT4UP; Vafidemstat; Vafidemstat [INN]; XBBRLCXCBCZIOI-DLBZAZTESA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CC-90011 | Phase 2 | [4] | ||
| Synonyms |
UNII-W6F4FRQ5QC; W6F4FRQ5QC; CC90011; 1821307-10-1; CC-90011 besylate; 4-[2-(4-aminopiperidin-1-yl)-5-(3-fluoro-4-methoxyphenyl)-1-methyl-6-oxo-1,6-dihydropyrimidin-4-yl]-2-fluorobenzonitrile; Pulrodemstat; 4-(2-(4-Aminopiperidin-1-yl)-5-(3-fluoro-4-methoxyphenyl)-1-methyl-6-oxo-1,6-dihydropyrimidin-4-yl)-2-fluorobenzonitrile; Pulrodemstat [INN]; CC-90011 Free base; SCHEMBL17222702; GTPL11284; US10023543, Example 7; BDBM283216; US10023543, Example 85; US10023543, Example 86; NSC822744; NSC-822744; compound 11 [PMID: 33034194]; Q67009340; NC1CCN(CC1)C=1N(C(C(=C(N1)C1=CC(=C(C#N)C=C1)F)C1=CC(=C(C=C1)OC)F)=O)C; 4-(2-(4-Amino-piperidin-1-yl)-5-(3-fluoro-4-methoxyphenyl)-1-methyl-6-oxo-1,6-dihydropyrimidin-4-yl)-2-fluorobenzonitrile; 4-[2-(4-amino-piperidin-1-yl)-5- (3-fluoro-4-methoxy-phenyl)-1- methyl-6-oxo-1,6-dihydro- pyrimidin-4-yl]-2-fluoro- benzonitrile; Benzonitrile, 4-(2-(4-amino-1-piperidinyl)-5-(3-fluoro-4-methoxyphenyl)-1,6-dihydro-1-methyl-6-oxo-4-pyrimidinyl)-2-fluoro-; V0Y
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ORY-2001 | Phase 2 | [5] | ||
| Synonyms |
(1R,2S)-2-(4-(Benzyloxy)phenyl)-N-((5-imino-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)cyclopropanamine; 1,3,4-Oxadiazole-2-methanamine, 4,5-dihydro-5-imino-N-((1R,2S)-2-(4-(phenylmethoxy)phenyl)cyclopropyl)-; 1,3,4-Oxadiazole-2-methanamine, 5-amino-N-((1R,2S)-2-(4-(phenylmethoxy)phenyl)cyclopropyl)-; 1357362-02-7; 5-((((1R,2S)-2-(4-(benzyloxy)phenyl)cyclopropyl)amino)methyl)-1,3,4-oxadiazol-2-amine; 5-(((trans)-2-(4-(benzyloxy)phenyl)cyclopropylamino)methyl)-1,3,4-oxadiazol-2-amine; 5-[[[(1R,2S)-2-(4-phenylmethoxyphenyl)cyclopropyl]amino]methyl]-1,3,4-oxadiazol-2-amine; A930244; AKOS040742807; BCP29383; BDBM50594947; CHEMBL4802155; CS-0058593; HY-112623; LZ82JLT4UP; MS-25100; ORY 2001; ORY2001; ORY-2001; ORY-2001; SCHEMBL528204; UNII-LZ82JLT4UP; Vafidemstat; Vafidemstat [INN]; XBBRLCXCBCZIOI-DLBZAZTESA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| INCB59872 | Phase 1/2 | [2] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| CC-90011 | Phase 1 | [2] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| GSK2879552 | Phase 1 | [6] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 24 nM | |||
| External Link | ||||
| Seclidemstat | Phase 1 | [7] | ||
| Synonyms |
UNII-TYH386V3WJ; SP-2577; TYH386V3WJ; 1423715-37-0; SP2577; CHEMBL4297641; SCHEMBL14697017; SCHEMBL14697019; EX-A3574; s6722; BS-15371; HY-103713; CS-0039281; Benzoic acid, 3-((4-methyl-1-piperazinyl)sulfonyl)-, (2E)-2-(1-(5-chloro-2-hydroxyphenyl)ethylidene)hydrazide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| TAS-1440 | Phase 1 | [8] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27019002-Compound-16 | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2220 nM | |||
| External Link | ||||
| PMID27019002-Compound-41 | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 19 nM | |||
| External Link | ||||
| Benzenamine derivative 2 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-35
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27019002-Compound-28a | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| Aryl cyclopropylamine derivative 5 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-25c
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 352000 nM | |||
| External Link | ||||
| Pyrimidine derivative 17 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-39
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100 nM | |||
| External Link | ||||
| PMID27019002-Compound-31b | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 31380 nM | |||
| External Link | ||||
| Benzenamine derivative 3 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-35a
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclopropylamine derivative 1 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-23
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1 to 100 nM | |||
| External Link | ||||
| N6-cyclopropyllydine derivative 1 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-30
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 to 1000 nM | |||
| External Link | ||||
| Tarnylcypromine derivative 1 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-18
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 1300 nM | |||
| External Link | ||||
| Cyclopropylamine derivative 7 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-29e
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Aryl cyclopropylamine derivative 4 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-25b
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 456000 nM | |||
| External Link | ||||
| PMID27019002-Compound-45 | Patented | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27019002-Compound-13 | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5270 nM | |||
| External Link | ||||
| PMID27019002-Compound-49 | Patented | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclopropylamine derivative 4 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-29b
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27019002-Compound-42b | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 3000 nM | |||
| External Link | ||||
| PMID27019002-Compound-31a | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 39380 nM | |||
| External Link | ||||
| PMID27019002-Compound-43c | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 81 nM | |||
| External Link | ||||
| Aryl cyclopropylamine derivative 3 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-25a
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 173000 nM | |||
| External Link | ||||
| Benzenamine derivative 4 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-36
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclopropylamine derivative 6 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-29d
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Pyrimidine derivative 16 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-38
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27019002-Compound-28 | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| Heteroaryl-cyclopropylamine derivative 2 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-22a
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 1000 nM | |||
| External Link | ||||
| Cyclopropylamine derivative 5 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-29c
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27019002-Compound-43b | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 273 nM | |||
| External Link | ||||
| Pyrimidine derivative 18 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-40
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclopropylamine derivative 3 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-29a
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27019002-Compound-20b | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1 to 100 nM | |||
| External Link | ||||
| PMID27019002-Compound-50 | Patented | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclopropylamine derivative 8 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-32
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 500 nM | |||
| External Link | ||||
| PMID27019002-Compound-43a | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 90 nM | |||
| External Link | ||||
| Benzenamine derivative 1 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-14
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27019002-Compound-7 | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 59 nM | |||
| External Link | ||||
| PMID27019002-Compound-42a | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 3000 nM | |||
| External Link | ||||
| PMID27019002-Compound-37a | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 11160 nM | |||
| External Link | ||||
| PMID27019002-Compound-44 | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1190 nM | |||
| External Link | ||||
| PMID27019002-Compound-21a | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID27019002-Compound-37b | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 10540 nM | |||
| External Link | ||||
| Heteroaryl-cyclopropylamine derivative 4 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-22c
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| Cyclopropylamine derivative 11 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-33c
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 180 nM | |||
| External Link | ||||
| PMID25399762-Compound-Table 6-11 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 200 nM | |||
| External Link | ||||
| PMID27019002-Compound-20a | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1 to 100 nM | |||
| External Link | ||||
| Cyclopropylamine derivative 9 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-33a
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 230 nM | |||
| External Link | ||||
| PMID25399762-Compound-Table 6-13 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 30 nM | |||
| External Link | ||||
| Heteroaryl-cyclopropylamine derivative 3 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-22b
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| Cyclopropylamine derivative 13 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-33e
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100 nM | |||
| External Link | ||||
| PMID27019002-Compound-21b | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1 to 100 nM | |||
| External Link | ||||
| PMID27019002-Compound-48 | Patented | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID25399762-Compound-Table 6-10 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 100 nM | |||
| External Link | ||||
| PMID25399762-Compound-Table 6-12 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 200 nM | |||
| External Link | ||||
| PMID25399762-Compound-Table 6-9 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 20 nM | |||
| External Link | ||||
| PMID25399762-Compound-Table 6-14 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 100 nM | |||
| External Link | ||||
| PMID25399762-Compound-Table 6-15 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 50 nM | |||
| External Link | ||||
| Cyclopropylamine derivative 12 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-33d
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 210 nM | |||
| External Link | ||||
| PMID27019002-Compound-21c | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1 to 100 nM | |||
| External Link | ||||
| Cyclopropylamine derivative 10 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-33b
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 240 nM | |||
| External Link | ||||
| PMID27019002-Compound-47 | Patented | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27019002-Compound-17 | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 13 nM | |||
| External Link | ||||
| N-(2-phenylcyclopropyl) amino acid derivative 2 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-19a
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 18 nM | |||
| External Link | ||||
| PMID27019002-Compound-46 | Patented | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclic peptide derivative 1 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-compound11
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5000 nM | |||
| External Link | ||||
| Heteroaryl-cyclopropylamine derivative 1 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-22d
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 8 nM | |||
| External Link | ||||
| N-(2-phenylcyclopropyl) amino acid derivative 3 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-19b
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 22 nM | |||
| External Link | ||||
| N-(2-phenylcyclopropyl) amino acid derivative 1 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-19
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki < 1000 nM | |||
| External Link | ||||
| Tarnylcypromine derivative 2 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-26a-h
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1100 to 1900 nM | |||
| External Link | ||||
| Tarnylcypromine derivative 3 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-27a-m
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1300 to 40000 nM | |||
| External Link | ||||
| OG-L002 | Investigative | [11] | ||
| Synonyms |
1357302-64-7; 4'-((1R,2S)-2-aminocyclopropyl)-[1,1'-biphenyl]-3-ol; SCHEMBL6837351; GTPL7023; OGL002; AOB2070; MolPort-035-395-885; BDBM179446; BCP12278; EX-A2117; s7237; 2610AH; ZINC114026926; AKOS027422749; SB19352; BC600435; 4'-((1R,2S)-2-aminocyclopropyl)biphenyl-3-ol; 3-{4-[(1R,2S)-2-aminocyclopropyl]phenyl}phenol; J-006764; US9676701, 4 4'-((trans)-2-aminocyclopropyl)biphenyl-3-ol hydrochloride
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 20 nM | |||
| External Link | ||||
| GSK-LSD1 | Investigative | [12] | ||
| Synonyms |
GSK-LSD1 2HCl; 1431368-48-7; N-[(1R,2S)-2-phenylcyclopropyl]piperidin-4-amine; GSK LSD1 Dihydrochloride; GTPL8241; SCHEMBL14880683; BDBM256459; 1431368-48-7(free base); ZINC44675892; AKOS030573682; GSK-LSD1, > NCGC00356416-07; US9487512, 3
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 24 nM | |||
| External Link | ||||
| NCL-1 | Investigative | [13] | ||
| Synonyms |
GTPL7024; ZINC94568752
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8A20: Alzheimer disease | 498 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Rivastigmine | Approved | [14] | ||
| Synonyms |
Rivastigmine (transdermal patch)
Click to Show/Hide
|
|||
| External Link | ||||
| Propentofylline propionate | Approved | [15] | ||
| Synonyms |
Hextol (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Dihydroergotoxine | Approved | [16] | ||
| Synonyms |
Ergotamine, dihydro, methanesulfonate (salt)
Click to Show/Hide
|
|||
| External Link | ||||
| Galantamine | Approved | [17] | ||
| Synonyms |
GNT; Galantamin; Galantamina; Galantaminum; Galanthamine; Galanthaminum; Jilkon; Lycoremin; Lycoremine; Reminyl; Razadyne ER; Galantamina [INN-Spanish]; Galantamine [USAN:INN]; Galantaminum [INN-Latin]; Nivalin (TN); Razadyne (TN); Razadyne ER (TN); Reminyl (TN); Galantamine (USAN/INN); (-)-Galanthamine; (4aS,6R,8aS)-4a,5,9,10,11,12-Hexahydro-3-methoxy-11-methyl-6H-benzofuro(3a,3,2-ef)(2)benzazepin-6-ol
Click to Show/Hide
|
|||
| External Link | ||||
| Eicosapentaenoic acid/docosa-hexaenoic acid | Approved | [18] | ||
| Synonyms |
Docosahexaenoic acid; Doconexent; Cervonic acid; 6217-54-5; all-cis-DHA; Doconexentum; Doconexento; Doxonexent; (4Z,7Z,10Z,13Z,16Z,19Z)-docosa-4,7,10,13,16,19-hexaenoic acid; AquaGrow Advantage; all-Z-Docosahexaenoic acid; Martek DHA HM; Ropufa 60; cis-4,7,10,13,16,19-Docosahexaenoic acid; Docosahexaenoate; UNII-ZAD9OKH9JC; (4Z,7Z,10Z,13Z,16Z,19Z)-Docosahexaenoic acid; Docosahexaenoic acid (all-Z); CCRIS 7670; all-cis-4,7,10,13,16,19-Docosahexaenoic acid; ZAD9OKH9JC; all-cis-docosa-4,7,10,13,16,19-hexaenoic acid; CHEMBL367149; Espanova (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Donepezil | Approved | [19] | ||
| Synonyms |
Donepezil (transdermal patch, Alzheimer's disease)
Click to Show/Hide
|
|||
| External Link | ||||
| Thiazolidinedione | Approved | [20] | ||
| Synonyms |
2295-31-0; 1,3-Thiazolidine-2,4-dione; thiazolidine-2,4-dione; 2,4-Dioxothiazolidine; 2,4(3H,5H)-Thiazoledione; USAF EK-5496; Thiazolidindione; UNII-AA68LXK93C; Thiazolidinedione-2,4; NSC 6745; EINECS 218-941-2; BRN 0110700; AA68LXK93C; AI3-61185; CHEBI:50992; NSC6745; ZOBPZXTWZATXDG-UHFFFAOYSA-N; MFCD00005478; 2,4-Thiazolidinedione, 99%; C3H3NO2S; thiazolidine-dione; 2,4-thiazolidindione; 2,5H)-Thiazoledione; PubChem17487
Click to Show/Hide
|
|||
| External Link | ||||
| Rosiglitazone XR | Approved | [21] | ||
| Synonyms |
Avandia; Nyracta; Venvia; Rosiglitazone Maleate [USAN]; Rosiglitazone maleate; BRL 49653C; Avandia (TN); Avandiaadministration for 6-12 weeks; BRL 49653-C; BRL-49653C; SB-206846; SB-210232; Rosiglitazone maleate (JAN/USAN); (+-)-5-(p-(2-(Methyl-2-pyridylamino)ethoxy)benzyl)-2,4-thiazolidinedione maleate (1:1); (+-)-5-[[4-2-(methyl]-2-pyridinylamino)ethoxy]phenyl]methyl]-2,4-thiazolidinedione,(Z)-2-butenedioate (1:1); 2,4-Thiazolidinedione, 5-((4-(2-(methyl-2-pyridinylamino)ethoxy)phenyl)methyl)-, (2Z)-2-butenedioate (1:1); 2,4-Thiazolidinedione, 5-[[4-[2-(methyl-2-pyridinylamino) ethoxy]phenyl]methyl]-, (2Z)-2-butenedioate (1:1); 2,4-Thiazolidinedione,5-((4-(2-(methyl-2-pyridinylamino)ethoxy)phenyl)methyl)-,(2Z)-2-butenedioate; 5-[[4-[2-[methyl(pyridin-2-yl)amino]ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione; 5-{[4-({2-[methyl(pyridin-2-yl)amino]ethyl}oxy)phenyl]methyl}-1,3-thiazolidine-2,4-dione (2Z)-but-2-enedioate
Click to Show/Hide
|
|||
| External Link | ||||
| GV-971 | Approved in China | [22] | ||
| External Link | ||||
| Tacrine | Approved | [23] | ||
| Synonyms |
Cognex; Romotal; Tacrina; Tacrinal; Tacrinum; Tenakrin; Tetrahydroaminacrine; Tetrahydroaminoacridine; Tetrahydroaminocrin; Tetrahydroaminocrine; Tha; Tacrine hydrochloride; BBL001044; CS 12602; Cognex (TN); Tacrina [INN-Spanish]; Tacrinal (TN); Tacrine (INN); Tacrine [INN:BAN]; Tacrinum [INN-Latin]; 1,2,3,4-TETRAHYDRO-9-ACRIDINAMINE; 1,2,3,4-TETRAHYDRO-9-ACRIDINAMINE (SEE ALSO 1684-40-8); 1,2,3,4-Tetrahydro-9-acridineamine; 1,2,3,4-Tetrahydro-acridin-9-ylamine; 1,2,3,4-Tetrahydroaminoacridine; 1,2,3,4-tetrahydroacridin-9-amine; 5-Amino-6,7,8,9-tetrahydroacridine (European); 9-AMINOTETRAHYDROACRIDINE; 9-Acridinamine, 1,2,3,4-tetrahydro-(9CI); 9-Amino-1,2,3,4-Tetrahydroacridine Hydrate Hydrochloride Hydrate; 9-amino-1,2,3,4-tetrahydroacridine
Click to Show/Hide
|
|||
| External Link | ||||
| Lemborexant | Phase 2 | [24] | ||
| Synonyms |
E2006
Click to Show/Hide
|
|||
| External Link | ||||
| Pexidartinib | Approved | [25] | ||
| Synonyms |
PLX-3397
Click to Show/Hide
|
|||
| External Link | ||||
| F18-florbetaben | Approved | [26] | ||
| External Link | ||||
| Colostrinin | Approved | [15] | ||
| Synonyms |
Colostrinin (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Huperzine A | Approved | [15] | ||
| Synonyms |
Fordine; ( inverted exclamation markA)-Huperazine A; ( inverted exclamation markA)-Huperzine A; C15H18N2O; 5,9-Methanocycloocta(b)pyridin-2(1H)-one, 5-amino-11-ethylidene-5,6,9,10-tetrahydro-7-methyl-; rac-huperzine A; NCGC00159362-02; SCHEMBL679315; SCHEMBL1047469; CHEMBL394259; CHEBI:91724; MolPort-003-941-680
Click to Show/Hide
|
|||
| External Link | ||||
| Lecanemab | Approved | [27] | ||
| External Link | ||||
| F18-flutemetamol | Approved | [28] | ||
| External Link | ||||
| Ergoloid mesylate | Approved | [29] | ||
| Synonyms |
Alkergot; Circanol; Gerimal; Hydergin; Ischelium; Redergin; Trigot; Dihydroergotoxin Mesilat; Dihydroergotoxinmesylate; Dihydroergotoxin methanesulfonate; Dihydroergotoxine methanesulfonate; Dihydroergotoxine methanesulphonate; Ergoloid Mesylates [USAN]; Hydergine LC; Hydrogenated Ergot Alkaloids; Alkergot (TN); Cicanol (TN); Deapril-ST; Gerimal (TN); Hydergina (TN); Hydergine (TN); Niloric (TN); Redergin (TN); Redizork (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Immune globulin | Approved | [30] | ||
| External Link | ||||
| Memantine | Approved | [31] | ||
| Synonyms |
Memantine ER; Namenda XR; Memantine (extended release); Memantine (extended release), Forest
Click to Show/Hide
|
|||
| External Link | ||||
| Aducanumab | Approved | [32] | ||
| External Link | ||||
| Nirogacestat | Phase 2 | [33] | ||
| Synonyms |
Nirogacestat; 1290543-63-3; PF-3084014; UNII-QZ62892OFJ; 865773-15-5; PF 3084014; PF-03084014 (PF-3084014); QZ62892OFJ; Z-3181; PF03084014; Nirogacestat [USAN]; Nirogacestat (USAN/INN); GTPL7746; SCHEMBL13184754; CHEMBL1770916; EX-A855; DTXSID60235679; MolPort-039-193-852; (2S)-2-[[(2S)-6,8-difluoro-1,2,3,4-tetrahydronaphthalen-2-yl]amino]-N-[1-[1-(2,2-dimethylpropylamino)-2-methylpropan-2-yl]imidazol-4-yl]pentanamide; ZINC38217837; s8018; AKOS030526383; SB16726; DB12005; CS-1689; NCGC00378713-01
Click to Show/Hide
|
|||
| External Link | ||||
| 18F-AV-1451 | Phase 4 | [34] | ||
| Synonyms |
Tau imaging agent)
Click to Show/Hide
|
|||
| External Link | ||||
| NE3107 | Phase 3 | [35] | ||
| External Link | ||||
| Guanfacine | Phase 3 | [36] | ||
| Synonyms |
(2,6-dichlorophenylacetyl)-guanidine; [(2,6-dichlorophenyl)acetyl]guanidine; [(2,6-Dichlorophenyl)acetyl]guanidine hydrochloride; 29110-47-2; 30OMY4G3MK; A902647; A918619; AB01563079_01; AKOS030255657; AKOS030526130; BCP09647; BDBM81984; Benzeneacetamide, N-(aminoiminomethyl)-2,6-dichloro-; BPBio1_000415; BRD-K32830106-003-03-0; BRD-K32830106-003-11-3; BSPBio_000377; C02AC02; C07037; CAS_29110-47-2; CAS-29110-48-3; CCG-204609; CHEBI:5558; CHEMBL862; D08031; DB01018; DTXCID7026944; DTXSID9046944; EINECS 249-442-8; EN300-243924; Estulic; Estulic (Salt/Mix); Estulic (TN); FT-0669067; FT-0669068; GTPL522; Guanfacina; Guanfacina (INN-Spanish); Guanfacina [INN-Spanish]; GUANFACINE; Guanfacine (INN); Guanfacine [INN:BAN]; GUANFACINE [INN]; GUANFACINE [MI]; GUANFACINE [VANDF]; GUANFACINE [WHO-DD]; Guanfacine HCl; Guanfacine hydrochloride (Salt/Mix); Guanfacinum; Guanfacinum (INN-Latin); Guanfacinum [INN-Latin]; HY-17416A; J-017394; L000286; L013430; LON 798 (Salt/Mix); Lopac0_000519; Lopac-G-1043; MRF-0000019; N-(Diaminomethyliden)-2-(2,6-dichlorophenyl)acetamid; N-(diaminomethylidene)-2-(2,6-dichlorophenyl)acetamide; N-amidino-2-(2,6-dichlorophenyl)acetamide; N-carbamimidoyl-2-(2,6-dichlorophenyl)acetamide; NCGC00015469-01; NCGC00015469-02; NCGC00015469-03; NCGC00015469-04; NCGC00015469-05; NCGC00015469-06; NCGC00015469-07; NCGC00024950-01; NCGC00024950-02; NCGC00024950-03; NSC 759121; NSC_3519; NSC-759121; Prestwick0_000339; Prestwick1_000339; Prestwick2_000339; Prestwick3_000339; Q5613599; RASPBERRYKETONEGLUCOSIDE; SCHEMBL35094; SDCCGSBI-0050502.P002; SPBio_002298; Tenex (Salt/Mix); Tocris-1030; UNII-30OMY4G3MK
Click to Show/Hide
|
|||
| External Link | ||||
| BPDO-1603 | Phase 3 | [37] | ||
| External Link | ||||
| TRx0237 | Phase 3 | [38] | ||
| Synonyms |
951131-15-0; UNII-E79ZM68IOZ; E79ZM68IOZ; Leucomethylene Blue dihydrobromide; TRX0237 dihydrobromide; TRX 0237 dihydrobromide; TRX-0237 dihydrobromide; TRx0237(LMTX); TRX-0237 2HBr; Leukomethylene Blue dihydrobromide; Hydromethylthionine HBr(TRX0237); BCP24159; EX-A4299; Reduced methylene Blue dihydrobromide; N3,N3,N7,N7-Tetramethyl-10H-phenothiazine-3,7-diamine dihydrobromide; Leucomethylene Blue 2HBr;TRX0237 dihydrobromide;TRX 0237 dihydrobromide;TRX-0237 dihydrobromide
Click to Show/Hide
|
|||
| External Link | ||||
| AXS-05 | Phase 2/3 | [39] | ||
| External Link | ||||
| Remternetug | Phase 3 | [40] | ||
| Synonyms |
LY3372993
Click to Show/Hide
|
|||
| External Link | ||||
| Donanemab | Phase 3 | [41] | ||
| External Link | ||||
| AR1001 | Phase 3 | [42] | ||
| Synonyms |
(-)-methylbenzylamine; (+)-2-Methylbutylp-aminocinnamate; 103-67-3; 7KN7F4X49E; AI3-26793; AKOS000119094; AR1001; Benzenemethanamine, N-methyl-; benzyl methyl amine; benzyl methylamine; Benzyl(methyl)amine; BENZYLAMINE, N-METHYL; Benzylamine, N-methyl-; Benzylmethylamine; benzyl-methylamine; Benzyl-methyl-amine; Benzylmethyl-d3-amine; BRD-K44558320-003-01-7; CHEMBL1338; CS-W007426; DTXSID9048439; EC 203-133-4; EINECS 203-133-4; EN300-18191; F2190-0316; FT-0631560; HNMeBzl; HY-W007426; InChI=1/C8H11N/c1-9-7-8-5-3-2-4-6-8/h2-6,9H,7H2,1H; KBio2_001842; KBio2_004410; KBio2_006978; KBioGR_002247; KBioSS_001842; M0164; methyl benzylamine; Methyl(phenylmethyl)amine; methyl-benzyl amine; Methylbenzylamine; Methylbenzylamine, N-; methylbezylamine; MFCD00008289; MLS004773900; N-(Phenylmethyl)methylamine; N-benzyl methyl amine; N-benzyl methylamine; N-benzylmethanamine; N-benzyl-methyl amine; N-Benzylmethylamine; N-benzyl-methylamine; N-Benzylmethylamine, 97%; N-Benzyl-N-methylamine; N-benzyl-N-methyl-amine; NCGC00166047-01; N-methyl benzyl amine; N-methyl benzylamine; N-methyl -benzylamine; N-Methyl(phenyl)methanamine; N-methyl-1-phenylmethanamine; N-methyl-1-phenyl-methanamine; N-methylbenzenemethanamine; N-methyl-benzenemethanamine; N-methylbenzyl amine; N-methyl-benzyl amine; N-METHYLBENZYLAMINE; N-methyl-benzylamine; N-methyl-N-(phenylmethyl)amine; N-methyl-N-benzyl amine; N-methyl-N-benzylamine; N-Methy-N-benzylamine; NSC 8059; NSC8059; NSC-8059; omega-Methylaminotoluene; Q23978278; Racemic methylbenzyl amine; SCHEMBL2271; SDCCGMLS-0066901.P001; SMR000112361; Spectrum_001362; Spectrum4_001764; Spectrum5_000347; STR02536; UNII-7KN7F4X49E; W-108843; Z57327124
Click to Show/Hide
|
|||
| External Link | ||||
| GV-971 | Phase 3 | [43] | ||
| Synonyms |
sodium oligomannate
Click to Show/Hide
|
|||
| External Link | ||||
| AVP-786 | Phase 3 | [39] | ||
| External Link | ||||
| NI-101 | Phase 3 | [44] | ||
| Synonyms |
BART; Ch12F6A; MAb (AD), Neurimmune/Biogen Idec; Monoclonal antibodies (Alzheimer! s disease), Neurimmune Therapeutics/Biogen Idec; Anti-beta amyloid mAbs (Alzheimer! s disease), Neurimmune Therapeutics/Biogen Idec
Click to Show/Hide
|
|||
| External Link | ||||
| AC-1204 | Phase 3 | [24] | ||
| Synonyms |
isoindoline hydrochloride; 32372-82-0; 2,3-Dihydroisoindole hydrochloride; 2,3-dihydro-1H-isoindole hydrochloride; 2,3-Dihydro-1H-isoindole HCl; Isoindoline HCl salt; 1H-Isoindole, 2,3-dihydro-, hydrochloride; Isoindoline hydrochloride, 97%; Isoindolinehydrochloride; Isoindoline, HCl; ISOINDOLINE HCL; AC1Q38WR; dihydroisoindole hydrochloride; KSC491I3F; AMBZ0192; SCHEMBL4702076; CTK3J1432; DTXSID50487241; MolPort-003-986-749; NOVIRODZMIZUPA-UHFFFAOYSA-N; BH168; CS-D1516; ACT08858; ACN-S003258; KS-000001RA
Click to Show/Hide
|
|||
| External Link | ||||
| Lanabecestat | Phase 3 | [24] | ||
| Synonyms |
1383982-64-6; UNII-X8SPJ492VF; X8SPJ492VF; LY3314814; Lanabecestat [USAN]; Lanabecestat (USAN); SCHEMBL9947930; GTPL7789; SCHEMBL9947926; CHEMBL3261045; SCHEMBL10249890; CHEMBL3989948; CHEMBL3349234; BDBM41542; BDBM41537; MolPort-044-560-403; BDBM136733; EX-A1471; s8193; ZINC95576075; BDBM50012629; US8865911, 20a Isomer 1; CS-7494; Lanabecestat(AZD3293,LY-3314814); HY-100740; LY 3314814; D10946; US8865911, 122; US8865911, 114; 4-methoxy-5'-methyl-6'-(5-(prop-1-yn-1-yl)pyridin-3-yl)-3'H-dispiro(cyclohexane
Click to Show/Hide
|
|||
| External Link | ||||
| Davunetide | Phase 3 | [45] | ||
| Synonyms |
NAP; AL-108; AL-208; Davunetide (intranasal spray), Allon; NAPVSIPQ eight amino acid peptide (intranasal spray), Allon; Davunetide (intravenous-infused), Allon Therapeutics; NAP eight amino acid peptide (neuroprotection/cognitive impairment), Allon; NAPVSIPQ eight amino acid peptide (neuroprotection/cognitive impairment), Allon; Central nervous system therapeutic (Alzheimer's disease/schizophrenia), Allon; Central nervous system therapeutic (post-cardiac artery bypass graft/mild cognitive impairment), Allon; NAPVSIPQ eight amino acid peptide (intravenous-infused/subcutaneous depot formulation), Allon Therapeutics; Davunetide (iv/sc, Alzheimer's disease), Allon Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| SB-742457 | Phase 3 | [46] | ||
| Synonyms |
GSK 742457; SB 742457; GSK-742457; 3-(benzenesulfonyl)-8-piperazin-1-yl-quinoline
Click to Show/Hide
|
|||
| External Link | ||||
| Crenezumab | Phase 3 | [24] | ||
| Synonyms |
MABT5102A; RG7412
Click to Show/Hide
|
|||
| External Link | ||||
| verubecestat | Phase 3 | [47] | ||
| Synonyms |
example 25 (US8940748)
Click to Show/Hide
|
|||
| External Link | ||||
| Gantenerumab | Phase 3 | [48] | ||
| Synonyms |
RG1450
Click to Show/Hide
|
|||
| External Link | ||||
| PF-4494700 | Phase 3 | [24] | ||
| Synonyms |
PF-04494700; TTP-488; TTP-488); Alzheimers therapy, TransTech/Pfizer; Diabetic nephropathy therapy, Transtech/Pfizer; Alzheimer'streatment, TransTech/Pfizer; Alzheimer's therapy (RAGE), Transtech/Pfizer
Click to Show/Hide
|
|||
| External Link | ||||
| acelarin | Phase 2 | [49] | ||
| Synonyms |
NUC-1031
Click to Show/Hide
|
|||
| External Link | ||||
| AMG 386 | Phase 3 | [50] | ||
| Synonyms |
7440-23-5; Natrium; Sodium-23; Sodio; Sodium metal; Sodio [Spanish]; Sodium (liquid alloy); UNII-9NEZ333N27; HSDB 687; EINECS 231-132-9; UN1428; UNII-23J3BHR95O; Sodium, dry stick; 9NEZ333N27; MFCD00085307; Sodium, 998%, oiled sticks, wrapped in aluminium foil; monosodium; sodium atom; mono sodium; mono-sodium; Sodium, CP; SodiuR4733m hydride, CP; AC1NSENP; ACMC-1BKTZ; Sodium, ACS reagent, dry; 11Na; EC 231-132-9; AC1Q1W6R; SODIUM, LARGE PIECES; Sodium hydride, dry, 95%; NAH 80; HSDB 745; CTK2H7876; CHEBI:26708; Oravescent fentanyl; R3487; PT-15; R4930
Click to Show/Hide
|
|||
| External Link | ||||
| SI-657 | Phase 3 | [51] | ||
| Synonyms |
AC1L9R2T; 6-[3-acetamido-2-[6-[3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid
Click to Show/Hide
|
|||
| External Link | ||||
| EVP-6124 | Phase 3 | [52] | ||
| Synonyms |
Encenicline; 550999-75-2; UNII-5FI5376A0X; EVP6124; EVP 6124; CHEMBL2151572; (r)-7-chloro-n-(quinuclidin-3-yl)benzo[b]thiophene-2-carboxamide; 5FI5376A0X; C16H17ClN2OS; 550999-74-1; Encenicline [USAN:INN]; FRM-6124; Encenicline (USAN/INN); SCHEMBL744767; GTPL6926; SSRDSYXGYPJKRR-ZDUSSCGKSA-N; ZINC95579362; BDBM50393255; 3662AH; AKOS027322165; DB11726; CS-0933; MT-4666; Benzo(b)thiophene-2-carboxamide, N-(3R)-1-azabicyclo(2.2.2)oct-3-yl-7-chloro-; NCGC00378871-01; HY-15430; W-5978; D10626
Click to Show/Hide
|
|||
| External Link | ||||
| ALZT-OP1 | Phase 3 | [24] | ||
| Synonyms |
ALZT-OP1a+ALZT-OP1b; cromolyn + ibuprofen
Click to Show/Hide
|
|||
| External Link | ||||
| MIM-D3 | Phase 3 | [53] | ||
| Synonyms |
TrkA agonist (ocular disease/Alzheimer's disease), Mimetogen
Click to Show/Hide
|
|||
| External Link | ||||
| LCS 16 | Phase 3 | [54] | ||
| External Link | ||||
| Bapineuzumab | Phase 3 | [55] | ||
| Synonyms |
AAB-001; Monoclonal antibody (Alzheimer's disease), Elan/Pfizer; Monoclonal antibody (Alzheimer's disease), Elan/Wyeth; Monoclonal antibody (Alzheimer's disease), Wyeth/Elan
Click to Show/Hide
|
|||
| External Link | ||||
| Tirapazamine | Phase 3 | [56] | ||
| Synonyms |
TPZ; Tirazone; SR-259075; SR-4233; SR-4317; SR-4330; SR-4482; Win-59075
Click to Show/Hide
|
|||
| External Link | ||||
| AV 133 | Phase 3 | [57] | ||
| External Link | ||||
| Tramiprosate | Phase 3 | [58] | ||
| Synonyms |
Alzhemed; Cerebril; Homotaurine; Vivimind; LU-02659; NC-531; NC-758; Tramiprosate (stroke), Neurochem; Tramiprosate (Alzheimer's disease), Neurochem; 3APS
Click to Show/Hide
|
|||
| External Link | ||||
| E-2609 | Phase 3 | [24] | ||
| Synonyms |
Beta secretase inhibitor (Alzheimer's disease), Eisai
Click to Show/Hide
|
|||
| External Link | ||||
| (-)-Phenserine | Phase 3 | [59] | ||
| Synonyms |
CHEMBL54727; SCHEMBL5464366; BDBM10622; (3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo[2,3-b]indol-5-yl N-phenylcarbamate
Click to Show/Hide
|
|||
| External Link | ||||
| ARC029 | Phase 3 | [60] | ||
| Synonyms |
Nilvadipine; 75530-68-6; Escor; Nivadil; Nivadipine; FR-34235; Nilvadipinum [Latin]; Nilvadipino [Spanish]; FR 34235; FK 235; Nilvadipine [USAN:INN:JAN]; Nilvadipine (ARC029); FK-235; F-102362; BRN 3572609; F 102,362; CL-287389; CL 287,389; FAIIFDPAEUKBEP-UHFFFAOYSA-N; C19H19N3O6; NCGC00167435-01; 5-Isopropyl 3-methyl 2-cyano-1,4-dihydro-6-methyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate; 3,5-Pyridinedicarboxylic acid, 2-cyano-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-, 3-methyl 5-(1-methylethyl) ester; Nilvadipino
Click to Show/Hide
|
|||
| External Link | ||||
| Eltoprazine | Phase 2 | [24] | ||
| Synonyms |
Piperazine derivative (ADHD), PsychoGenics/ReqMed Company; 5-HT1A/5-HT1B agonist and 5-HT2C antagonist (ADHD), PsychoGenics/ReqMed Company
Click to Show/Hide
|
|||
| External Link | ||||
| LMT-X | Phase 3 | [61] | ||
| Synonyms |
Second-generation tau aggregation inhibitor (Alzheimer's disease); Second-generation tau aggregation inhibitor (Alzheimer's disease), TauRx
Click to Show/Hide
|
|||
| External Link | ||||
| INM-176 | Phase 3 | [62] | ||
| Synonyms |
WIN-026; Win-025; Alzheimer's therapeutic, Whanin Pharmaceuticals; KR-WAP-026; Anti-beta amyloid/AChE inhibitor (Alzheimer's), WhanIn; Anti-beta amyloid/acetylcholinesterase inhibitor (Alzheimer's), WhanIn
Click to Show/Hide
|
|||
| External Link | ||||
| Xaliproden | Phase 3 | [63] | ||
| Synonyms |
Xaliproden (USAN); 1,2,3,6-tetrahydro-1-(2-(2-naphthalenyl)ethyl)-4-(3-(trifluoromethyl)phenyl)-pyridine; 1,2,3,6-tetrahydro-1-(2-(2-naphthyl)ethyl)-4-(alpha,alpha,alpha-trifluoro-m-tolyl)pyridine; 1-(2-(2-Naphthyl)ethyl)-4-(3-trifluoromethylphenyl)-1,2,3,6-tetrahydropyridine; 1-(2-naphthalen-2-ylethyl)-4-[3-(trifluoromethyl)phenyl]-3,6-dihydro-2H-pyridine; 1-[2-(2-naphthyl)ethyl]-4-[3-(trifluoromethyl)phenyl]-1,2,3,6-tetrahydropyridine
Click to Show/Hide
|
|||
| External Link | ||||
| Tamibarotene | Phase 3 | [64] | ||
| Synonyms |
Tamibarotene (oral, Alzheimer's disease)
Click to Show/Hide
|
|||
| External Link | ||||
| Immune globulin + albumin | Phase 3 | [24] | ||
| External Link | ||||
| Solanezumab | Phase 3 | [65] | ||
| External Link | ||||
| E2814 | Phase 2/3 | [66] | ||
| External Link | ||||
| CAD106 | Phase 2/3 | [67] | ||
| External Link | ||||
| Icosapent ethyl | Phase 2/3 | [68] | ||
| Synonyms |
(5Z,8Z,11Z,14Z,17Z)-Eicosapentaenoic acid ethyl ester; (5Z,8Z,11Z,14Z,17Z)-Eicosapetaenoic acid ethyl ester; (all-Z)-5,8,11,14,17-Eicosapentaenoic acid ethyl ester; 5,8,11,14,17-eicosapentaenoic acid, ethyl ester, (5Z,8Z,11Z,14Z,17Z)-; 5,8,11,14,17-Eicosapentaenoic acid, ethyl ester, (all-Z)-; 5-8-11-14-17-all cis-eicosapentaenoic acid ethyl ester; 5Z,8Z,11Z,14Z,17Z-Eicosapentaenoic acid, ethyl ester; 6GC8A4PAYH; 73310-10-8; 86227-47-6; 86227-47-6 for all ''Z'' compound, 73310-10-8 for ''undefined'' compound; AB01563352_01; AC-33765; AKOS025295847; all cis-5,8,11,14,17-Eicosapentaenoic Acid Ethyl Ester; all-cis-ethyl 5,8,11,14,17-icosapentaenoate; AMR 101; AMR101; AMR-101; BS-48985; CCG-213714; CHEBI:80366; CHEBI:84883; CHEMBL2095209; cis-5,8,11,14,17-Eicosapentaenoic acid ethyl ester; cis-5,8,11,14,17-Eicosapentaenoic acidethyl ester; cis-Eicosapentaenoic acid ethyl ester; CS-5304; D01892; DB08887; DTXSID601018686; E0442; E0853; E-EPA; Eicosapentaenoic acid (ethyl ester); Eicosapentaenoic acid ethyl ester; EICOSAPENTAENOIC ACID ETHYL ESTER (MART.); EICOSAPENTAENOIC ACID ETHYL ESTER (USP-RS); EICOSAPENTAENOIC ACID ETHYL ESTER [MART.]; EICOSAPENTAENOIC ACID ETHYL ESTER [MI]; EICOSAPENTAENOIC ACID ETHYL ESTER [USP-RS]; EICOSAPENTAENOIC ACID ETHYL ESTER [WHO-DD]; Eicosapentaenoic acid, ethyl ester; Eicosapentaenoicacidethylester; EICOSAPENTAENOICACIDETHYLESTER(EPAEE)(SG); EN300-25951782; EPA ethyl ester; Epadel; Epadel S; Epadel S (TN); EPA-E; Ethyl (5Z,8Z,11Z,14Z,17Z)-eicosa-5,8,11,14,17-pentaenoate; ethyl (5Z,8Z,11Z,14Z,17Z)-eicosapentaenoate; ethyl (5Z,8Z,11Z,14Z,17Z)-icosa-5,8,11,14,17-pentaenoate; ethyl (5Z,8Z,11Z,14Z,17Z)-icosapentaenoate; Ethyl (all cis)-5,8,11,14,17-icosapentaenoate; Ethyl all cis-5,8,11,14,17-Eicosapentaenoate; Ethyl all cis-5,8,11,14,17-Icosapentaenoate; ethyl all-cis-5,8,11,14,17-icosapentaenoate; Ethyl all-cis-5,8,11,14,17-icosapentaenoic acid; ethyl eicosapentaenoate; Ethyl eicosapentaenoic acid; Ethyl EPA; Ethyl ester(all-Z)-5,8,11,14,17-Eicosapentaenoic acid; Ethyl icosapent; ethyl icosapentaenoate; ethyl icosapentate; Ethyl icosapentate (JP17); Ethyl icosapentate [JAN]; Ethyl icosapentate, JAN; ethyl-eicosapentaenoic acid; ethyl-EPA; GTPL7441; HMS2094K19; HY-B0747; ICOSAPENT ETHYL; Icosapent ethyl (USAN); ICOSAPENT ETHYL [ORANGE BOOK]; Icosapent ethyl [USAN]; ICOSAPENT ETHYL [VANDF]; Icosapent ethyl ester; LMFA07010877; MFCD00673476; MND 21; NSC 759597; NSC759597; NSC-759597; Pharmakon1600-01300030; Q5404453; s6466; SBI-0206684.P002; SCHEMBL123305; SR-05000002595; SR-05000002595-1; Timnodonic acid ethyl ester; UNII-6GC8A4PAYH; Vascepa; Vascepa (TN); Vazkepa
Click to Show/Hide
|
|||
| External Link | ||||
| Fosgonimeton | Phase 2/3 | [69] | ||
| Synonyms |
2093305-05-4; AKOS040757261; ATH-1017; ATH-1017 FREE ACID; CHEMBL5095419; CS-0204081; dihydrogen 4-[(2S)-3-({(2S,3S)-1-[(6-amino-6-oxohexyl)amino]-3-methyl-1-oxopentan-2-yl}amino)-2-hexanamido-3-oxopropyl]phenyl phosphate; Fosgonimeton; Fosgonimeton [INN]; Fosgonimeton [USAN:INN]; Fosgonimeton [USAN]; H91OA9858J; HY-132814; L-Isoleucinamide, O-(phosphono-kappaO)-N-(1-oxohexyl)-L-tyrosyl-N-(6-amino-6-oxohexyl)-; L-Isoleucinamide, O-(phosphono-kappaO)-N-(1-oxohexyl)-L-tyrosyl-N-(6-amino-6-oxohexyl)-,; NDX-1017; NDX-1017 FREE ACID; UNII-H91OA9858J; WHO 11782
Click to Show/Hide
|
|||
| External Link | ||||
| Ginkgo biloba | Phase 2/3 | [70] | ||
| External Link | ||||
| Atuzaginstat | Phase 2/3 | [71] | ||
| Synonyms |
2211981-76-7; Atuzaginstat; Atuzaginstat [INN]; AtuzaginstatCOR388; BDBM453275; CHEMBL5095230; COR388; COR-388; Cyclopentanecarboxamide, N-((1S)-5-amino-1-(2-(2,3,6-trifluorophenoxy)acetyl)pentyl)-; DGN7ROZ8EN; EX-A6081; N-((3S)-7-Amino-2-oxo-1-(2,3,6- trifluorophenoxy)heptan-3-yl)cyclopentanecarboxamide; N-[(3S)-7-amino-2-oxo-1-(2,3,6-trifluorophenoxy)heptan-3-yl]cyclopentanecarboxamide; SCHEMBL19972758; UNII-DGN7ROZ8EN; US10730826, Compound 1a-non-racemic; US10730826, Compound 1a-racemic
Click to Show/Hide
|
|||
| External Link | ||||
| JNJ-54861911 | Phase 2/3 | [72] | ||
| External Link | ||||
| Plasminogen | Phase 2/3 | [73] | ||
| External Link | ||||
| CAD-106 | Phase 2/3 | [74] | ||
| Synonyms |
Amilomotide; Alzheimers disease vaccine, Cytos/Novartis; Immunodrug vaccines (Alzheimers disease), Cytos/Novartis; Beta amyloid 1-6 peptide/Qbeta virus-like particle conjugate (Alzheimer's disease), Cytos/Novartis
Click to Show/Hide
|
|||
| External Link | ||||
| AMG520 | Phase 2/3 | [47] | ||
| Synonyms |
CNP520
Click to Show/Hide
|
|||
| External Link | ||||
| SI-614 | Phase 2/3 | [75] | ||
| Synonyms |
Modified Hyaluronate
Click to Show/Hide
|
|||
| External Link | ||||
| AZD3293 | Phase 2/3 | [76] | ||
| Synonyms |
CHEMBL2152914; SCHEMBL9948518; SCHEMBL9948271; SCHEMBL18562845; BDBM50393099
Click to Show/Hide
|
|||
| External Link | ||||
| ABvac40 | Phase 2 | [77] | ||
| External Link | ||||
| AL002 | Phase 2 | [78] | ||
| External Link | ||||
| MLC901 | Phase 2 | [79] | ||
| Synonyms |
NeuroAiD
Click to Show/Hide
|
|||
| External Link | ||||
| ABBV-916 | Phase 2 | [80] | ||
| External Link | ||||
| TB006 | Phase 2 | [81] | ||
| External Link | ||||
| LY3372689 | Phase 2 | [82] | ||
| Synonyms |
2241514-56-5; Acetamide, N-(4-fluoro-5-(((2S,4S)-2-methyl-4-((5-methyl-1,2,4-oxadiazol-3-yl)methoxy)-1-piperidinyl)methyl)-2-thiazolyl)-; CHEMBL5095251; CS-0433932; EX-A6549; GLXC-25705; GTPL11953; HY-144681; LY3372689; LY-3372689; MS-26295; N-(4-Fluoro-5-(((2S,4S)-2-methyl-4-((5-methyl-1,2,4-oxadiazol-3-yl)methoxy)piperidin-1-yl)methyl)thiazol-2-yl)acetamide; N-[4-fluoro-5-[[(2S,4S)-2-methyl-4-[(5-methyl-1,2,4-oxadiazol-3-yl)methoxy]piperidin-1-yl]methyl]-1,3-thiazol-2-yl]acetamide; SCHEMBL20421995; U0SGP6ZX2V; UNII-U0SGP6ZX2V
Click to Show/Hide
|
|||
| External Link | ||||
| MP-101 | Phase 2 | [83] | ||
| External Link | ||||
| DHP1401 | Phase 2 | [84] | ||
| External Link | ||||
| CY6463 | Phase 2 | [85] | ||
| Synonyms |
2201048-82-8; 8-(2-Fluorobenzyl)-6-(3-(trifluoromethyl)-1H-1,2,4-triazol-5-yl)imidazo(1,2-a)pyrazine; 8-[(2-fluorophenyl)methyl]-6-[5-(trifluoromethyl)-1H-1,2,4-triazol-3-yl]imidazo[1,2-a]pyrazine; 8-[(2-fluorophenyl)methyl]-6-[5-(trifluoromethyl)-4H-1,2,4-triazol-3-yl]imidazo[1,2-a]pyrazine; AKOS040757400; CS-0376702; CY6463; CY-6463; EX-A7620; GTKNNCQKFKGSHR-UHFFFAOYSA-N; H7KEN3O8AI; HY-145607; Imidazo(1,2-a)pyrazine, 8-((2-fluorophenyl)methyl)-6-(5-(trifluoromethyl)-1H-1,2,4-triazol-3-yl)-; Imidazo[1,2-a]pyrazine, 8-[(2-fluorophenyl)methyl]-6-[5-(trifluoromethyl)-1H-1,2,4-triazol-3-yl]-; IW-6463; MS-25746; SCHEMBL19922804; UNII-H7KEN3O8AI; Zagociguat; Zagociguat [INN]; ZAGOCIGUAT [USAN]
Click to Show/Hide
|
|||
| External Link | ||||
| ID1201 | Phase 2 | [86] | ||
| External Link | ||||
| Benfotiamine | Phase 2 | [87] | ||
| Synonyms |
(3Z)-4-{N-[(4-amino-2-methylpyrimidin-5-yl)methyl]carbonylamino}-3-(phenylcarb onylthio)pent-3-enyl dihydrogen phosphate; (Z)-S-(2-(N-((4-amino-2-methylpyrimidin-5-yl)methyl)formamido)-5-(phosphonooxy)pent-2-en-3-yl) benzothioate; {[(3Z)-4-{N-[(4-amino-2-methylpyrimidin-5-yl)methyl]formamido}-3-[(Z)-benzoylsulfanyl]pent-3-en-1-yl]oxy}phosphonic acid; 22457-89-2; 22457-89-2 (free acid); 775256-41-2; AC-8280; AKOS015920320; Benfotamine; Benfotiamina; benfotiamine; Benfotiamine (JAN/INN); Benfotiaminum; Benphothiamine; Benzoylthiamine monophosphate; Betivina; Biotamin (TN); BPBio1_000757; BSPBio_000687; CAS-22457-89-2; CCG-220654; CHEBI:41039; CHEMBL4303665; D01255; DB11748; DTXCID1025433; DTXSID3045433; EN300-21694383; HMS1570C09; HMS2097C09; HMS3714C09; MFCD00057343; N-((4-Amino-2-methyl-5-pyrimidinyl)methyl)-N-(4-hydroxy-2-mercapto-1-methyl-1-butenyl)formamide S-benzoate O-phosphate; NCGC00016764-01; NCGC00016764-04; NCGC00179477-01; Prestwick_68; Prestwick2_000654; Prestwick3_000654; S-(2-(N-((4-Amino-2-methylpyrimidin-5-yl)methyl)formamido)-5-(phosphonooxy)pent-2-en-3-yl)benzothioa; S-[(Z)-2-[(4-amino-2-methylpyrimidin-5-yl)methyl-formylamino]-5-phosphonooxypent-2-en-3-yl] benzenecarbothioate; s-[2-{[(4-amino-2-methylpyrimidin-5-yl)methyl](formyl)amino}-5-(phosphonooxy)pent-2-en-3-yl] benzenecarbothioate; s-{(1z)-2-[[(4-amino-2-methyl-5-pyrimidinyl)methyl](formyl)amino]-1-[2-(phosphonooxy)ethyl]-1-propenyl} benzenecarbothioate; S-{(1Z)-2-[[(4-Amino-2-methyl-5-pyrimidinyl)methyl](formyl)amino]-1-[2-(phosphonooxy)ethyl]-1-propenyl} benzenecarbothioate, AldrichCPR; S-Benzoylthiamine monophosphate; S-Benzoylthiamine O-monophosphate; S-Benzoylthiamine-O-monophosphate; SCHEMBL188070; SCHEMBL19184708; SR-01000872627; SR-01000872627-1; SR-01000872627-2; Tox21_110597; Tox21_110597_1
Click to Show/Hide
|
|||
| External Link | ||||
| ORY-2001 | Phase 2 | [88] | ||
| Synonyms |
(1R,2S)-2-(4-(Benzyloxy)phenyl)-N-((5-imino-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)cyclopropanamine; 1,3,4-Oxadiazole-2-methanamine, 4,5-dihydro-5-imino-N-((1R,2S)-2-(4-(phenylmethoxy)phenyl)cyclopropyl)-; 1,3,4-Oxadiazole-2-methanamine, 5-amino-N-((1R,2S)-2-(4-(phenylmethoxy)phenyl)cyclopropyl)-; 1357362-02-7; 5-((((1R,2S)-2-(4-(benzyloxy)phenyl)cyclopropyl)amino)methyl)-1,3,4-oxadiazol-2-amine; 5-(((trans)-2-(4-(benzyloxy)phenyl)cyclopropylamino)methyl)-1,3,4-oxadiazol-2-amine; 5-[[[(1R,2S)-2-(4-phenylmethoxyphenyl)cyclopropyl]amino]methyl]-1,3,4-oxadiazol-2-amine; A930244; AKOS040742807; BCP29383; BDBM50594947; CHEMBL4802155; CS-0058593; HY-112623; LZ82JLT4UP; MS-25100; ORY 2001; ORY2001; ORY-2001; ORY-2001; SCHEMBL528204; UNII-LZ82JLT4UP; Vafidemstat; Vafidemstat [INN]; XBBRLCXCBCZIOI-DLBZAZTESA-N
Click to Show/Hide
|
|||
| External Link | ||||
| Bepranemab | Phase 2 | [89] | ||
| Synonyms |
RG6416
Click to Show/Hide
|
|||
| External Link | ||||
| JNJ-63733657 | Phase 2 | [90] | ||
| External Link | ||||
| SAGE-718 | Phase 2 | [91] | ||
| External Link | ||||
| APH-1105 | Phase 2 | [92] | ||
| External Link | ||||
| Nuplazid | Phase 2 | [93] | ||
| Synonyms |
Pimavanserin tartrate; UNII-NA83F1SJSR; 706782-28-7; ACP 103; ACP-103; 706782-28-7 (tartrate); NA83F1SJSR; Pimavanserin tartrate [USAN]; Bis(1-(4-Fluorobenzyl)-1-(1-methylpiperidin-4-yl)-3-(4-(2-methylpropoxy)benzyl)urea) (2R,3R)-2,3-dihydroxybutanedioate; Pimavanserin tartrate (USAN); 1-(4-fluorobenzyl)-3-(4-isobutoxybenzyl)-1-(1-methylpiperidin-4-yl)urea, ((2R,3R)-2,3-dihydroxysuccinate) (2:1); Nuplazide (TN); pimavanserin hemitartrate; DTXSID50220958; CHEBI:133014; HMS3886L06; HY-14557A; Pimavanserin Dihydroxysuccinate(2:1); AKOS027327334; CCG-270608; CS-7954; 1-(4-fluorobenzyl)-3-(4-isobutoxybenzyl)-1-(1-methylpiperidin-4-yl)urea (2R,3R)-2,3-dihydroxysuccinate; AC-29901; AS-56699; N-(4-Fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N'-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1); Urea, N-((4-fluorophenyl)methyl)-N-(1-methyl-4-piperidinyl)-N'-((4-(2-methylpropoxy)phenyl)methyl)-, (2R,3R)-2,3-dihydroxybutanedioate (2:1); D08969; Q27284759; bis(4-{[(4-fluorophenyl)methyl]({[4-(2-methylpropoxy)phenyl]methyl}carbamoyl)amino}-1-methylpiperidin-1-ium) (2R,3R)-2,3-dihydroxybutanedioate; bis{N-[(4-fluorophenyl)methyl]-N-(1-methylpiperidin-4-yl)-N'-{[4-(2-methylpropoxy)phenyl]methyl}urea} (2R,3R)-2,3-dihydroxybutanedioate
Click to Show/Hide
|
|||
| External Link | ||||
| MK-1942 | Phase 2 | [94] | ||
| External Link | ||||
| Bryostatin-1 | Phase 2 | [95] | ||
| Synonyms |
(1S-(1R*,3R*,5Z,7S*,8E,11R*,12R*(2E,4E),13E,15R*,17S*(S*),21S*,23S*,25R*))-25-(Acetyloxy)-1,11,21-trihydroxy-17-(1-hydroxyethyl)-5,13-bis(2-methoxy-2-oxoethylidene)-10,10,26,26-tetramethyl-19-oxo-18,27,28,29-tetraoxatetracyclo(21.3.1.13,7.111,15)nonacos-8-en-12-yl 2,4-octadienoate; (1S,3S,5Z,7R,8E,11S,12S,13E,15S,17R,21R,23R,25S)-25-(acetyloxy)-1,11,21-trihydroxy-17-[(1R)-1-hydroxyethyl]-5,13-bis(2-methoxy-2-oxoethylidene)-10,10,26,26-tetramethyl-19-oxo-18,27,28,29-tetraoxatetracyclo[21.3.1.1(3,7).1(11,15)]nonacos-8-en-12-yl (2E,4E)-octa-2,4-dienoate; (1S,3S,5Z,7R,8E,11S,12S,13E,15S,17R,21R,23R,25S)-25-(Acetyloxy)-1,11,21-trihydroxy-17-[(1R)-1-hydroxyethyl]-5,13-bis(2-methoxy-2-oxoethylidene)-10,10,26,26-tetramethyl-19-oxo-18,27,28,29-tetraoxatetracyclo[21.3.1.13,7.111,15]nonacos-8-en-12-yl-(2E, 4E)-2,4-octadienoic acid ester; [(1S,3S,5Z,7R,8E,11S,12S,13E,15S,17R,21R,23R,25S)-25-acetyloxy-1,11,21-trihydroxy-17-[(1R)-1-hydroxyethyl]-5,13-bis(2-methoxy-2-oxoethylidene)-10,10,26,26-tetramethyl-19-oxo-18,27,28,29-tetraoxatetracyclo[21.3.1.13,7.111,15]nonacos-8-en-12-yl] (2E,4E)-octa-2,4-dienoate; 2,4-Octadienoic acid, (1S,3S,5Z,7R,8E,11S,12S,13E,15S,17R,21R,23R,25S)-25-(acetyloxy)-1,11,21-trihydroxy-17-((1R)-1-hydroxyethyl)-5,13-bis(2-methoxy-2-oxoethylidene)-10,10,26,26-tetramethyl-19-oxo-18,27,28,29-tetraoxatetracyclo(21.3.1.13,7.111,15)nonacos-8-en-12-yl ester, (2E,4E)-; 2,4-Octadienoic acid, 25-(acetyloxy)-1,11,21-trihydroxy-17-(1-hydroxyethyl)-5,13-bis(2-methoxy-2-oxoethylidene)-10,10,26,26-tetramethyl-19-oxo-18,27,28,29-tetraoxatetracyclo(21.3.1.13,7.111,15)nonacos-8-en-12-yl ester, (1S-(1R*,3R*,5Z,7S*,8E,11R*,12R*(2E,4E),13E,15R*,17S*(S*),21S*,23S*,25R*))-; 37O2X55Y9E; 83314-01-6; BDBM50258529; BMY-45618; BRN 4349157; BRYOSTATIN; Bryostatin 1; Bryostatin 1 - CAS 83314-01-6; BRYOSTATIN 1 [MI]; BRYOSTATIN 1 [WHO-DD]; Bryostatin 1, >=99%, solid; Bryostatin-1; CHEBI:88353; CHEMBL449158; CS-0025440; DTXSID8046876; HY-105231; MFCD00893832; MJQUEDHRCUIRLF-TVIXENOKSA-N; NSC 339555; NSC-339555; Q27095907; SCHEMBL182960; UNII-37O2X55Y9E
Click to Show/Hide
|
|||
| External Link | ||||
| AADvac-1 | Phase 2 | [96] | ||
| External Link | ||||
| Semorinemab | Phase 2 | [97] | ||
| Synonyms |
RO7105705
Click to Show/Hide
|
|||
| External Link | ||||
| NLY01 | Phase 2 | [98] | ||
| External Link | ||||
| PTI-125 | Phase 2 | [99] | ||
| Synonyms |
UNII-6NV440YIO0; 6NV440YIO0; PTI-910; Simufilam; Simufilam [USAN]; SCHEMBL12627054; C0105M; WHO 11778; 1-benzyl-8-methyl-1,4,8-triazaspiro(4.5)decan-2-one; 1,4,8-Triazaspiro(4.5)decan-2-one, 8-methyl-1-(phenylmethyl)-; 1224591-33-6
Click to Show/Hide
|
|||
| External Link | ||||
| AD-35 | Phase 2 | [100] | ||
| Synonyms |
1531586-58-9; 6'-(2-(1-(Pyridin-2-ylmethyl)piperidin-4-yl)ethyl)spiro[cyClopropane-1,5'-[1,3]dioxolo[4,5-f]isoindol]-7'(6'H)-one; AD-35; BDBM231544; CHEMBL3949886; GLXC-15057; IND-120499; SCHEMBL15598869; starbld0021420; US9346818, I-29; US9346818, I-33; US9346818, I-35
Click to Show/Hide
|
|||
| External Link | ||||
| Vafidemstat | Phase 2 | [88] | ||
| Synonyms |
(1R,2S)-2-(4-(Benzyloxy)phenyl)-N-((5-imino-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)cyclopropanamine; 1,3,4-Oxadiazole-2-methanamine, 4,5-dihydro-5-imino-N-((1R,2S)-2-(4-(phenylmethoxy)phenyl)cyclopropyl)-; 1,3,4-Oxadiazole-2-methanamine, 5-amino-N-((1R,2S)-2-(4-(phenylmethoxy)phenyl)cyclopropyl)-; 1357362-02-7; 5-((((1R,2S)-2-(4-(benzyloxy)phenyl)cyclopropyl)amino)methyl)-1,3,4-oxadiazol-2-amine; 5-(((trans)-2-(4-(benzyloxy)phenyl)cyclopropylamino)methyl)-1,3,4-oxadiazol-2-amine; 5-[[[(1R,2S)-2-(4-phenylmethoxyphenyl)cyclopropyl]amino]methyl]-1,3,4-oxadiazol-2-amine; A930244; AKOS040742807; BCP29383; BDBM50594947; CHEMBL4802155; CS-0058593; HY-112623; LZ82JLT4UP; MS-25100; ORY 2001; ORY2001; ORY-2001; ORY-2001; SCHEMBL528204; UNII-LZ82JLT4UP; Vafidemstat; Vafidemstat [INN]; XBBRLCXCBCZIOI-DLBZAZTESA-N
Click to Show/Hide
|
|||
| External Link | ||||
| T3D-959 | Phase 2 | [101] | ||
| External Link | ||||
| RPh201 | Phase 2 | [102] | ||
| External Link | ||||
| Trontinemab | Phase 2 | [103] | ||
| Synonyms |
RG6102
Click to Show/Hide
|
|||
| External Link | ||||
| TPI-287 | Phase 1 | [104] | ||
| Synonyms |
849213-15-6; (1S,2S,4S,7S,7aR,7a1S,10aS,11aR,13aS,13bR)-1-(benzoyloxy)-4-(((2R,3S)-3-((tert-butoxycarbonyl)amino)-2-hydroxy-5-methylhexanoyl)oxy)-2-hydroxy-5,7a1,14,14-tetramethyl-9-vinyl-2,3,4,7,7a,7a1,10a,11,11a,13,13a,13b-dodecahydro-1H-8,10,12-trioxa-2,6-methanocyclobuta[b]cyclodeca[de]naphthalene-7,13a-diyl diacetate; TPI 287; SCHEMBL10000720; DTXSID50233967; Q27273546
Click to Show/Hide
|
|||
| External Link | ||||
| MW150 | Phase 2 | [105] | ||
| Synonyms |
1628502-91-9; 3GF; 6-(4-methyl-1-piperazinyl)-3-(2-naphthalenyl)-4-(4-pyridinyl)-pyridazine; 6-(4-Methyl-piperazin-1-yl)-(2-naphthalen-2-yl)-4-pyridin-4-ylpyridazine; 6-(4-Methylpiperazin-1-Yl)-3-(Naphthalen-2-Yl)-4-(Pyridin-4-Yl)pyridazine; 6-(4-methylpiperazin-1-yl)-3-naphthalen-2-yl-4-pyridin-4-ylpyridazine; AKOS040758765; BDBM50537600; CHEMBL4129018; CIIVUDIZZJLXCN-UHFFFAOYSA-N; compound 11 [PMID: 30978288]; compound 27 [WO2014145485A2]; compound 8 [PMID: 25676389]; CS-0069509; EPZ68T461K; EX-A3206A; GTPL10524; HY-120111; MS-26255; MW 150; MW01-18-150SRM; MW150; MW-150; NSC785340; NSC-785340; Pyridazine, 6-(4-methyl-1-piperazinyl)-3-(2-naphthalenyl)-4-(4-pyridinyl)-; Q27453797; SCHEMBL16061104; UNII-EPZ68T461K
Click to Show/Hide
|
|||
| External Link | ||||
| NP001 | Phase 1 | [24] | ||
| External Link | ||||
| Nuedexta | Phase 2 | [24] | ||
| External Link | ||||
| SUVN-502 | Phase 2 | [24] | ||
| Synonyms |
SVN-502; 5-HT 6 receptor antagonist (Alzheimer's disease), Suven; 5-HT 6 receptor antagonist (cognitive/memory disorder), Suven
Click to Show/Hide
|
|||
| External Link | ||||
| S-38093 | Phase 2 | [106] | ||
| Synonyms |
S-41150; S-38471-1; S-750-1; Histamine H3 antagonists (sleep/cognitive disorders), Servier
Click to Show/Hide
|
|||
| External Link | ||||
| LY3202626 | Phase 2 | [24] | ||
| External Link | ||||
| AN-1792 | Phase 2 | [107] | ||
| Synonyms |
Betabloc; AIP-001
Click to Show/Hide
|
|||
| External Link | ||||
| BI-409306 | Phase 2 | [24] | ||
| External Link | ||||
| Methanesulfonyl fluoride | Phase 2 | [108] | ||
| Synonyms |
Fluoride; Methanesulfonyl fluoride (oral, Alzheimer's disease/cognitive disorder)
Click to Show/Hide
|
|||
| External Link | ||||
| NIC5-15 | Phase 2 | [109] | ||
| Synonyms |
D-Pinitol; Pinitol; 10284-63-6; 3-O-Methyl-D-chiro-inositol; (+)-Pinitol; D-(+)-Pinitol; Inzitol; Methylinositol; Sennitol; 1-D-4-O-METHYL-MYO-INOSITOL; Pinit; D-ononitol; Ononitol; 1D-4-O-Methyl-myo-inositol; 1D-3-O-methyl-chiro-inositol; D-chiro-Inositol, 3-O-methyl-; 4-O-Methyl-myo-inositol; UNII-TF9HZN9T0M; UNII-A998ME07KR; 5D-5-O-Methyl-chiro-inositol; 6090-97-7; (1r,2s,4s,5s)-6-methoxycyclohexane-1,2,3,4,5-pentol; TF9HZN9T0M; 484-68-4; Matezit; Sennit; CHEMBL493737; A998ME07KR; CHEBI:28548; DSCFFEYYQKSRSV-KLJZZCKASA-N; NSC 43336
Click to Show/Hide
|
|||
| External Link | ||||
| SAM-531 | Phase 2 | [110] | ||
| Synonyms |
PF-05212365; PF-5212365; WAY-262531; 5-HT6 receptor antagonist (Alzheimer's disease, schizophrenia), Pfizer
Click to Show/Hide
|
|||
| External Link | ||||
| IMD-1041 | Phase 2 | [111] | ||
| Synonyms |
IMD-1041 (oral, COPD/interstitial cystis/type 2 diabetics/chronic inflammatory disease/metabolic syndrome/AD), IMMD
Click to Show/Hide
|
|||
| External Link | ||||
| GSK933776A | Phase 2 | [112] | ||
| External Link | ||||
| LY3002813 | Phase 2 | [113] | ||
| External Link | ||||
| AD02 vaccine | Phase 2 | [114] | ||
| External Link | ||||
| E2027 | Phase 1 | [24] | ||
| External Link | ||||
| TTP-448 | Phase 2 | [115] | ||
| External Link | ||||
| CPC-201 | Phase 2 | [116] | ||
| External Link | ||||
| UE-2343 | Phase 2 | [24] | ||
| External Link | ||||
| EVP-0962 | Phase 2 | [117] | ||
| External Link | ||||
| R-phenserine | Phase 2 | [118] | ||
| Synonyms |
Posiphen (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| FK-962 | Phase 2 | [119] | ||
| Synonyms |
FK-960 analog, Fujisawa
Click to Show/Hide
|
|||
| External Link | ||||
| SAR-110894 | Phase 2 | [120] | ||
| Synonyms |
SAR-110894D
Click to Show/Hide
|
|||
| External Link | ||||
| UB-311 | Phase 2 | [121] | ||
| External Link | ||||
| ABT-288 | Phase 2 | [122] | ||
| Synonyms |
UNII-5MEI1M3NHH; 5MEI1M3NHH; ABT 288; GNIRITULTPTAQWKNQAVFIVSAN; SCHEMBL2406947; ABT288; GTPL6927; GNIRITULTPTAQW-KNQAVFIVSA-N; 948845-91-8; J3.497.401B; 2-[4'-(3aR,6aR)-(5-Methylhexahydropyrrolo[3,4-b]pyrrol-1(2H)-yl)-1,1'-biphenyl-4-yl]pyridazin-3(2H)-one; 2-{4'-[(3aR,6aR)-5-Methylhexahydropyrrolo[3,4-b]pyrrol-1(2H)-yl]-1,1-biphenyl-4-yl}pyridazin-3(2H)-one; 2-[4'-(3aR,6aR)-(5-methylhexahydropyrrolo[3,4-b]pyrrol-1(2H)-yl)-1,1'-biphenyl-4-yl ]pyridazin-3(2H)-one; (3aR, 6aR)-2-[4'-(5-Methyl-hexahydro-pyrrolo[3,4
Click to Show/Hide
|
|||
| External Link | ||||
| Oleoyl-estrone | Phase 2 | [24] | ||
| Synonyms |
Anti-obesity hormone-based therapy, Manhattan
Click to Show/Hide
|
|||
| External Link | ||||
| CERE-110 | Phase 2 | [123] | ||
| External Link | ||||
| HSRx-888 | Phase 2 | [24] | ||
| External Link | ||||
| AVN 322 | Phase 1 | [124] | ||
| External Link | ||||
| Nefiracetam | Phase 2 | [125] | ||
| Synonyms |
Motiva (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| JOT106 | Phase 2 | [47] | ||
| External Link | ||||
| BPN14770 | Phase 2 | [126] | ||
| Synonyms |
KRRGWHSEDYQKDQ-UHFFFAOYSA-N; SCHEMBL15659026; 2-(4-((2-(3-Chlorophenyl)-6-(trifluoromethyl)pyrimidin-4-yl)amino)phenyl)acetamide
Click to Show/Hide
|
|||
| External Link | ||||
| BIIB092 | Phase 2 | [24] | ||
| External Link | ||||
| AZD-5213 | Phase 2 | [127] | ||
| External Link | ||||
| ABBV-8E12 | Phase 2 | [128] | ||
| Synonyms |
C2N-8E12
Click to Show/Hide
|
|||
| External Link | ||||
| LymPro | Phase 2 | [129] | ||
| External Link | ||||
| AQW-051 | Phase 2 | [130] | ||
| Synonyms |
669770-29-0; (R)-3-((6-(p-Tolyl)pyridin-3-yl)oxy)quinuclidine; AQW051; NPDLTEZXGWRMLQ-IBGZPJMESA-N; UNII-JQH481R778; SCHEMBL1459285; GTPL7371; SCHEMBL19522679; JQH481R778; ZINC3942685; AKOS030628482; SB17130; AS-35273; (R)-3-(6-p-tolyl-pyridin-3-yloxy)-1-aza-bicyclo[2.2.2]octane
Click to Show/Hide
|
|||
| External Link | ||||
| CX-717 | Preclinical | [131] | ||
| Synonyms |
CX-1763; CX-546; CX-614; CX-727; CX-729; CX-743; CX-815; S-40929; First generation AMPA receptor modulators, Cortex/University of California; First-generation AMPAKINE compounds, University of California/Cortex
Click to Show/Hide
|
|||
| External Link | ||||
| PF-05212377 | Phase 2 | [132] | ||
| Synonyms |
SAM-760
Click to Show/Hide
|
|||
| External Link | ||||
| CT1812 | Phase 2 | [133] | ||
| Synonyms |
Elayta
Click to Show/Hide
|
|||
| External Link | ||||
| LY2886721 | Phase 2 | [134] | ||
| Synonyms |
1262036-50-9; LY-2886721; UNII-2CQ62IWB67; LY 2886721; 2CQ62IWB67; N-(3-((4aS,7aS)-2-amino-4a,5,7,7a-tetrahydro-4H-furo[3,4-d][1,3]thiazin-7a-yl)-4-fluorophenyl)-5-fluoropicolinamide; CHEMBL2396989; N-{3-[(4as,7as)-2-Amino-4a,5-Dihydro-4h-Furo[3,4-D][1,3]thiazin-7a(7h)-Yl]-4-Fluorophenyl}-5-Fluoropyridine-2-Carboxamide; n-(3-((4as,7as)-2-amino-4a,5-dihydro-4h-furo(3,4-d)(1,3)thiazin-7a(7h)-yl)-4-fluorophenyl)-5-fluoro-2-pyridinecarboxamide; NIDRNVHMMDAAIK-YPMLDQLKSA-N; MLS006011070; SCHEMBL966802; GTPL6475
Click to Show/Hide
|
|||
| External Link | ||||
| CSTC1 | Phase 2 | [24] | ||
| Synonyms |
BAC; D-Bacillosamine; Bacillosamine; D-Bac; 2,4-Diamino-2,4,6-Trideoxy-D-Glucopyranose; 2,4-Diamino-2,4,6-Trideoxy-Glucose; 2,4,6-Trideoxy-2,4-Diamino-Glucose; 2,4-Diamino-2,4,6-Trideoxy-D-Glucose; 2,4,6-Trideoxy-2,4-Diamino-D-Glucose; 2,4,6-Trideoxy-2,4-Diamino-Glucopyranose; 2,4-Diamino-2,4,6-Trideoxy-Glucopyranose; 2,4-Diamino-2,4,6-Trideoxy-Glucopyranoside; 2,4,6-Trideoxy-2,4-Diamino-Glucopyranoside; 2,4,6-Trideoxy-2,4-Diamino-D-Glucopyranose; 2,4,6-Trideoxy-2,4-Diamino-D-Glucopyranoside; 2,4-Diamino-2,4,6-Trideoxy-D-Gluc
Click to Show/Hide
|
|||
| External Link | ||||
| T-817MA | Phase 2 | [135] | ||
| Synonyms |
Edonerpic maleate; UNII-0LB9F7I5P3; 0LB9F7I5P3; 519187-97-4; T-817; T-817 maleate; SCHEMBL48064; RLUCYBFCLXANSO-BTJKTKAUSA-N; HY-17631A; DC10762; CS-8069; J2.179.155E; 1-(3-(2-(1-benzothiophen-5-yl)ethoxy)propyl)azetidin-3-ol maleate; 1-{3-[2-(1-benzothiophen-5-yl)ethoxy]propyl}-3-azetidinol maleate; 1-(3-(2-(Benzo(b)thiophene-5-yl)ethoxy)propyl)azetidine-3-ol maleate; 1-(3-(2-(1-benzothiophene-5-yl)ethoxy)propyl)-3-azetidinol maleate; 3-Azetidinol, 1-(3-(2-benzo(b)thien-5-ylethoxy)propyl)-, (2Z)-2-butenedioate (1:
Click to Show/Hide
|
|||
| External Link | ||||
| KPAX002 | Phase 2 | [24] | ||
| External Link | ||||
| Bryostatin-1 | Phase 2 | [24] | ||
| Synonyms |
Bryostatin 1; 83314-01-6
Click to Show/Hide
|
|||
| External Link | ||||
| MIQ-001 | Phase 2 | [136] | ||
| Synonyms |
M-IQ 001; Fatty acid metabolism inhibitor (Alzheimer's disease), Meta-IQ
Click to Show/Hide
|
|||
| External Link | ||||
| Basmisanil | Phase 2 | [137] | ||
| Synonyms |
1159600-41-5; UNII-788PET5SUA; 788PET5SUA; (1,1-Dioxidothiomorpholino)(6-((3-(4-fluorophenyl)-5-methylisoxazol-4-yl)methoxy)pyridin-3-yl)methanone; Basmisanil [INN]; Basmisanil [USAN:INN]; Basmisani; Basmisanil(RG1662); Basmisanil (USAN/INN); SCHEMBL2685527; CHEMBL3681419; MolPort-044-561-818; VCGRFBXVSFAGGA-UHFFFAOYSA-N; BDBM133427; EX-A1272; AKOS032947142; ZINC145814743; DB11877; CS-6046; HY-16716; (1,1-Dioxo-4-thiomorpholinyl)(6-((3-(4-fluorophenyl)-5-methylisoxazol-4-yl)methoxy)pyridin-3-yl)metha
Click to Show/Hide
|
|||
| External Link | ||||
| Ladostigil | Phase 2 | [138] | ||
| Synonyms |
Ladostigil tartrate; Alzheimer disease therapeutics, Teva; TV-3219; TV-3279; TV-3326
Click to Show/Hide
|
|||
| External Link | ||||
| NDD-094 | Phase 2 | [139] | ||
| Synonyms |
NDD-094A; SDZ-NDD-094
Click to Show/Hide
|
|||
| External Link | ||||
| Ponezumab | Phase 2 | [140] | ||
| Synonyms |
Neurological disease and injury therapeutics, Pfizer; PF-04360365; PF-4360365; RI-1014; RI-1219; RI-409; RN-1219
Click to Show/Hide
|
|||
| External Link | ||||
| ORM-12741 | Phase 2 | [141] | ||
| Synonyms |
Alpha 2c adrenoceptor antagonist (neurological diseases), Orion; Alpha 2c adrenoceptor antagonist (psychiatric disorders), Orion
Click to Show/Hide
|
|||
| External Link | ||||
| PF-4447943 | Phase 2 | [142] | ||
| Synonyms |
BCP16255
Click to Show/Hide
|
|||
| External Link | ||||
| DCB-AD1 | Phase 2 | [143] | ||
| External Link | ||||
| ABT-126 | Phase 2 | [144] | ||
| External Link | ||||
| AZD4694 | Phase 2 | [145] | ||
| External Link | ||||
| GSK239512 | Phase 2 | [146] | ||
| Synonyms |
720691-69-0; GSK-239512; 1-(6-((3-CYCLOBUTYL-2,3,4,5-TETRAHYDRO-1H-BENZO[D]AZEPIN-7-YL)OXY)PYRIDIN-3-YL)PYRROLIDIN-2-ONE; UNII-4I7U5C459M; CHEMBL3092650; 4I7U5C459M; YFRBKEVUUCQYOW-UHFFFAOYSA-N; SCHEMBL167578; MolPort-035-776-189; ZINC3961802; BDBM50444496; AKOS025291102; SB16754; KS-0000063Q; AS-42474; AK171368; 2-Pyrrolidinone, 1-(6-((3-cyclobutyl-2,3,4,5-tetrahydro-1H-3-benzazepin-7-yl)oxy)-3-pyridinyl)-; J3.497.402K
Click to Show/Hide
|
|||
| External Link | ||||
| ALZ-801 | Phase 2 | [24] | ||
| External Link | ||||
| SYN-120 | Phase 2 | [147] | ||
| External Link | ||||
| VI-1121 | Phase 2 | [148] | ||
| External Link | ||||
| TPI 287 | Phase 1 | [47] | ||
| Synonyms |
FDTAUJJRHBRHIJ-BBGIBMRQSA-N; 849213-15-6; SCHEMBL19374618; Hexanoic acid, 3-(((1,1-dimethylethoxy)carbonyl)amino)-2-hydroxy-5-methyl-, (1S,2S,4S,7S,7aR,10aS,11aR,13aS,13bR,13cS)-7,13a-bis(acetyloxy)-1-(benzoyloxy)-9-ethenyl-1,3,4,7,7a,10a,11,11a,13,13a,13b,13c-dodecahydro-2-hydroxy-5,13c,14,14-tetramethyl-2,6-methano-2H-cyclodec(de)oxeto(3,2-g)(1,3)benzodioxin-4-yl ester, (2R,3S)-
Click to Show/Hide
|
|||
| External Link | ||||
| ST-101 | Phase 2 | [149] | ||
| Synonyms |
ST 101 [French]; 7-Chloro-1,3-dihydro-5-phenyl-1-trimethylgermyl-2H-1,4-benzodiazepin-2-one; Trimethyl germyl-1, chloro-7, dihydro-1-3, phenyl-5 2H benzodiazepine-1-4 one-2 [French]; 2H-1,4-Benzodiazepin-2-one, 1,3-dihydro-7-chloro-5-phenyl-1-trimethylgermyl-; AC1O4GHW; ST 101; LS-34275; Trimethyl germyl-1, chloro-7, dihydro-1-3, phenyl-5 2H benzodiazepine-1-4 one-2
Click to Show/Hide
|
|||
| External Link | ||||
| EVT302 | Phase 2 | [24] | ||
| External Link | ||||
| DM-99 | Phase 2 | [150] | ||
| External Link | ||||
| RP5063 | Phase 1 | [39] | ||
| External Link | ||||
| ABT-384 | Phase 2 | [151] | ||
| Synonyms |
UNII-R5TH77F919; CHEMBL222670; ABT 384; R5TH77F919; SCHEMBL231595; GTPL7357; ABT384; SCHEMBL20457214; BDBM50195291; DB12501; 868623-40-9; 4-{2-methyl-2-[4-(5-trifluoromethyl-pyridin-2-yl)-piperazin-1-yl]-propionylamino}-adamantane-1-carboxylic acid amide
Click to Show/Hide
|
|||
| External Link | ||||
| PRX-3140 | Phase 2 | [152] | ||
| External Link | ||||
| Myo-inositol | Phase 2 | [39] | ||
| Synonyms |
Scyllo-inositol; inositol; Muco-Inositol; epi-Inositol; i-Inositol; meso-Inositol; Allo-inositol; 87-89-8; 1D-Chiro-inositol; 1L-Chiro-inositol; 488-59-5; Scyllitol; 643-12-9; D-(+)-chiro-Inositol; cis-Inositol; mesoinositol; Quercinitol; Myoinosite; Dambose; Cyclohexane-1,2,3,4,5,6-hexaol; Neo-inositol; Meat sugar; 6917-35-7; Phaseomannite; Inositina; Cocositol; D-chiro-Inositol; Inositene; Inosital; Iso-inositol; cyclohexane-1,2,3,4,5,6-hexol; Phaseomannitol; Cyclohexitol; Mesoinosit; Scyllite; Mesoinosite; Inosite
Click to Show/Hide
|
|||
| External Link | ||||
| CHF-5074 | Phase 2 | [153] | ||
| Synonyms |
CHF 5074; CHF5074; 749269-83-8; Itanapraced; UNII-C35RF1MWQZ; GHF-5074; C35RF1MWQZ; CHEMBL196945; 1-(3',4'-dichloro-2-fluoro(1,1'-biphenyl)-4-yl)cyclopropanecarboxylic acid; 1-[4-(3,4-dichlorophenyl)-3-fluorophenyl]cyclopropane-1-carboxylic acid; 1-(3',4'-Dichloro-2-Fluorobiphenyl-4-Yl)cyclopropanecarboxylic Acid; H50; SCHEMBL407631; GTPL7339; DTXSID30225901; AOB5325; LIYLTQQDABRNRX-UHFFFAOYSA-N; ZINC3986651; EX-A1963; BDBM50172482; AKOS026750398; SB16945; CS-5022; NCGC00408905-01; AS-16850; HY-14399; BC600569; FT-0708261
Click to Show/Hide
|
|||
| External Link | ||||
| FRM-0962 | Phase 2 | [154] | ||
| External Link | ||||
| MK-7622 | Phase 2 | [155] | ||
| Synonyms |
M1 receptor modulator; 1227923-29-6; MK7622; MK 7622; 3-[(1S,2S)-2-Hydroxycyclohexyl]-6-[(6-methyl-3-pyridinyl)methyl]benzo[h]quinazolin-4(3H)-one; SCHEMBL2399084; EX-A804; JUVQLZBJFOGEEO-GOTSBHOMSA-N; BCP27739; ZINC95930184; AKOS028113668; DB12897; CS-5442; HY-15618; Benzo(H)quinazolin-4(3H)-one, 3-((1S,2S)-2-hydroxycyclohexyl)-6-((6-methyl-3-pyridinyl)methyl)-; AS-35236; KB-145903; J-690076; UNII-57R7D1Q49R component JUVQLZBJFOGEEO-GOTSBHOMSA-N
Click to Show/Hide
|
|||
| External Link | ||||
| RG1577 | Phase 2 | [156] | ||
| External Link | ||||
| VX-745 | Phase 2 | [24] | ||
| Synonyms |
5-(2,6-Dichlorophenyl)-2-((2,4-difluorophenyl)thio)-6H-pyrimido[1,6-b]pyridazin-6-one; 209410-46-8; Neflamapimod; VX 745; VX745; VRT-031745; UNII-TYL52QM320; TYL52QM320; CHEBI:90528; 5-(2,6-dichlorophenyl)-2-(2,4-difluorophenylthio)-6H-pyrimido[1,6-b]pyridazin-6-one; 5-(2,6-dichlorophenyl)-2-(2,4-difluorophenyl)sulfanylpyrimido[1,6-b]pyridazin-6-one; Neflamapimod (USAN); Neflamapimod [USAN]; AK-44905; C19H9Cl2F2N3OS; 5-(2,6-Dichlorophenyl)-2-[(2,4-Difluorophenyl)sulfanyl]-6h-Pyrimido[1,6-B]pyridazin-6-One; VX745, VX-745; 5-(2,6-Dichlorophenyl)-2-((2,4-difluorophenyl)thio)-6H-pyrimido(1,6-b)pyridazin-6-one; Vertex 745 (VX745)
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-708163 | Phase 2 | [157] | ||
| Synonyms |
Avagacestat
Click to Show/Hide
|
|||
| External Link | ||||
| Tau-binding PET tracer | Phase 2 | [158] | ||
| Synonyms |
T-777; T-807; T-808; Tau-binding PET tracer (Alzheimer disease); Tau-binding PET tracer (Alzheimer disease), Siemens
Click to Show/Hide
|
|||
| External Link | ||||
| MK-0752 | Phase 2 | [159] | ||
| Synonyms |
471905-41-6; MK0752; UNII-9JD9B4S53T; MK 0752; cis-4-[(4-Chlorophenyl)sulfonyl]-4-(2,5-difluorophenyl)cyclohexanepropanoic acid; 9JD9B4S53T; 3-((1r,4s)-4-((4-chlorophenyl)sulfonyl)-4-(2,5-difluorophenyl)cyclohexyl)propanoic acid; 3-(cis-4-((4-Chlorophenyl)sulfonyl)-4-(2,5-difluorophenyl)cyclohexyl)propanoic acid; 3-((1r,4s)-4-(4-chlorophenylsulfonyl)-4-(2,5-difluorophenyl)cyclohexyl)propanoic acid; 952578-68-6; cc-14; C21H21ClF2O4S; MLS006011072; SCHEMBL756249; SCHEMBL756248; SCHEMBL756247; CHEMBL3392635
Click to Show/Hide
|
|||
| External Link | ||||
| GM-602 | Phase 2 | [160] | ||
| Synonyms |
Alirinetide; UNII-6BK9OEG8CC; 6BK9OEG8CC; 725715-18-4; GM-603; GM-605; GM-604; GM-607; GM-609; GM-606; L-Arginine, L-phenylalanyl-L-seryl-L-arginyl-L-tyrosyl-L-alanyl-; (2S)-2-(((2S)-2-(((2S)-2-(((2S)-2-(((2S)-2-(((2S)-2-Amino-3-phenyl-propanoyl)amino)-3-hydroxy-propanoyl)amino)-5-guanidino-pentanoyl)amino)-3-(4-hydroxyphenyl)propanoyl)amino)propanoyl)amino)-5-guanidino-pentanoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| HF-0220 | Phase 2 | [161] | ||
| Synonyms |
Cytoprotective steroid (Alzheimer's disease), Newron; Prostaglandin D synthase stimulator (Alzheimer's disease), Newron; Prostaglandin J2 synthesis stimulator (Alzheimer's disease), Newron; Cytoprotective steroid (Alzheimer's disease), Hunter-Fleming; Prostaglandin D synthase stimulator (Alzheimer's disease), Hunter-Fleming; Prostaglandin J2 synthesis stimulator (Alzheimer's disease), Hunter-Fleming
Click to Show/Hide
|
|||
| External Link | ||||
| Mibampator | Phase 2 | [162] | ||
| Synonyms |
LY-450108; LY-451395; AMPA agonists (Alzheimer's Disease), Lilly; AMPA receptor agonist (agitation in Alzheimer's Disease), Eli Lilly
Click to Show/Hide
|
|||
| External Link | ||||
| AVN 101 | Phase 2 | [163] | ||
| External Link | ||||
| Coluracetam | Phase 2 | [164] | ||
| Synonyms |
BCI-540; MKC-231; High-affinity choline uptake facilitator (CNS disorders), Mitsubishi; Neurons growth promoting compound (major depressive disorder/anxiety), BrainCells; High-affinity choline uptake facilitator (depression/ anxiety), BrainCells
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-1446 | Phase 2 | [165] | ||
| Synonyms |
TC-6683; Alpha-4 beta-2 neuronal nicotinic receptor modulator (oral, cognitive disorder), Targacept/AstraZeneca
Click to Show/Hide
|
|||
| External Link | ||||
| AUS-131 | Phase 2 | [24] | ||
| External Link | ||||
| TD-8954 | Phase 1/2 | [166] | ||
| Synonyms |
UNII-35F0Y2W16Q; CHEMBL2402904; 35F0Y2W16Q; 916075-84-8; compound 18 [PMID 23756062]; SCHEMBL390795; GTPL8426; BDBM50436989; SB17471; DB12725; 1-Piperidinecarboxylic acid, 4-((4-((((2-(1-methylethyl)-1H-benzimidazol-7-yl)carbonyl)amino)methyl)-1-piperidinyl)methyl)-, methyl ester; methyl 4-[[4-[[(2-propan-2-yl1H-benzimidazole-4-carbonyl)amino]methyl]piperidin-1-yl]methyl]piperidine-1-carboxylate
Click to Show/Hide
|
|||
| External Link | ||||
| Vanutide cridificar | Phase 2 | [167] | ||
| Synonyms |
ACC-001
Click to Show/Hide
|
|||
| External Link | ||||
| RG6100 | Phase 2 | [168] | ||
| External Link | ||||
| Mitoglitazone | Phase 2 | [24] | ||
| Synonyms |
MSDC-0160; Mitoglitazone (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| PBT-2 | Phase 2 | [169] | ||
| Synonyms |
AD/HD therapy, Prana; Alzheimers/Huntingtons disease therapy (chelating agent), Prana
Click to Show/Hide
|
|||
| External Link | ||||
| Neu-P11 | Phase 2 | [24] | ||
| Synonyms |
Piromelatine; UNII-S3UN2146K9; 946846-83-9; S3UN2146K9; Piromelatine [INN]; NEU-P-11; SCHEMBL8235551; DTXSID90241566; N-(2-(5-Methoxy-1H-indol-3-yl)ethyl)-4-oxo-4H-pyran-2-carboxamide; 4H-Pyran-2-carboxamide, N-(2-(5-methoxy-1H-indol-3-yl)ethyl)-4-oxo-; SB19819
Click to Show/Hide
|
|||
| External Link | ||||
| ICA-17043 | Phase 2 | [170] | ||
| Synonyms |
Senicapoc; 289656-45-7; 2,2-bis(4-fluorophenyl)-2-phenylacetamide; UNII-TS6G201A6Q; TS6G201A6Q; 2,2-BIS(4-FLUOROPHENYL)-2-PHENYL-ACETAMIDE; AK-79332; Senicapoc (USAN); Bis(4-fluorophenyl)phenylacetamide; Senicapoc [USAN:INN]; ICA 17043; C20H15F2NO; PubChem19381; 4-Fluoro-alpha-(4-fluorophenyl)-alpha-phenylbenzeneacetamide; AC1Q4NLN; AC1L50IL; CHEMBL405821; SCHEMBL1443805; GTPL2331; CTK4G2492; DTXSID60276906; SCTZUZTYRMOMKT-UHFFFAOYSA-N; MolPort-019-996-128; BCP14507; ACT06676; ZINC3816408; KS-00000XJ3; BBL102413; STL556215
Click to Show/Hide
|
|||
| External Link | ||||
| MIB-626 | Phase 1/2 | [171] | ||
| External Link | ||||
| Xanamem | Phase 1/2 | [172] | ||
| Synonyms |
(5-(1H-Pyrazol-4-yl)thiophen-3-yl)((1R,3r,5S)-3-hydroxy-3-(pyrimidin-2-yl)-8-azabicyclo[3.2.1]octan-8-yl)methanone; [(1S,5R)-3-hydroxy-3-pyrimidin-2-yl-8-azabicyclo[3.2.1]octan-8-yl]-[5-(1H-pyrazol-4-yl)thiophen-3-yl]methanone; 1346013-80-6; compound 4 [PMID: 28012176]; GLXC-26582; GTPL12037; SCHEMBL23207782; SCHEMBL23798797; starbld0030988; UE2343; UE-2343; Xanamem
Click to Show/Hide
|
|||
| External Link | ||||
| VGH-AD1 | Phase 1/2 | [173] | ||
| External Link | ||||
| GB301 | Phase 1/2 | [174] | ||
| External Link | ||||
| LM11A-31 | Phase 1/2 | [175] | ||
| Synonyms |
N-[2-(Morpholin-4-yl)ethyl]-L-isoleucinamide; 1243259-19-9; (2S,3S)-2-amino-3-methyl-N-(2-morpholin-4-ylethyl)pentanamide; 102562-74-3; SCHEMBL1723692; DTXSID50595097; ZINC4239960; (2s,3s)-2-amino-3-methyl-N-(2-morpholinoethyl) pentanamide; (2S,3S)-2-amino-3-methyl-N-(2-morpholinoethyl)-pentanamide; (2S,3S)-2-Amino-3-methyl-N-(2-morpholinoethyl)pentanamide
Click to Show/Hide
|
|||
| External Link | ||||
| LX1001 | Phase 1/2 | [176] | ||
| Synonyms |
AAVrh.10hAPOE2
Click to Show/Hide
|
|||
| External Link | ||||
| VAC20121 | Phase 1/2 | [177] | ||
| Synonyms |
ACI-35
Click to Show/Hide
|
|||
| External Link | ||||
| AL 001 | Phase 1/2 | [178] | ||
| Synonyms |
Latozinemab
Click to Show/Hide
|
|||
| External Link | ||||
| T3D-959 | Phase 1/2 | [179] | ||
| External Link | ||||
| AstroStem | Phase 1/2 | [24] | ||
| External Link | ||||
| EB-101 | Phase 1/2 | [180] | ||
| Synonyms |
Beta amyloid vaccine (Alzheimer's disease), Atlas Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| IONIS-MAPTRx | Phase 1/2 | [47] | ||
| External Link | ||||
| ACI-24 | Phase 1/2 | [181] | ||
| Synonyms |
Beta amyloid vaccine (liposomal/SupraAntigen, Alzheimer's disease/Down syndrome), AC Immune
Click to Show/Hide
|
|||
| External Link | ||||
| RVX-208 | Phase 1/2 | [182] | ||
| External Link | ||||
| VLP norovirus bivalent vaccine | Phase 1/2 | [183] | ||
| Synonyms |
VLP norovirus bivalent vaccine (intranasal, norovirus-induced gastroenteritis)
Click to Show/Hide
|
|||
| External Link | ||||
| APH-0703 | Phase 1/2 | [184] | ||
| External Link | ||||
| LM11A-31 | Phase 1/2 | [24] | ||
| External Link | ||||
| BIIB080 | Phase 1/2 | [24] | ||
| External Link | ||||
| BIL010t | Phase 1/2 | [170] | ||
| External Link | ||||
| MK-2461 | Phase 1/2 | [185] | ||
| Synonyms |
MK 2461
Click to Show/Hide
|
|||
| External Link | ||||
| PF-06648671 | Phase 1 | [186] | ||
| Synonyms |
1587727-31-8; 2-[(1S)-1-[(2S,5R)-5-[4-Chloro-5-fluoro-2-(trifluoromethyl)phenyl]tetrahydro-2-furanyl]ethyl]-3,4-dihydro-7-(4-methyl-1H-imidazol-1-yl)-2H-pyrido[1,2-a]pyrazine-1,6-dione; CHEMBL3951810; SCHEMBL15611304; BDBM193081; HY-120789; CS-0079177; US9193726, 69; Q29213634; 2-[(1S)-1-[(2S,5R)-5-[4-chloro-5-fluoro-2-(trifluoromethyl)phenyl]oxolan-2-yl]ethyl]-7-(4-methylimidazol-1-yl)-3,4-dihydropyrido[1,2-a]pyrazine-1,6-dione
Click to Show/Hide
|
|||
| External Link | ||||
| AD101 | Phase 1 | [187] | ||
| Synonyms |
(2,4-dimethyl-3-pyridyl)-[4-methyl-4-[(3S)-3-methyl-4-[(1S)-1-[4-(trifluoromethyl)phenyl]ethyl]piperazin-1-yl]-1-piperidyl]methanone; (2,4-Dimethylpyridin-3-yl)(4-methyl-4-((S)-3-methyl-4-((S)-1-(4-(trifluoromethyl)phenyl)ethyl)piperazin-1-yl)piperidin-1-yl)methanone; (2,4-Dimethyl-pyridin-3-yl)-(4-methyl-4-{(S)-3-methyl-4-[(S)-1-(4-trifluoromethyl-phenyl)-ethyl]-piperazin-1-yl}-piperidin-1-yl)-methanone; (2,4-Dimethyl-pyridin-3-yl)-(4-methyl-4-{3-methyl-4-[1-(4-trifluoromethyl-phenyl)-ethyl]-piperazin-1-yl}-piperidin-1-yl)-methanone; (2,4-dimethylpyridin-3-yl)-[4-methyl-4-[(3S)-3-methyl-4-[(1S)-1-[4-(trifluoromethyl)phenyl]ethyl]piperazin-1-yl]piperidin-1-yl]methanone; 306293-36-7; AD101; BDBM50104946; CHEMBL113436; DTKUANPECHGGBY-UNMCSNQZSA-N; PD084304; Piperazine, 4-[1-[(2,4-dimethyl-3-pyridinyl)carbonyl]-4-methyl-4-piperidinyl]-2-methyl-1-[(1S)-1-[4-(trifluoromethyl)phenyl]ethyl]-, (2S)-; SCHEMBL4453513
Click to Show/Hide
|
|||
| External Link | ||||
| IGCAD1 | Phase 1 | [188] | ||
| External Link | ||||
| AVN-322 | Phase 1 | [189] | ||
| Synonyms |
1194574-68-9; N,7-dimethyl-3-(phenylsulfonyl)-6,7,8,9-tetrahydropyrazolo[1,5-a]pyrido[3,4-e]pyrimidin-2-amine hydrochloride; SB17475
Click to Show/Hide
|
|||
| External Link | ||||
| J147 | Phase 1 | [190] | ||
| Synonyms |
(E)-N-(2,4-Dimethylphenyl)-2,2,2-trifluoro-N'-(3-methoxybenzylidene)acetohydrazide; 1146963-51-0; 1807913-16-1; 2,2,2-Trifluoroacetic acid 1-(2,4-Dimethylphenyl)-2-[(3-methoxyphenyl)methylene]hydrazide; A894156; AC-35232; Acetic acid, 2,2,2-trifluoro-, (2E)-1-(2,4-dimethylphenyl)-2-((3-methoxyphenyl)methylene)hydrazide; Acetic acid, 2,2,2-trifluoro-, 1-(2,4-dimethylphenyl)-2-((3-methoxyphenyl)methylene)hydrazide; Acetic acid, 2,2,2-trifluoro-, 1-(2,4-dimethylphenyl)-2-[(3-methoxyphenyl)methylene]hydrazide; AKOS024458485; AS-16718; CCG-268030; CHEBI:192601; CHEMBL2387144; CS-3688; DB13957; DTXSID501045787; EX-A2235; HMS3886O17; HY-13779; HYMZAYGFKNNHDN-SSDVNMTOSA-N; j147; J-147; MFCD25976644; N-(2,4-Dimethylphenyl)-2,2,2-trifluoro-N-((E)-(3-methoxyphenyl)methylideneamino)acetamide; N-(2,4-Dimethylphenyl)-2,2,2-trifluoro-N'-(3-methoxybenzylidene)acetohydrazide; N-(2,4-DIMETHYLPHENYL)-2,2,2-TRIFLUORO-N'-[(1E)-(3-METHOXYPHENYL)METHYLIDENE]ACETOHYDRAZIDE; N-(2,4-dimethylphenyl)-2,2,2-trifluoro-N'-[(3-methoxyphenyl)methylidene]acetohydrazide; N-(2,4-Dimethylphenyl)-2,2,2-trifluoro-N'-[(E)-(3-methoxyphenyl)methylene]acetohydrazide; N-(2,4-dimethylphenyl)-2,2,2-trifluoro-N'-[(E)-(3-methoxyphenyl)methylidene]acetohydrazide; N-(2,4-dimethylphenyl)-2,2,2-trifluoro-N-[(E)-(3-methoxyphenyl)methylideneamino]acetamide; N-(2,4-dimethylphenyl)-2,2,2-triluoro-N-[(E)-(3-methoxyphenyl)methylideneamino]acetamide; s5269; SCHEMBL12995834; SCHEMBL21294999; UNII-Z41H3C5BT9; Z41H3C5BT9
Click to Show/Hide
|
|||
| External Link | ||||
| RG6289 | Phase 1 | [191] | ||
| External Link | ||||
| ASN51 | Phase 1 | [192] | ||
| External Link | ||||
| E2511 | Phase 1 | [193] | ||
| External Link | ||||
| SHR-1707 | Phase 1 | [194] | ||
| External Link | ||||
| Lu AF87908 | Phase 1 | [195] | ||
| External Link | ||||
| APNmAb005 | Phase 1 | [196] | ||
| External Link | ||||
| APH-1104 | Phase 1 | [197] | ||
| External Link | ||||
| DNL919 | Phase 1 | [198] | ||
| Synonyms |
TAK-920
Click to Show/Hide
|
|||
| External Link | ||||
| PRX-005 | Phase 1 | [199] | ||
| External Link | ||||
| NTRX-07 | Phase 1 | [200] | ||
| Synonyms |
MDA7
Click to Show/Hide
|
|||
| External Link | ||||
| AAV-hTERT | Phase 1 | [201] | ||
| External Link | ||||
| DNL104 | Phase 1 | [202] | ||
| External Link | ||||
| DNL747 | Phase 1 | [203] | ||
| External Link | ||||
| BEY2153 | Phase 1 | [204] | ||
| External Link | ||||
| REM0046127 | Phase 1 | [205] | ||
| External Link | ||||
| ACU193 | Phase 1 | [206] | ||
| External Link | ||||
| Protollin | Phase 1 | [207] | ||
| External Link | ||||
| BMS-984923 | Phase 1 | [208] | ||
| External Link | ||||
| ALN-APP | Phase 1 | [209] | ||
| External Link | ||||
| MK-4334 | Phase 1 | [210] | ||
| External Link | ||||
| NNI-362 | Phase 1 | [211] | ||
| External Link | ||||
| COR588 | Phase 1 | [212] | ||
| External Link | ||||
| AL003 | Phase 1 | [213] | ||
| External Link | ||||
| LY3372993 | Phase 1 | [214] | ||
| External Link | ||||
| MER5101 | Phase 1 | [24] | ||
| External Link | ||||
| BMS-984923 | Phase 1 | [215] | ||
| Synonyms |
(4R,5R)-5-(2-Chlorophenyl)-4-(5-(phenylethynyl)pyridin-3-yl)oxazolidin-2-one; (4R,5R)-5-(2-chlorophenyl)-4-[5-(2-phenylethynyl)pyridin-3-yl]-1,3-oxazolidin-2-one; 1375752-78-5; 2-Oxazolidinone, 5-(2-chlorophenyl)-4-(5-(2-phenylethynyl)-3-pyridinyl)-, (4R,5R)-; 3I1803DK5Z; AKOS040756698; BDBM50536712; BMS984923; BMS-984923; CHEMBL4571075; CS-0087012; HY-122559; SCHEMBL4541143; UNII-3I1803DK5Z
Click to Show/Hide
|
|||
| External Link | ||||
| BIIB113 | Phase 1 | [216] | ||
| External Link | ||||
| 11C-6-Me-BTA-1 | Phase 1 | [217] | ||
| Synonyms |
Carbon-11-6-Me-BTA-1; 11C-BTA-1
Click to Show/Hide
|
|||
| External Link | ||||
| RVT-104 | Phase 1 | [24] | ||
| External Link | ||||
| V950 vaccine | Phase 1 | [218] | ||
| External Link | ||||
| PF-06751979 | Phase 1 | [47] | ||
| Synonyms |
ZLZUHACSRMOLLV-RAALSFIWSA-N; 1818339-66-0; UNII-1Y0Y126GUG; 1Y0Y126GUG; US9315520, Example 1; CHEMBL3952064; US9315520, Example 2; US9315520, Comparator 1; SCHEMBL17162186; BDBM223395; HY-112157; CS-0043501; US9315520, 1; N-(2-((4aR,6S,8aR)-2-Amino-6-methyl-4a,5,6,8-tetrahydro-4H-pyrano(3,4-d)(1,3)thiazin-8a-yl)thiazol-4-yl)-5-(difluoromethoxy)pyridine-2-carboxamide; N-{2-[(4aR,6S,8aR)-2-Amino-6-methyl-4,4a,5,6-tetrahydropyrano[3,4-d][1,3]thiazin-8a(8H)-yl]-1,3-thiazol-4-yl}-5-(difluoromethoxy)pyridine-2-carb
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-932481 | Phase 1 | [115] | ||
| External Link | ||||
| ABT-354 | Phase 1 | [219] | ||
| External Link | ||||
| Affitope AD-01 | Phase 1 | [220] | ||
| Synonyms |
AD-01; Amyloid beta 40-42 peptide vaccine (sc, Alzheimer's), AFFiRiS
Click to Show/Hide
|
|||
| External Link | ||||
| PTI-125 | Phase 1 | [24] | ||
| External Link | ||||
| CPC-212 | Phase 1 | [24] | ||
| External Link | ||||
| COR-388 | Phase 1 | [24] | ||
| External Link | ||||
| BIIB076 | Phase 1 | [24] | ||
| External Link | ||||
| CPC-250 | Phase 1 | [24] | ||
| External Link | ||||
| ACI-35 | Phase 1 | [221] | ||
| External Link | ||||
| PU-AD | Phase 1 | [24] | ||
| External Link | ||||
| BMS-241027 | Phase 1 | [222] | ||
| External Link | ||||
| AD03 vaccine | Phase 1 | [223] | ||
| External Link | ||||
| 11C-AZD-2184 | Phase 1 | [224] | ||
| Synonyms |
AZD-2184-[11C]; AZD-2184-[3H]; Tritium-AZD-2184; Beta amyloid modulator (iv PET ligand, AD), AstraZeneca; Carbon-11-AZD-2184; Hydrogen-3-AZD-2184; Beta amyloid modulator (iv PET ligand, Alzheimer's disease), AstraZeneca; 3H-AZD-2184
Click to Show/Hide
|
|||
| External Link | ||||
| GSI-136 | Phase 1 | [225] | ||
| Synonyms |
WAY-179642; WAY-208983; WAY-GSI-A; WAY-GSI-B; Gamma-secretase inhibitors (oral, Alzheimer's disease), Wyeth
Click to Show/Hide
|
|||
| External Link | ||||
| 18F-FEDAA-1106 | Phase 1 | [226] | ||
| Synonyms |
ZK-6032924; FEDAA-1106-[18F]; Fluorine-18-FEDAA-1106; 18F-labeled PET imaging agent (Alzheimer's disease), Bayer; 18F-labeled PET imaging agent (multiple sclerosis), Bayer
Click to Show/Hide
|
|||
| External Link | ||||
| PF-06648671 | Phase 1 | [47] | ||
| External Link | ||||
| BCI-632 | Phase 1 | [227] | ||
| Synonyms |
LFAGGDAZZKUVKO-JAGWWQSPSA-N; MGS-0039; CHEMBL186453; 569686-87-9; MGS0039; MGS 0039; SCHEMBL234576; GTPL1397; DTXSID40432407; BDBM50151435; (1R,2R,3R,5R,6R)-2-amino-3-[(3,4-dichlorophenyl)methoxy]-6-fluorobicyclo[310]hexane-2,6-dicarboxylic acid; (1r,2r,3r,5r,6r)-2-amino-3-(3,4-dichlorobenzyloxy)6-fluorobicyclo[310] hexane-2,6-dicarboxylic acid; (1R,2R,3R,5R,6R)-2-Amino-3-(3,4-dichlorobenzyloxy)-6-fluorobicyclo[310]hexane-2,6-dicarboxylic acid; (1R, 2R, 3R, 5R,
Click to Show/Hide
|
|||
| External Link | ||||
| ARC031 | Phase 1 | [228] | ||
| External Link | ||||
| RVT-103+RVT-104 | Phase 1 | [47] | ||
| External Link | ||||
| Exebryl-1 | Phase 1 | [229] | ||
| Synonyms |
Exebryl-1 (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| TAu mAb | Phase 1 | [24] | ||
| External Link | ||||
| PTI-80 | Phase 1 | [230] | ||
| External Link | ||||
| BAY-1006578 | Phase 1 | [231] | ||
| Synonyms |
Diagnostic PET imaging agent (Alzheimer's disease), Bayer
Click to Show/Hide
|
|||
| External Link | ||||
| RG7129 | Phase 1 | [232] | ||
| External Link | ||||
| SAR228810 | Phase 1 | [233] | ||
| External Link | ||||
| GRF6019 | Phase 1 | [24] | ||
| External Link | ||||
| 123I-MNI-168 | Phase 1 | [234] | ||
| Synonyms |
Alzheimer's diagnostic, IND; 123I-SPECT diagnostic (Alzheimer's disease), IND
Click to Show/Hide
|
|||
| External Link | ||||
| LY-2811376 | Phase 1 | [235] | ||
| Synonyms |
LY2811376; 1194044-20-6; (S)-4-(2,4-Difluoro-5-(pyrimidin-5-yl)phenyl)-4-methyl-5,6-dihydro-4H-1,3-thiazin-2-amine; LY 2811376; UNII-UR18YJ97SJ; UR18YJ97SJ; CHEMBL2333941; (4s)-4-[2,4-Difluoro-5-(Pyrimidin-5-Yl)phenyl]-4-Methyl-5,6-Dihydro-4h-1,3-Thiazin-2-Amine; (4S)-4-[2,4-Difluoro-5-(5-pyrimidinyl)phenyl]-5,6-dihydro-4-methyl-4H-1,3-thiazin-2-amine; J-501480; MJQMRGWYPNIERM-HNNXBMFYSA-N; 4H-1,3-Thiazin-2-amine, 4-(2,4-difluoro-5-(5-pyrimidinyl)phenyl)-5,6-dihydro-4-methyl-, (4S)-; 4H-1,3-Thiazin-2-amine,
Click to Show/Hide
|
|||
| External Link | ||||
| 123I-MNI-330 | Phase 1 | [236] | ||
| External Link | ||||
| SUVN-D4010 | Phase 1 | [24] | ||
| Synonyms |
DWTFBJGTRBMHPG-UHFFFAOYSA-N; UNII-GNQ25KYD72; GNQ25KYD72; 1428862-32-1; SCHEMBL14810657; 1-isopropyl-3-{5-[1-(3-methoxy propyl)piperidin-4-yl]-[1,3,4]oxadiazol-2-yl}-1H-indazole; 1-isopropyl-3-{5-[1-(3-methoxy propyl) piperidin-4-yl]-[1,3,4]oxadiazol-2-yl}-1H-indazole
Click to Show/Hide
|
|||
| External Link | ||||
| Mesenchymal stem cell therapy | Phase 1 | [24] | ||
| External Link | ||||
| AZD2184 | Phase 1 | [237] | ||
| External Link | ||||
| ASP-0777 | Phase 1 | [238] | ||
| External Link | ||||
| NPT088 | Phase 1 | [24] | ||
| External Link | ||||
| Aleplasinin | Phase 1 | [239] | ||
| Synonyms |
PAZ-417; PAI inhibitors (Alzheimers disease), Wyeth; Plasminogen activator inhibitor inhibitors (Alzheimers disease),Wyeth
Click to Show/Hide
|
|||
| External Link | ||||
| MEDI1841 | Phase 1 | [240] | ||
| External Link | ||||
| E-2212 | Phase 1 | [241] | ||
| External Link | ||||
| TTP-4000 | Phase 1 | [242] | ||
| Synonyms |
Extracellular RAGE domain (diabetic nephropathy), TransTech; Extracellular RAGE domain (Alzheimer's disease), TransTech
Click to Show/Hide
|
|||
| External Link | ||||
| ANVS301 | Phase 1 | [243] | ||
| Synonyms |
Bisnormcerysine
Click to Show/Hide
|
|||
| External Link | ||||
| E2012 | Phase 1 | [244] | ||
| Synonyms |
870843-42-8; E 2012; (E)-1-[(1S)-1-(4-FLUOROPHENYL)ETHYL]-3-[3-METHOXY-4-(4-METHYL-1H-IMIDAZOL-1-YL)BENZYLIDENE]PIPERIDIN-2-ONE; E-2012; UNII-3LSD4Y5F0F; 3LSD4Y5F0F; CHEMBL1224151; J-501810; (3E)-1-[(1S)-1-(4-fluorophenyl)ethyl]-3-[[3-methoxy-4-(4-methylimidazol-1-yl)phenyl]methylidene]piperidin-2-one; (e)-1-((1s)-1-(4-fluorophenyl)ethyl)-3-(3-methoxy-4-(4-methyl-1h-imidazol-1-yl)benzylidene)piperidin-2-one; PUOAETJYKQITMO-LANLRWRYSA-N
Click to Show/Hide
|
|||
| External Link | ||||
| NGP 555 | Phase 1 | [47] | ||
| Synonyms |
WDEKUGNKKOGFOA-UHFFFAOYSA-N; NGP555; 1304630-27-0; UNII-1XA7T7L527; CHEMBL2151100; SCHEMBL16198584; NGP-455; 1XA7T7L527; HY-108714; CS-0030521; 2-Thiazolamine, N-(5-ethyl-2,4-dimethylphenyl)-4-(3-fluoro-4-(4-methyl-1H-imidazol-1-yl)phenyl)-; N-(5-Ethyl-2,4-dimethylphenyl)-4-(3-fluoro-4-(4-methylimidazol-1-yl)phenyl)-1,3-thiazol-2-amine
Click to Show/Hide
|
|||
| External Link | ||||
| AV-965 | Phase 1 | [245] | ||
| External Link | ||||
| GSK2647544 | Phase 1 | [246] | ||
| Synonyms |
GSK-2647544
Click to Show/Hide
|
|||
| External Link | ||||
| NDX-1017 | Phase 1 | [24] | ||
| External Link | ||||
| GC021109 | Phase 1 | [24] | ||
| External Link | ||||
| NsG-0202 | Phase 1 | [247] | ||
| Synonyms |
ECB-AD; ECT-AD; ECT-NGF; ECT (Alzheimers Disease), NsGene; Encapsulated cell technology (Alzheimers Disease), NsGene; Encapsulated ARPE-19 cells (Alzheimers disease), NsGene
Click to Show/Hide
|
|||
| External Link | ||||
| SGC-1061 | Phase 1 | [248] | ||
| External Link | ||||
| NBXT-001+Nobilis inhalation device | Phase 1 | [47] | ||
| External Link | ||||
| Lu AF20513 | Phase 1 | [24] | ||
| External Link | ||||
| Begacestat | Phase 1 | [249] | ||
| Synonyms |
GSI-953; PF-05212362; PF-5212362; WAY-201953; WAY-210953
Click to Show/Hide
|
|||
| External Link | ||||
| BCI-838 | Discontinued in Phase 1 | [250] | ||
| External Link | ||||
| MK-3328 | Phase 1 | [251] | ||
| Synonyms |
UNII-4I12ES557C; MK-3328 F-18; 4I12ES557C; 1201324-21-1; (18F)MK-3328; [18F]MK-3328; CHEMBL2203396; SCHEMBL12268073
Click to Show/Hide
|
|||
| External Link | ||||
| JES-9501 | Phase 1 | [252] | ||
| Synonyms |
DHED; Dehydroevodiamine; Dehydroevodiamine hydrochloride
Click to Show/Hide
|
|||
| External Link | ||||
| TAK-071 | Phase 1 | [24] | ||
| Synonyms |
WFSARWQASFQZMG-FGZHOGPDSA-N; 1820812-16-5
Click to Show/Hide
|
|||
| External Link | ||||
| ANAVEX 2-73 | Phase 1 | [24] | ||
| External Link | ||||
| SPI-014 | Phase 1 | [253] | ||
| Synonyms |
SPI-1802; SPI-1810; Amyloid beta peptide 1-42 deposition inhibitor (AD), Satori; Gamma-secretase modulators (Alzheimers disease), Satori Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| HPP-854 | Phase 1 | [254] | ||
| External Link | ||||
| JNJ-63733657 | Phase 1 | [24] | ||
| External Link | ||||
| DSP-8658 | Phase 1 | [255] | ||
| External Link | ||||
| SOR-C13 | Phase 1 | [170] | ||
| External Link | ||||
| DWP-09031 | Phase 1 | [256] | ||
| Synonyms |
DWJ-301
Click to Show/Hide
|
|||
| External Link | ||||
| AZD2995 | Phase 1 | [237] | ||
| External Link | ||||
| Palomid-529 | Phase 1 | [257] | ||
| Synonyms |
P-529; P-529 (inhaled), Paloma; P-529 (ophthalmic), Paloma; P-529 (oral), Paloma; P-529 (topical), Paloma; P-529 drug eluting stent (cardiovascular disease), Paloma; Palomid-529 (inhaled), Paloma; Palomid-529 (ophthalmic), Paloma; Palomid-529 (oral), Paloma; Palomid-529 (topical), Paloma; Palomid-529 drug eluting stent (cardiovascular disease), Paloma; P-529 (intravitreal/subconjunctival), Paloma; Palomid-529 (intravitreal/subconjunctival), Paloma
Click to Show/Hide
|
|||
| External Link | ||||
| Protexia | Phase 1 | [258] | ||
| Synonyms |
RBChE; PEG-rBChE; PEGylated butyrylcholinesterase (recombinant human, nerve agent exposure), PharmAthene
Click to Show/Hide
|
|||
| External Link | ||||
| FGLL | Phase 1 | [259] | ||
| Synonyms |
NCAM mimetics (Alzheimer's disease), Enkam
Click to Show/Hide
|
|||
| External Link | ||||
| Posiphen R-phenserine | Phase 1 | [260] | ||
| External Link | ||||
| Anti-N3pG-Abeta antibody | Phase 1 | [261] | ||
| External Link | ||||
| CTS-21166 | Phase 1 | [262] | ||
| External Link | ||||
| MCD-386 | Phase 1 | [263] | ||
| Synonyms |
CDD-0102; CDD-0262; CDD-0264; CDD-102; MCD-386 Forte; MCD-386 Transderm; MCD-386CR; MCD-386 Forte/Transderm; Tetrahydropyrimidine muscarinic M1 agonists (Alzeimer's disease), Cognitive Pharmaceuticals; Tetrahydropyrimidine muscarinic M1 agonists (Alzeimer's disease), University of Toledo; Tetrahydropyrimidine muscarinicM1 agonists (Alzheimer's disease), Mithridion; MCD-386 (oral controlled release, CNS disorders), Mithridion; MCD-386 (high dose transdermal, Alzheimer's disease/schizophrenia), Mithridion; MCD-386 (high dose, Alzheimer's disease/schizophrenia), Mithridion; MCD-386 (transdermal, Alzheimer's disease/schizophrenia), Mithridion
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-3839 | Phase 1 | [264] | ||
| Synonyms |
GTPL6931; 4b05; (3S)-3-[2-(difluoromethyl)pyridin-4-yl]-7-fluoro-3-[3-(pyrimidin-5-yl)phenyl]-2,3-dihydro-1H-isoindol-1-amine; 32D
Click to Show/Hide
|
|||
| External Link | ||||
| MEDI1814 | Phase 1 | [24] | ||
| External Link | ||||
| ABT-957 | Phase 1 | [265] | ||
| External Link | ||||
| XEL 001HP | Phase 1 | [266] | ||
| External Link | ||||
| PF-05251749 | Phase 1 | [47] | ||
| External Link | ||||
| PF-04995274 | Phase 1 | [267] | ||
| Synonyms |
UNII-XI179PG9LV; PF 04995274; XI179PG9LV; 1331782-27-4; CHEMBL2152922; (R)-4-((4-(((4-(Tetrahydrofuran-3-yloxy)-1,2-benzisoxazol-3-yl)oxy)methyl)piperidin-1-yl)methyl)tetrahydro-2H-pyran-4-ol; PF04995274; compound 2d [PMID: 22974325]; SCHEMBL619629; GTPL9059; WLLOFQROROXOMO-GOSISDBHSA-N; ZINC95577747; BDBM50398598; DB12675; NCGC00386746-01; PF-04995274, > 4-[[4-[[4-[(3R)-oxolan-3-yl]oxy-1,2-benzoxazol-3-yl]oxymethyl]piperidin-1-yl]methyl]oxan-4-ol
Click to Show/Hide
|
|||
| External Link | ||||
| LY3303560 | Phase 1 | [47] | ||
| External Link | ||||
| AAB-003/PF-05236812 | Phase 1 | [268] | ||
| External Link | ||||
| GSK2981710 | Phase 1 | [269] | ||
| External Link | ||||
| ASP3662 | Phase 1 | [270] | ||
| External Link | ||||
| AAD-2004 | Phase 1 | [271] | ||
| Synonyms |
927685-43-6; UNII-11VWK61J69; 11VWK61J69; 2-Hydroxy-5-((4-(trifluoromethyl)phenethyl)amino)benzoic acid; Benzoic acid, 2-hydroxy-5-((2-(4-(trifluoromethyl)phenyl)ethyl)amino)-; Benzoic acid, 2-hydroxy-5-[[2-[4-(trifluoromethyl)phenyl]ethyl]amino]-; UTMVACIBQLDZLP-UHFFFAOYSA-N; SCHEMBL608498; ZINC34885635; AKOS027338686; SB16954; AS-35180; 2-HYDROXY-5-(2-(4-TRIFLUOROMETHYL-PHENYL)-ETHYLAMINO)-BENZOIC ACID
Click to Show/Hide
|
|||
| External Link | ||||
| TT-301 | Phase 1 | [272] | ||
| Synonyms |
Minozac; MW01-9-034WH; Anti-neuroinflammatories (Alzheimer's disease), NeuroMedix/Transition Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| methyl 5-[(4-tert-butylbenzoyl)amino]-2H-1,2,4-triazole-3-carboxylate | Clinical trial | [273] | ||
| Synonyms |
MLS001003740; SMR000347588; methyl 3-[(4-tert-butylbenzoyl)amino]-1H-1,2,4-triazole-5-carboxylate; AC1M4T6W; Oprea1_530669; Oprea1_180448; GTPL6553; SCHEMBL14688402; CHEMBL1595992; cid_2314952; BDBM33902; CHEBI:109731; HMS2702F21; AB00577675-02; SR-01000028101; SR-01000028101-1; 3-[[(4-tert-butylphenyl)-oxomethyl]amino]-1H-1,2,4-triazole-5-carboxylic acid methyl ester; methyl 3-[(4-tert-butylphenyl)carbonylamino]-1H-1,2,4-triazole-5-carboxylate
Click to Show/Hide
|
|||
| External Link | ||||
| bimatoprost (free acid form) | Clinical trial | [274] | ||
| Synonyms |
17-phenyl-omega-trinor-PGF2alpha
Click to Show/Hide
|
|||
| External Link | ||||
| JNJ-479655 | Clinical trial | [275] | ||
| Synonyms |
1428327-31-4; N-((4-(4-phenylpiperazin-1-yl)tetrahydro-2H-pyran-4-yl)methyl)-2-(phenylthio)nicotinamide; JNJ-47965567; JNJ 47965567; N-{[4-(4-Phenylpiperazin-1-Yl)oxan-4-Yl]methyl}-2-(Phenylsulfanyl)pyridine-3-Carboxamide; P2X Antagonist III; antagonist JNJ47965567; GTPL7538; CHEMBL2338352; MolPort-035-941-198; ZINC95590396; AKOS025142079; JNJ47965567; NCGC00387264-01; J-115; JNJ-47965567, > Z2235332565; N-[[4-(4-phenylpiperazin-1-yl)oxan-4-yl]methyl]-2-phenylsulfanylpyridine-3-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| alpha-ketothiazole analogue 36 | Clinical trial | [276] | ||
| Synonyms |
GTPL6549; CHEMBL273653; BDBM12976; (2S)-2-[[(1R)-1-(4-bromophenyl)ethyl]carbamoylamino]-5-(carbamoylamino)-N-[(2S)-1-[[(2S)-5-(diaminomethylideneamino)-1-oxo-1-(1,3-thiazol-2-yl)pentan-2-yl]amino]-3-methyl-1-oxobutan-2-yl]pentanamide
Click to Show/Hide
|
|||
| External Link | ||||
| PMID27998201-Compound-22 | Patented | [277] | ||
| External Link | ||||
| PMID28350212-Compound-20 | Patented | [278] | ||
| External Link | ||||
| PMID27998201-Compound-2 | Patented | [277] | ||
| External Link | ||||
| Schiff base compound 2 | Patented | [279] | ||
| Synonyms |
PMID29324067-Compound-53
Click to Show/Hide
|
|||
| External Link | ||||
| PMID29334795-Compound-22 | Patented | [280] | ||
| External Link | ||||
| PMID28350212-Compound-19 | Patented | [278] | ||
| External Link | ||||
| Thiadiazolyl carboxamide derivative 1 | Patented | [281] | ||
| Synonyms |
PMID28270010-Compound-Figure18-1
Click to Show/Hide
|
|||
| External Link | ||||
| Schiff base compound 1 | Patented | [279] | ||
| Synonyms |
PMID29324067-Compound-43
Click to Show/Hide
|
|||
| External Link | ||||
| PMID28350212-Compound-18 | Patented | [278] | ||
| External Link | ||||
| Zanapezil | Discontinued in Phase 3 | [282] | ||
| Synonyms |
TAK-147
Click to Show/Hide
|
|||
| External Link | ||||
| PF-1913539 | Discontinued in Phase 3 | [283] | ||
| Synonyms |
Sch-58261; Sch 58261; 160098-96-4; Sch58261; 2-(Furan-2-yl)-7-phenethyl-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine; UNII-4309023MAH; CHEMBL17127; 4309023MAH; 7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine; 2-(2-Furanyl)-7-(2-phenylethyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine; 5-Amino-7-(2-phenylethyl)-2-(2-furyl)-pyrazolo(4,3-e)-1,2,4-triazolo(1,5-c)pyrimidine; [3H]SCH 58261
Click to Show/Hide
|
|||
| External Link | ||||
| AZD0328 | Discontinued in Phase 2 | [284] | ||
| Synonyms |
AR-R23465XX; AZD-0328; Spiro[1-azabicyclo[2.2.2]octane-8,8'-7-oxa-5-azabicyclo[4.3.0]nona-2,4,10-triene]
Click to Show/Hide
|
|||
| External Link | ||||
| S-18986 | Discontinued in Phase 2 | [285] | ||
| Synonyms |
S 18986; UNII-IA262432Y9; 175340-20-2; S18986; IA262432Y9; MNTIJYGEITVWHU-SNVBAGLBSA-N; AC1LCV4Z; S18986-1; SCHEMBL6622911; S 18986-1; GTPL4304; CHEMBL320642; EX-A818; MolPort-023-276-986; (3aS)-2,3,3a,4-tetrahydro-1H-pyrrolo[2,1-c][1,2,4]benzothiadiazine 5,5-dioxide; ZINC3827020; AKOS024457867; (S)-2,3-dihydro-(3,4)cyclopentano-1,2,4-benzothiadiazine-1,1-dioxide; B5493; (3aS)-5,5Dioxo-2,3,3a,4-tetrahydro-1H-pyrrolo[2,1-c][1,2,4]benzothiadiazine
Click to Show/Hide
|
|||
| External Link | ||||
| AVN 397 | Discontinued in Phase 2 | [286] | ||
| External Link | ||||
| DPC-543 | Discontinued in Phase 2 | [287] | ||
| External Link | ||||
| NGD-97-1 | Discontinued in Phase 2 | [288] | ||
| Synonyms |
CP-457,920
Click to Show/Hide
|
|||
| External Link | ||||
| IPENOXAZONE | Discontinued in Phase 2 | [289] | ||
| Synonyms |
MLV-6976; NC-1200; (+)-(4S,5R)-4-(2-Methylpropyl)-3-[3-(perhydroazepin-1-yl)propyl]-5-phenyl-1,3-oxazolidin-2-one
Click to Show/Hide
|
|||
| External Link | ||||
| Ispronicline | Discontinued in Phase 2 | [290] | ||
| Synonyms |
RJR 1734; TC 01734; AZD-3480; RJR-1734; Ispronicline (INN/USAN); (E)-N-methyl-5-(5-propan-2-yloxypyridin-3-yl)pent-4-en-2-amine
Click to Show/Hide
|
|||
| External Link | ||||
| ABT-418 | Discontinued in Phase 2 | [291] | ||
| Synonyms |
Abt 418; 147402-53-7; 3-methyl-5-(1-methyl-2-pyrrolidinyl)isoxazole; CHEMBL274525; (S)-3-Methyl-5-(1-methyl-2-pyrrolidinyl)isoxazole; (S)-3-methyl-5-(1-methylpyrrolidin-2-yl)isoxazole; C9H14N2O; Isoxazole, 3-methyl-5-((2S)-1-methyl-2-pyrrolidinyl)-; Isoxazole, 3-methyl-5-(1-methyl-2-pyrrolidinyl)-, (S)-; Isoxazole, 3-methyl-5-[(2S)-1-methyl-2-pyrrolidinyl]-; 3-methyl-5-[(2S)-1-methylpyrrolidin-2-yl]-1,2-oxazole; AC1L3OPH; SCHEMBL194161; DTXSID10163711; ILLGYRJAYAAAEW-QMMMGPOBSA-N; ZINC3786099; BDBM50035398
Click to Show/Hide
|
|||
| External Link | ||||
| Talsaclidine fumarate | Discontinued in Phase 2 | [292] | ||
| Synonyms |
Wal 2014 FU; Talsaclidine fumarate (USAN); 1-Azabicyclo(2.2.2)octane, 3-(2-propynyloxy)-, (R)-, (E)-2-butenedioate (1:1); 3-(2-Propynyloxy)-1-azabicyclo(2.2.2)octane 2-butenedioate; 3-prop-2-ynoxy-1-azabicyclo[2.2.2]octane
Click to Show/Hide
|
|||
| External Link | ||||
| Affitope AD-02 | Discontinued in Phase 2 | [293] | ||
| Synonyms |
Alzheimer's disease vaccine 2 (AFFITOPE), AFFiRiS; Alzheimer's disease vaccine 2 (AFFITOPE), GlaxoSmithKline Biologicals
Click to Show/Hide
|
|||
| External Link | ||||
| SIB-1553A | Discontinued in Phase 2 | [294] | ||
| Synonyms |
191611-89-9; SIB 1553A hydrochloride; SIB 1553A Hyrdrochloride; SIB 1553A HCl; SCHEMBL6333252; SIB 1553A; MolPort-035-765-827; AKOS024458336; LS-104963; J-012387; (+/-)-4-[[2-(1-methyl-2-pyrrolidinyl)ethyl]thio]phenol hydrochloride; (+-)-4-((2-(1-Methyl-2-pyrrolidinyl)ethyl)thio)phenol hydrochloride; Phenol, 4-((2-(1-methyl-2-pyrrolidinyl)ethyl)thio)-, hydrochloride
Click to Show/Hide
|
|||
| External Link | ||||
| VP-025 | Discontinued in Phase 1 | [295] | ||
| Synonyms |
Inflammation therapeutics, Vasogen; VP-015
Click to Show/Hide
|
|||
| External Link | ||||
| TRx-0037 | Discontinued in Phase 1 | [296] | ||
| External Link | ||||
| EVT-301 | Discontinued in Phase 1 | [297] | ||
| Synonyms |
MAO B inhibitors (Alzheimer's disease), Evotec/Roche; Monoamineoxidase B inhibitors (Alzheimer's disease),Evotec/Roche
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-1080 | Discontinued in Phase 1 | [298] | ||
| External Link | ||||
| PF-05236812 | Discontinued in Phase 1 | [299] | ||
| Synonyms |
AAB-003
Click to Show/Hide
|
|||
| External Link | ||||
| C-9138 | Discontinued in Phase 1 | [300] | ||
| External Link | ||||
| SL-25.1188 | Discontinued in Phase 1 | [301] | ||
| External Link | ||||
| GR-253035 | Discontinued in Phase 1 | [302] | ||
| External Link | ||||
| TAK-065 | Discontinued in Phase 1 | [303] | ||
| Synonyms |
Neuroregeneration enhancer (oral, Alzheimers/Parkinsons disease), Takeda
Click to Show/Hide
|
|||
| External Link | ||||
| NP-61 | Discontinued in Phase 1 | [304] | ||
| Synonyms |
Dual binding site acetylcholinesterase inhibitor, Neuropharma; NP-00361; NP-0361; AChE inhibitor (Alzheimer's) Neuropharma (Zeltia); AChE inhibitor/beta amyloid secretion inhibitor (oral formulation, Alzheimer's disease), Noscira
Click to Show/Hide
|
|||
| External Link | ||||
| ACC-002 | Phase 0 | [305] | ||
| External Link | ||||
| INP102 | Phase 0 | [24] | ||
| External Link | ||||
| AAB-002 | Phase 0 | [306] | ||
| External Link | ||||
| SD1003 | Preclinical | [307] | ||
| External Link | ||||
| Z-Phe-Ala-diazomethylketone | Preclinical | [307] | ||
| Synonyms |
71732-53-1; CHEMBL2179950; carbobenzoxycarbonyl-phenylalanyl-alaninyldiazomethane; ZPAD; Z-FA-DMK; SCHEMBL9364460; SCHEMBL17747846; SCHEMBL17747847; ZINC4899534; BDBM50400264; MFCD00077029; (Z,3S)-1-diazonio-3-[[(2S)-3-phenyl-2-(phenylmethoxycarbonylamino)propanoyl]amino]but-1-en-2-olate; Q27278127; benzyl (S)-1-((S)-4-diazo-3-oxobutan-2-ylamino)-1-oxo-3-phenylpropan-2-ylcarbamate; benzyl N-[(2S)-1-[[(2S)-4-diazo-3-oxobutan-2-yl]amino]-1-oxo-3-phenylpropan-2-yl]carbamate
Click to Show/Hide
|
|||
| External Link | ||||
| SD1002 | Preclinical | [307] | ||
| External Link | ||||
| TPT-43 | Preclinical | [308] | ||
| External Link | ||||
| CX-1501 | Preclinical | [309] | ||
| Synonyms |
AMPA receptor modulators (ADHD, AD, sleep disorders), Cortex Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| SPH-1285 | Preclinical | [310] | ||
| Synonyms |
Galantamine derivatives, Sanochemia; SPH-1286; SPH-1359
Click to Show/Hide
|
|||
| External Link | ||||
| ANAVEX 1-41 | Preclinical | [311] | ||
| External Link | ||||
| VER-155008 | Preclinical | [307] | ||
| Synonyms |
Heat shock protein 70 inhibitor (cancer), Vernalis; Hsp 70 inhibitor (cancer), Vernalis
Click to Show/Hide
|
|||
| External Link | ||||
| Pepticlere | Preclinical | [312] | ||
| Synonyms |
DP-68; DP-74; Small peptide beta amyloid protein fibril formation inhibitors, Proteotech; Alzheimers disease therapy (nasal spray), ProteTech; Small peptide A-beta protein fibril formation inhibitors, Proteotech; Laminin alpha chain derivatives (Alzheimer's disease), Proteotech; 6-9 mer peptide analogs (Alzheimer's disease), Proteotech
Click to Show/Hide
|
|||
| External Link | ||||
| SCH-1359113 | Preclinical | [313] | ||
| Synonyms |
SCH-1341030; SCH-1682496; SCH-745966; SCH-747123; SCH-785532; BACE-1 inhibitors (Alzheimer's disease); BACE-1 inhibitors (Alzheimer's disease), Merck & Co; BACE-1 inhibitors (Alzheimer's disease), Schering-Plough; Beta-secretase inhibitors (Alzheimer's disease), Schering-Plough
Click to Show/Hide
|
|||
| External Link | ||||
| humanin | Preclinical | [314] | ||
| Synonyms |
formyl humanin
Click to Show/Hide
|
|||
| External Link | ||||
| CDD-0199-J | Preclinical | [315] | ||
| External Link | ||||
| Alvameline | Terminated | [316] | ||
| Synonyms |
Alvameline maleate; LU-25077; LU-25109; LU-31126; LU-32181; Lu-25109M
Click to Show/Hide
|
|||
| External Link | ||||
| Ro-46-5934 | Terminated | [317] | ||
| External Link | ||||
| IDRA-21 | Terminated | [318] | ||
| Synonyms |
IDRA-21 analogs; IDRA-5
Click to Show/Hide
|
|||
| External Link | ||||
| BIBN-99 | Terminated | [319] | ||
| Synonyms |
Bibn 99; 145301-48-0; AC1L31GK; SCHEMBL194898; DTXSID00162975; N-(3-(1-(2-(8-Chloro-5,6-dihydro-6-oxo-11H-pyrido(2,3-b)(1,4)benzodiazepin-11-yl)-2-oxoethyl)-4-piperidinyl)propyl)-N-ethyl-2,2-dimethylpentanamide; L008252; N-[3-[1-[2-(8-chloro-6-oxo-5H-pyrido[2,3-b][1,4]benzodiazepin-11-yl)-2-oxoethyl]piperidin-4-yl]propyl]-N-ethyl-2,2-dimethylpentanamide; Pentanamide, N-(3-(1-(2-(8-chloro-5,6-dihydro-6-oxo-11H-pyrido(2,3-b)(1,4)benzodiazepin-11-yl)-2-oxoethyl)-4-piperidinyl)propyl)-N-ethyl-2,2-dimethyl-
Click to Show/Hide
|
|||
| External Link | ||||
| RS-66252 | Terminated | [320] | ||
| External Link | ||||
| ZK-93426 | Terminated | [321] | ||
| Synonyms |
ZK 93426; ZK93426; ZK 93 426; CHEMBL1271047; 89592-45-0; 5-Isopropoxy-4-methyl-beta-carboline-3-carboxylic acid ethyl ester; Ethyl 4-methyl-5-(1-methylethoxy)-9H-pyrido(3,4-b)indole-3-carboxylate; 9H-Pyrido(3,4-b)indole-3-carboxylic acid, 4-methyl-5-(1-methylethoxy)-, ethyl ester; AC1L3GQB; SCHEMBL195334; GTPL4347; CHEBI:93201; ZINC5857864; BDBM50329658; NCGC00161396-01; LS-178094; BRD-K68392338-003-01-2; ethyl 5-isopropoxy-4-methyl-9H-pyrido[3,4-b]indole-3-carboxylate; ethyl 4-methyl-5-propan-2-yloxy-9H-pyrido[5,4-
Click to Show/Hide
|
|||
| External Link | ||||
| CEP-427 | Terminated | [322] | ||
| External Link | ||||
| EHT-1864 | Terminated | [323] | ||
| Synonyms |
EHT-0101; EHT-0206; EHT-101; EHT-206; Rac1 inhibitor (Alzheimer's disease/cancer),Exonhit
Click to Show/Hide
|
|||
| External Link | ||||
| CI-1002 | Terminated | [324] | ||
| Synonyms |
PD-142676
Click to Show/Hide
|
|||
| External Link | ||||
| BU-4514N | Terminated | [325] | ||
| Synonyms |
BU 4514N; AC1Q6BPU; AC1L4UFU; SCHEMBL194669; 4-[(2-{2-[(5-amino-6-methyltetrahydro-2h-pyran-2-yl)oxy]propyl}-1,3,6-trimethyl-1,2,4a,5,6,7,8,8a-octahydronaphthalen-1-yl)carbonyl]-5-hydroxy-1,2-dihydro-3h-pyrrol-3-one
Click to Show/Hide
|
|||
| External Link | ||||
| DNP-004089 | Terminated | [326] | ||
| Synonyms |
BACE inhibitors, De Novo; Beta-amyloid converting enzyme inhibitors,De Novo; Beta-secretase inhibitors, De Novo
Click to Show/Hide
|
|||
| External Link | ||||
| MF-8615 | Terminated | [327] | ||
| External Link | ||||
| FR-152558 | Terminated | [328] | ||
| External Link | ||||
| NS-649 | Terminated | [329] | ||
| External Link | ||||
| MDL-28170 | Terminated | [330] | ||
| Synonyms |
MDL-2170
Click to Show/Hide
|
|||
| External Link | ||||
| NS-377 | Terminated | [331] | ||
| External Link | ||||
| R-1315 | Terminated | [332] | ||
| External Link | ||||
| L-698544 | Terminated | [333] | ||
| Synonyms |
7-Chloro-3-nitro-3,4-dihydroquinolin-2(1H)-one; 147778-05-0; 7-chloro-3-nitro-3,4-dihydro-1H-quinolin-2-one; CHEMBL102574; 2(1H)-Quinolinone,7-chloro-3,4-dihydro-3-nitro-; ACMC-1C9KO; SCHEMBL8271408; CTK4C5567; DTXSID50436916; BDBM50038176; 3697AJ; AKOS024260378; AB06846; AK153524; DB-063735; AX8026622; FT-0703007; Z-7553; CNDQ
Click to Show/Hide
|
|||
| External Link | ||||
| GYKI-52466 | Terminated | [334] | ||
| Synonyms |
102771-26-6; GYKI 52466; UNII-471V8NZ5X3; GYKI 52466 HCl; CHEMBL275006; CHEBI:79560; 471V8NZ5X3; 1-(p-Aminophenyl)-4-methyl-7,8-methylenedioxy-5H-2,3-benzodiazepine hydrochloride; 4-(8-methyl-9h-[1,3]dioxolo[4,5-h][2,3]benzodiazepin-5-yl)aniline; 4-(8-Methyl-9H-1,3-dioxolo(4,5-h)(2,3)benzodiazepin-5-yl)benzenamine; Benzenamine,4-(8-methyl-9H-1,3-dioxolo[4,5-h][2,3]benzodiazepin-5-yl)-; Benzenamine, 4-(8-methyl-9H-1,3-dioxolo(4,5-h)(2,3)benzodiazepin-5-yl)-; C17H15N3O2
Click to Show/Hide
|
|||
| External Link | ||||
| Nerve growth factor | Terminated | [335] | ||
| Synonyms |
Neuleze; Nerve growth factor (recombinant); RhNGF, Genentech; NGF-beta, Genentech; Nerve growth factor (recombinant), Genentech; Nerve growth factor, Mitsubishi/Dompe/Fidia
Click to Show/Hide
|
|||
| External Link | ||||
| F-3796 | Terminated | [336] | ||
| External Link | ||||
| NNC-90-0270 | Terminated | [337] | ||
| Synonyms |
Nicotinic ACh agonists, Novo Nordisk
Click to Show/Hide
|
|||
| External Link | ||||
| F-14413 | Terminated | [338] | ||
| Synonyms |
Alpha-2 adrenoceptor antagonist (Alzheimer's disease), Pierre Fabre
Click to Show/Hide
|
|||
| External Link | ||||
| GT-4001 | Terminated | [339] | ||
| External Link | ||||
| SAN-61 | Terminated | [340] | ||
| Synonyms |
SAN-AL-61; Oral beta amyloid inhibitor (Alzheimer's disease), Sanomune
Click to Show/Hide
|
|||
| External Link | ||||
| Milacemide | Terminated | [341] | ||
| Synonyms |
Glyzac; Glyzan; Milacemide hydrochloride; CP-1552S
Click to Show/Hide
|
|||
| External Link | ||||
| Sch-57790 | Terminated | [342] | ||
| Synonyms |
CHEMBL73341; SCH57790; GTPL350; SCHEMBL194921; SCH 57790; BDBM50092317; 2-(4-cyclohexylpiperazin-1-yl)-2-[4-(4-methoxyphenyl)sulfinylphenyl]acetonitrile; L018294
Click to Show/Hide
|
|||
| External Link | ||||
| MF268 | Terminated | [343] | ||
| Synonyms |
MF 268; 154619-51-9; 5-O-[8-(cis-2,6-dimethylmorpholino)octylcarbamoyl]eseroline; (3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydropyrrolo[2,3-b]indol-5-yl {8-[(2R,6S)-2,6-dimethylmorpholin-4-yl]octyl}carbamate; Carbamic acid, (8-(2,6-dimethyl-4-morpholinyl)octyl)-, 1,2,3,3a,8,8a-hexahydro-1,3a,8-trimethylpyrrolo(2,3-b)indol-5-yl ester, (3aS-(3a-alpha,5(2R*,6S*),8a-alpha))-; AC1MINCK; SCHEMBL2405111; CHEBI:43927; LS-49610; [(3aR,8bS)-3,4,8b-trimethyl-2,3a-dihydro-1H-pyrrolo[2,3-b]indol-7-yl] N-[8-[(2S,6R)-2,6-dimet
Click to Show/Hide
|
|||
| External Link | ||||
| Z-4105 | Terminated | [344] | ||
| Synonyms |
119737-52-9; Butanoic acid, 4-(((3-methyl-5-isoxazolyl)carbonyl)amino)-; Butanoic acid,4-[[(3-methyl-5-isoxazolyl)carbonyl]amino]-; ACMC-20cew8; AC1Q5P7F; SCHEMBL195378; AC1L4P39; CTK4B1474; 4-[(3-methyl-1,2-oxazole-5-carbonyl)amino]butanoic acid; DTXSID50152568; 4-{[(3-methyl-1,2-oxazol-5-yl)carbonyl]amino}butanoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| RU-33965 | Terminated | [345] | ||
| Synonyms |
RU 33965; 122321-05-5; AC1L2W1Z; SCHEMBL195494; DTXSID50153570; 6H-Imidazo(1,5-a)(1,4)benzodiazepin-6-one, 3-(cyclopropylcarbonyl)-4,5-dihydro-5-methyl-
Click to Show/Hide
|
|||
| External Link | ||||
| RS 86 | Terminated | [346] | ||
| Synonyms |
RS-86; UNII-5ASV6M91QU; 5ASV6M91QU; 2-Ethyl-8-methyl-2,8-diazaspiro(4,5)decane-1,3-dione hydrobromide; 2,8-Diazaspiro(4,5)decane-1,3-dione, 2-ethyl-8-methyl-, hydrobromide; 2-ethyl-8-methyl-2,8-diazaspiro[4.5]decane-1,3-dione hydrobromide(1:1); CHEMBL542883; RS-86 hydrobromide; RS 86, hydrobromide; 2-ethyl-8-methyl-2,8-diazaspiro[4,5]decane-1,3-dione hydrobromide; AC1L3EP8; 7524-74-5; C11H18N2O2.HBr; AC1Q23N4; CTK8D5439; 2-Nitrophenyl -D-galactopyranoside; 2-Nitrophenyl; A-D-galactopyranoside
Click to Show/Hide
|
|||
| External Link | ||||
| FR-121196 | Terminated | [347] | ||
| Synonyms |
FR 121196; 133920-65-7; N-(4-Acetyl-1-piperazinyl)-4-fluorobenzenesulfonamide; PZQKOVUNWPDCCQ-UHFFFAOYSA-N; FR121196; ACMC-20mv5f; AC1L2ZLZ; SCHEMBL195526; CTK4B8875; DTXSID90158423; Piperazine, 1-acetyl-4-(((4-fluorophenyl)sulfonyl)amino)-
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-2858 | Terminated | [348] | ||
| Synonyms |
AZD2858; 486424-20-8; AZD 2858; CHEMBL2177161; 3-amino-6-[4-(4-methylpiperazin-1-yl)sulfonylphenyl]-N-pyridin-3-ylpyrazine-2-carboxamide; 3-amino-6-(4-((4-methylpiperazin-1-yl)sulfonyl)phenyl)-N-(pyridin-3-yl)pyrazine-2-carboxamide; 3-Amino-6-{4-[(4-Methylpiperazin-1-Yl)sulfonyl]phenyl}-N-Pyridin-3-Ylpyrazine-2-Carboxamide; GSK-3 inhibition; 3-amino-6-{4-[(4-methylpiperazin-1-yl)sulfonyl]phenyl}-N-(pyridin-3-yl)pyrazine-2-carboxamide; GTPL8478; SCHEMBL3327702; C21H23N7O3S; EX-A707; AOB6850; MolPort-035-395-808
Click to Show/Hide
|
|||
| External Link | ||||
| CEP-431 | Terminated | [349] | ||
| Synonyms |
Clipsin inhibitor, Cephalon
Click to Show/Hide
|
|||
| External Link | ||||
| NP-7557 | Terminated | [350] | ||
| Synonyms |
Alzheimers disease therapy, Nastech; AChEI therapy (intranasal), Nastech
Click to Show/Hide
|
|||
| External Link | ||||
| GT-2016 | Terminated | [351] | ||
| Synonyms |
GT 2016; CHEMBL14812; 152241-24-2; SCHEMBL3395544; BDBM86490; MolPort-023-276-421; ZINC1537834; PDSP2_001477; PDSP1_001493; AKOS024457088; API0010299; NCGC00371075-01; LS-193753; L009653
Click to Show/Hide
|
|||
| External Link | ||||
| A-72055 | Terminated | [352] | ||
| External Link | ||||
| ABS-301 | Terminated | [353] | ||
| Synonyms |
ABS-302; ABS-303; ABS-304; Tacrine analogs, ABS
Click to Show/Hide
|
|||
| External Link | ||||
| ZK-91296 | Terminated | [354] | ||
| Synonyms |
ZK 91296; 83910-34-3; Ethyl 5-benzyloxy-4-methoxymethyl-beta-carboline-3-carboxylate; 9H-Pyrido(3,4-b)indole-3-carboxylic acid, 4-(methoxymethyl)-5-(phenylmethoxy)-, ethyl ester; WFPXOWCKXUEKCA-UHFFFAOYSA-N; AC1Q64YJ; SCHEMBL8845201; AC1L3X51; zk91296; BDBM85040; PDSP2_001753; ethyl 5-(benzyloxy)-4-(methoxymethyl)-9h-; PDSP1_001770; CAS_123700; NSC_123700; LS-187464; LS-186794; 5-benzyloxy-4-methoxymethyl-beta-carboline-3-carboxylic acid-ethyl ester
Click to Show/Hide
|
|||
| External Link | ||||
| BIBN-140 | Terminated | [355] | ||
| Synonyms |
Bibn 140; 145301-79-7; SCHEMBL8879830; Pentanamide, N-(3-(1-(2-(10,11-dihydro-11-oxo-5H-dibenzo(b,e)(1,4)diazepin-5-yl)-2-oxoethyl)-4-piperidinyl)propyl)-N-ethyl-2,2-dimethyl-
Click to Show/Hide
|
|||
| External Link | ||||
| Bifemelane | Terminated | [356] | ||
| Synonyms |
Alnert; Celeport; E-0687; MCI-2016; SON-216
Click to Show/Hide
|
|||
| External Link | ||||
| Sibopirdine | Terminated | [357] | ||
| Synonyms |
DuP-921; EXP-9121; EXP-921; 5H-Cyclopenta[2,1-b:3,4-b']dipyridine, 5,5-bis(4-pyridinylmethyl)-, monohydrate
Click to Show/Hide
|
|||
| External Link | ||||
| Tenilsetam | Terminated | [358] | ||
| Synonyms |
CAS-997
Click to Show/Hide
|
|||
| External Link | ||||
| AD-0802 | Investigative | [180] | ||
| Synonyms |
Humanized monoclonal antibody vaccine (Alzheimers disease), Bioarctic
Click to Show/Hide
|
|||
| External Link | ||||
| VK-11 | Investigative | [180] | ||
| Synonyms |
Imaging agent (Alzheimer's disease), Prana
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-6319 | Investigative | [359] | ||
| Synonyms |
Alpha 7 neuronal nicotinic receptor agonist (Alzheimer's disease), AstraZeneca
Click to Show/Hide
|
|||
| External Link | ||||
| PD-2015 | Investigative | [360] | ||
| Synonyms |
PD-2016; TNF alpha inhibitors (Alzheimers disease); TNF alpha inhibitors (Alzheimers disease), P2D Bioscience
Click to Show/Hide
|
|||
| External Link | ||||
| CBNU-06 | Investigative | [360] | ||
| External Link | ||||
| RAP-310 | Investigative | [361] | ||
| Synonyms |
Small stabilized receptor active peptide (Alzheimers disease), RAPID Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| Tideglusib | Phase 2 | [362] | ||
| External Link | ||||
| DWJ-209 | Investigative | [360] | ||
| External Link | ||||
| EVP-4473 | Investigative | [359] | ||
| External Link | ||||
| ReN-004 | Investigative | [360] | ||
| Synonyms |
Stem cell therapy (neurological disorders), ReNeuron
Click to Show/Hide
|
|||
| External Link | ||||
| KD-901 | Investigative | [360] | ||
| External Link | ||||
| NAChR APLs | Investigative | [360] | ||
| Synonyms |
NAChR APLs (Alzheimer's disease)
Click to Show/Hide
|
|||
| External Link | ||||
| COG-248 | Investigative | [360] | ||
| Synonyms |
Apolipoprotein E agonist series (Alzheimer's/Parkinsons disease); Neuroprotectant (Alzheimers/Parkinsons disease), Cognosci; Apolipoprotein E agonist series (Alzheimer's/Parkinsons disease), Cognosci
Click to Show/Hide
|
|||
| External Link | ||||
| ELND-007 | Investigative | [363] | ||
| Synonyms |
ELN-318463; ELN-318611; ELN-44989; ELN-475513; ELN-475516; ELN-480271; ELN-481090; ELN-481970; ELND-006; Gamma secretase inhibitors (Alzheimer's disease), Elan
Click to Show/Hide
|
|||
| External Link | ||||
| BAN-2203 | Investigative | [180] | ||
| Synonyms |
Beta amyloid modulator (Alzheimer's disease), BioArctic
Click to Show/Hide
|
|||
| External Link | ||||
| Turmeric extracts | Investigative | [180] | ||
| Synonyms |
HSS-808; HSS-818; HSS-838; HSS-848; HSS-888; Turmeric extracts (Alzheimer's disease); Turmeric extracts (Alzheimer's disease), HerbalScience
Click to Show/Hide
|
|||
| External Link | ||||
| Ginkgo biloba extract | Investigative | [360] | ||
| Synonyms |
YY-1224; YY-1824; Ginkgo biloba extract (Parkinson's disease/Alzheimer's disease); Ginkgo biloba extract (Parkinson's disease/Alzheimer's disease), Yuyu Inc
Click to Show/Hide
|
|||
| External Link | ||||
| SX-AZD1 | Investigative | [360] | ||
| External Link | ||||
| AIKb2 | Investigative | [360] | ||
| External Link | ||||
| NAChR alpha 7 APLs | Investigative | [360] | ||
| Synonyms |
NAChR alpha 7 APLs (Alzheimer's disease)
Click to Show/Hide
|
|||
| External Link | ||||
| 11A1 | Investigative | [180] | ||
| External Link | ||||
| Gamma-secretase modulators | Investigative | [364] | ||
| Synonyms |
Gamma-secretase modulators (Alzheimer's disease); Gamma-secretase modulators (Alzheimer's disease), F Hoffmann-La Roche
Click to Show/Hide
|
|||
| External Link | ||||
| MCD-386/glycopyrrolate | Investigative | [360] | ||
| Synonyms |
13283-82-4; (1,1-dimethylpyrrolidin-1-ium-3-yl) 2-cyclopentyl-2-hydroxy-2-phenylacetate; Glycopyrronium (USAN); NCGC00179456-02; Tovanor Breezhaler; zlchem 10; beta-1-Methyl-3-pyrrolidyl-alpha-cyclopentylmandelate methobromide; G00010-Watson-Int; Prestwick2_000746; Prestwick1_000746; Prestwick3_000746; Prestwick0_000746; AC1Q60WT; BSPBio_000732; SCHEMBL133002; SPBio_002671; AC1L1G28; BPBio1_000806; GTPL7459; Pyrrolidinium,
Click to Show/Hide
|
|||
| External Link | ||||
| HL-026 | Investigative | [360] | ||
| External Link | ||||
| ESN-XX | Investigative | [360] | ||
| External Link | ||||
| ETN-001 | Investigative | [360] | ||
| External Link | ||||
| DBT-1339 | Investigative | [180] | ||
| Synonyms |
DBTA-1339; DBTAI-1339; DWJ-501; DWK-1339; Beta-amyloid aggregation inhibitors (Alzheimer's), Digital Biotech; Beta-amyloid deposition inhibitors (Alzheimer's), Digital Biotech; Beta-amyloid aggregation/deposition inhibitors (Alzheimer's), Digital Biotech
Click to Show/Hide
|
|||
| External Link | ||||
| GRL-11097 | Investigative | [360] | ||
| Synonyms |
Memapsin 2 inhibitors (Alzheimers); Memapsin 2 inhibitors (Alzheimers), Purdue University/Astellas
Click to Show/Hide
|
|||
| External Link | ||||
| KNX-Monoclonal204 | Investigative | [180] | ||
| Synonyms |
KNX-Monoclonal205; KNX-Monoclonal205); Passive immunotherapy (Alzheimers disease), Kinexis
Click to Show/Hide
|
|||
| External Link | ||||
| KMS-88009 | Investigative | [180] | ||
| Synonyms |
KMS-88016; KMSB-600; Aminostyrylbenzofuran derivative beta amyloid fibril formation inhibitors (Alzheimer's disease), Korea Institute of Science and Technology/Seoul National University/Hanmi Pharmaceutical
Click to Show/Hide
|
|||
| External Link | ||||
| NGN-9079 | Investigative | [360] | ||
| Synonyms |
Alzheimer's disease therapy, NeuroGeneration
Click to Show/Hide
|
|||
| External Link | ||||
| PNB-04 | Investigative | [360] | ||
| Synonyms |
Alzheimer's disease therapy, PharmaNeuroBoost
Click to Show/Hide
|
|||
| External Link | ||||
| IMD-4482 | Investigative | [360] | ||
| Synonyms |
IMD-4690; PAI-1 inhibitor (oral, Alzheimers disease), IMMD; Plasminogen activator inhibitor-1 inhibitor (oral, Alzheimers disease), Institute of Medicinal Molecular Design
Click to Show/Hide
|
|||
| External Link | ||||
| Abloid | Investigative | [180] | ||
| Synonyms |
Amyloid protein deposition inhibitor (Alzheimer's disease), Virionics
Click to Show/Hide
|
|||
| External Link | ||||
| Memex | Investigative | [360] | ||
| Synonyms |
Nicotinomide adenine dinucleotide (NADH)
Click to Show/Hide
|
|||
| External Link | ||||
| CDD-0235-J | Investigative | [360] | ||
| External Link | ||||
| NP-103 | Investigative | [360] | ||
| Synonyms |
NP-060103; NP-60103; Glycogen synthase kinase-3 inhibitor (Alzheimer's disease), Neuropharma
Click to Show/Hide
|
|||
| External Link | ||||
| Leptin | Investigative | [365] | ||
| Synonyms |
Leptin (Alzheimer's disease)
Click to Show/Hide
|
|||
| External Link | ||||
| SP-08 | Investigative | [180] | ||
| Synonyms |
SP-008
Click to Show/Hide
|
|||
| External Link | ||||
| SPI-017 | Investigative | [360] | ||
| Synonyms |
SPI-017 (oral, Alzheimer's disease)
Click to Show/Hide
|
|||
| External Link | ||||
| RECALL-VAX | Investigative | [180] | ||
| Synonyms |
RV-01; RV-02; Beta-amyloid fragment-tetanus toxoid prophylactic vaccine (Alzheimer's disease); Beta-amyloid fragment-tetanus toxoid prophylactic vaccine (Alzheimer's disease), Intellect Neuroscience
Click to Show/Hide
|
|||
| External Link | ||||
| CWF-0804 | Investigative | [360] | ||
| External Link | ||||
| ACU-0101979 | Investigative | [180] | ||
| Synonyms |
HuC091; ACU-5A5; ADDL formation inhibitors, Acumen/Merck; Anti-ADDL antibodies (Alzheimers disease); Anti-ADDL antibodies (Alzheimers disease), Acumen/Merck & Co; Anti-ADDL vaccines (Alzheimers disease), Acumen/Merck & Co
Click to Show/Hide
|
|||
| External Link | ||||
| Cystatin C | Investigative | [360] | ||
| Synonyms |
Cystatin C (Alzheimer's disease)
Click to Show/Hide
|
|||
| External Link | ||||
| EDN-OL1 | Investigative | [180] | ||
| Synonyms |
Amyloid beta oligonucleotide (Alzheimer's disease/Down syndrome), Edunn
Click to Show/Hide
|
|||
| External Link | ||||
| ADepVac | Investigative | [360] | ||
| Synonyms |
DNA vaccine (Alzheimer's disease), University of California Irvine; DNA vaccine (TriGrid electroporation, Alzheimers), Ichor/UCI/IMM
Click to Show/Hide
|
|||
| External Link | ||||
| Anticalin | Investigative | [180] | ||
| Synonyms |
Anticalin (Alzheimer's disease)
Click to Show/Hide
|
|||
| External Link | ||||
| ACI-518 | Investigative | [360] | ||
| Synonyms |
AMPA glutamate receptor agonist (Alzheimer's disease), AC Immune
Click to Show/Hide
|
|||
| External Link | ||||
| SEN-1500 | Investigative | [180] | ||
| Synonyms |
SEN-1576; Beta-amyloid aggregation inhibitors (Alzheimer's disease); Beta-amyloid aggregation inhibitors (Alzheimer's disease), Senexis
Click to Show/Hide
|
|||
| External Link | ||||
| ARN-2966 | Investigative | [366] | ||
| Synonyms |
ARN-4261; Abeta aggregation inhibitors (Alzheimer's disease); Abeta aggregation inhibitors (Alzheimer's disease), New York University/Aria Neurosciences
Click to Show/Hide
|
|||
| External Link | ||||
| AZ-AAV9 | Investigative | [360] | ||
| Synonyms |
Alzheimers disease gene therapy, RegenX Biosciences; Adeno-associated virus vector-9 based gene therapy (injectable,AD), RegenX Biosciences
Click to Show/Hide
|
|||
| External Link | ||||
| ACI-636 | Investigative | [180] | ||
| Synonyms |
Morphomers; ACI-140; Beta amyloid beta sheet formation inhibitor (CNS diseases), AC Immune; Beta-amyloid oligomer inhibitors (Alzheimer's disease), AC Immune
Click to Show/Hide
|
|||
| External Link | ||||
| TKP-1001 | Investigative | [180] | ||
| Synonyms |
Amyloid-beta modulator (Alzheimer's disease), EUSA Pharma; Amyloid-beta modulator (Alzheimer's disease), Talisker Pharma; Amyloid-beta synthesis modulator (Alzheimer's disease), The Open University
Click to Show/Hide
|
|||
| External Link | ||||
| NP-17 | Investigative | [360] | ||
| Synonyms |
NP-21; NPM-01; NPM-05 series; NPM-05B1; NPM-05B2; Alpha-secreatse activators (oral, Alzheimer's disease), Noscira
Click to Show/Hide
|
|||
| External Link | ||||
| ANA-5 | Investigative | [180] | ||
| External Link | ||||
| NXD-9062 | Investigative | [360] | ||
| External Link | ||||
| LNK-3186 | Investigative | [360] | ||
| External Link | ||||
| A-887755 | Investigative | [180] | ||
| External Link | ||||
| ARC-069 | Investigative | [180] | ||
| Synonyms |
Gamma secretase inhibitors (Alzheimer's disease), Archer Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| NNI-AD | Investigative | [360] | ||
| Synonyms |
NNI-251; NNI-362; NNI-C; NNI-X01; AD therapy (oral), Neuronascent
Click to Show/Hide
|
|||
| External Link | ||||
| AZP-2006 | Investigative | [360] | ||
| Synonyms |
Amyloid precursor protein modulator (AD), AlzProtect/INSERM/University of Lille II
Click to Show/Hide
|
|||
| External Link | ||||
| AC-4402 | Investigative | [360] | ||
| Synonyms |
GABAA receptor inverse agonist (Alzheimer's disease), Dainippon Sumitomo Pharma
Click to Show/Hide
|
|||
| External Link | ||||
| GT 1061 | Investigative | [360] | ||
| External Link | ||||
| LNK-3248 | Investigative | [360] | ||
| External Link | ||||
| UC-1011 | Investigative | [367] | ||
| Synonyms |
UC-2021; UC-2025; GABA A receptor antagonists (Alzheimer's disease); GABA A receptor antagonists (Alzheimer's disease), Umecrine; 3beta-20beta-dihydroxy-5alpha-pregnane; 3beta-20beta-dihydroxy-5alpha-pregnane (Alzheimer's disease), Umecrine
Click to Show/Hide
|
|||
| External Link | ||||
| F-18 T808 | Investigative | [360] | ||
| External Link | ||||
| KD-501 | Investigative | [360] | ||
| External Link | ||||
| Haw-AD-14 | Investigative | [360] | ||
| External Link | ||||
| CDD-190 | Investigative | [360] | ||
| External Link | ||||
| 123I-DRM-106 | Investigative | [180] | ||
| Synonyms |
DRM-106-[123I]; Iodine-123-DRM-106; SPECT diagnostic (Alzheimer's disease), Fujifilm RI Pharma Co Ltd
Click to Show/Hide
|
|||
| External Link | ||||
| PN-403 | Investigative | [360] | ||
| External Link | ||||
| Mimovax | Investigative | [180] | ||
| Synonyms |
MV-01; AFFITOPE-based vaccine targeting truncated Abeta40/42 (Alzheimer's disease), AFFiRiS
Click to Show/Hide
|
|||
| External Link | ||||
| SEL-103 | Investigative | [360] | ||
| External Link | ||||
| Molecule 22 | Investigative | [360] | ||
| Synonyms |
MARK3 inhibitors (Alzheimer's disease); MARK3 inhibitors (Alzheimer's disease), Merck & Co
Click to Show/Hide
|
|||
| External Link | ||||
References