m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03149
|
[1] | |||
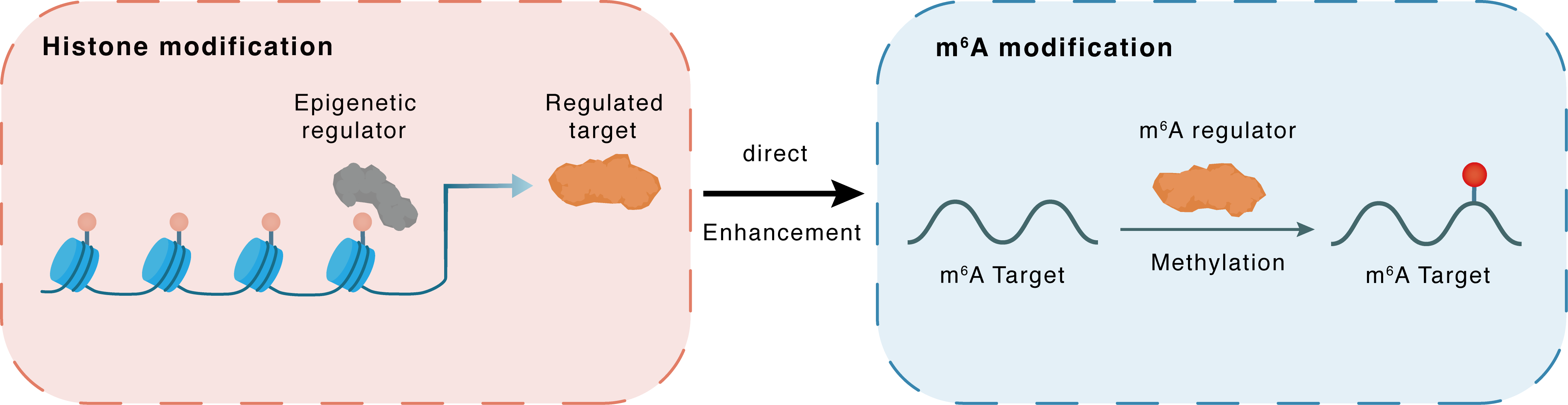 Histone modification
H3K9me2
G9a
METTL3
Direct
Enhancement
m6A modification
CD274
CD274
METTL3
Methylation
Histone modification
H3K9me2
G9a
METTL3
Direct
Enhancement
m6A modification
CD274
CD274
METTL3
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Programmed cell death 1 ligand 1 (CD274/PD-L1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone-lysine N-methyltransferase EHMT2 (EHMT2) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 9 dimethylation (H3K9me2) | View Details | |||
| Downstream Gene | METTL3 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification directly impacts m6A modification through modulating the level of m6A regulator | ||||
| Crosstalk Summary | EHMT2 promotes m6A methyltransferase activity of METTL3, which upregulates Programmed cell death 1 ligand 1 (CD274/PD-L1), CX3CR1 and CASP1, at translational/post-translational level by regulating Histone H3 lysine 9 dimethylation (H3K9me2) level during ET. | ||||
| Responsed Disease | Inflammatory response | ICD-11: MG46 | |||
In-vitro Model |
HEK293T | Normal | Homo sapiens | CVCL_0063 | |
| RAW 264.7 | Mouse leukemia | Mus musculus | CVCL_0493 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| CD274 molecule (CD274) | 55 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Avelumab | Approved | [2] | ||
| External Link | ||||
| Durvalumab | Approved | [3] | ||
| MOA | Modulator | |||
| External Link | ||||
| RG-7446 | Approved | [4] | ||
| External Link | ||||
| Bavencio | Approved | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Atezolizumab | Approved | [6] | ||
| External Link | ||||
| Sugemalimab | Approved in China | [7] | ||
| External Link | ||||
| MEDI4736 | Phase 3 | [8] | ||
| MOA | Modulator | |||
| External Link | ||||
| MPDL-3280A | Phase 3 | [9] | ||
| MOA | Modulator | |||
| External Link | ||||
| CS1001 | Phase 3 | [10] | ||
| External Link | ||||
| A167 | Phase 3 | [11] | ||
| Synonyms |
KL-A167
Click to Show/Hide
|
|||
| External Link | ||||
| KN046 | Phase 3 | [12] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Pidilizumab | Phase 2 | [13] | ||
| Synonyms |
CT-011
Click to Show/Hide
|
|||
| External Link | ||||
| KN035 | Phase 2 | [14] | ||
| Synonyms |
Envafolimab
Click to Show/Hide
|
|||
| External Link | ||||
| CX-072 | Phase 2 | [15] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| INCB86550 | Phase 2 | [16] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Bintrafusp alfa | Phase 2 | [17] | ||
| External Link | ||||
| M7824 | Phase 2 | [18] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| BGB-A333 | Phase 1/2 | [19] | ||
| External Link | ||||
| GS-4224 | Phase 1/2 | [20] | ||
| Synonyms |
Methyl Pyridazine-4-carboxylate; 34231-77-1; PYRIDAZINE-4-CARBOXYLIC ACID METHYL ESTER; 4-PYRIDAZINECARBOXYLIC ACID, METHYL ESTER; MFCD09953488; ACMC-1AJNN; methyl 4-pyridazinecarboxylate; methylpyridazine-4-carboxylate; SCHEMBL1421640; DTXSID30498310; AMY24958; BCP22435; ANW-50355; ZINC12359421; AKOS015854403; Methyl pyridazine-4-carboxylate, 97%; AC-4414; CS-W003697; PB31452; 4-Pyridazinecarboxylic acid methyl ester; AK-48857; SY004472; AB0024323; DB-030309; FT-0717698; W5569; S-2990; J-522632
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NM21-1480 | Phase 1/2 | [21] | ||
| MOA | Modulator | |||
| External Link | ||||
| LY3300054 | Phase 1 | [18] | ||
| External Link | ||||
| MSB2311 | Phase 1 | [14] | ||
| External Link | ||||
| Anti-PD-L1 | Phase 1 | [22] | ||
| Synonyms |
BMS-936559
Click to Show/Hide
|
|||
| External Link | ||||
| FAZ053 | Phase 1 | [15] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Anti-PD-L1 CSR T cells | Phase 1 | [23] | ||
| MOA | CAR-T-Cell-Therapy | |||
| External Link | ||||
| BMS-986189 | Phase 1 | [14] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Cosibelimab | Phase 1 | [24] | ||
| Synonyms |
CK-301/TG-1501
Click to Show/Hide
|
|||
| External Link | ||||
| CA-170 | Phase 1 | [25] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PD-L1 t-haNK | Phase 1 | [26] | ||
| External Link | ||||
| KD033 | Phase 1 | [27] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| C-Met/PD-L1 CAR-T Cell | Phase 1 | [28] | ||
| MOA | CAR-T-Cell-Therapy(Dual specific) | |||
| External Link | ||||
| CA-170 | Phase 1 | [18] | ||
| Synonyms |
3-Aminopyrrolidine dihydrochloride; 103831-11-4; pyrrolidin-3-amine dihydrochloride; 3-Pyrrolidinamine, dihydrochloride; 3-Aminopyrrolidine 2HCl; 3-Aminopyrrolidine diHCl; SCHEMBL555933; ACMC-2099s1; ACMC-2099s3; ACMC-20989g; 3-pyrrolidinamine dihydrochloride; CTK0H7226; DTXSID00583661; MolPort-002-343-989; NJPNCMOUEXEGBL-UHFFFAOYSA-N; 3-Amino-pyrrolidine dihydrochloride; KS-00000JI6; ACT01710; ANW-14978; SBB003982; ( -3-Aminopyrrolidine dihydrochloride; AKOS015844825; VP60158; AM85320; VP60228; TRA0055207; TRA0000843; TRA0097
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| FS118 | Phase 1 | [14] | ||
| External Link | ||||
| PF-07257876 | Phase 1 | [29] | ||
| External Link | ||||
| MCLA-145 | Phase 1 | [30] | ||
| MOA | Agonist | |||
| External Link | ||||
| GEN1046 | Phase 1 | [31] | ||
| MOA | Agonist | |||
| External Link | ||||
| ALPN-202 | Phase 1 | [32] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| TAK-252 | Phase 1 | [33] | ||
| Synonyms |
SL-279252
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| INBRX-105 | Phase 1 | [34] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| LY3415244 | Phase 1 | [35] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| A337 | Phase 1 | [11] | ||
| External Link | ||||
| IBI318 | Phase 1 | [36] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30107136-Compound-Example2 | Patented | [37] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 150 nM | |||
| External Link | ||||
| PMID30107136-Compound-Example1 | Patented | [37] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 18 nM | |||
| External Link | ||||
| PMID30247903-Compound-General structure7 | Investigative | [14] | ||
| Synonyms |
PMID30247903Compound7
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CA-327 | Investigative | [14] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30247903-Compound-General structure8 | Investigative | [14] | ||
| Synonyms |
PMID30247903Compound8
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30247903-Compound-General structure5 | Investigative | [14] | ||
| Synonyms |
PMID30247903Compound5
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30247903-Compound-General structure12 | Investigative | [14] | ||
| Synonyms |
PMID30247903Compound12
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30247903-Compound-General structure9 | Investigative | [14] | ||
| Synonyms |
PMID30247903Compound9
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30247903-Compound-General structure6 | Investigative | [14] | ||
| Synonyms |
PMID30247903Compound6
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30247903-Compound-General structure10 | Investigative | [14] | ||
| Synonyms |
PMID30247903Compound10
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| YH011 | Investigative | [38] | ||
| External Link | ||||
| YH010 | Investigative | [39] | ||
| External Link | ||||
| RG6084 | Phase 1 | [40] | ||
| External Link | ||||
| Histone-lysine N-methyltransferase EHMT2 (EHMT2) | 7 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| MS012 | Preclinical | [41] | ||
| Synonyms |
CHEMBL4086403; 2089617-83-2 (free base); N2-hexyl-6,7-dimethoxy-N4-(1-methylpiperidin-4-yl)quinazoline-2,4-diamine; BDBM50501525; N~2~-hexyl-6,7-dimethoxy-N~4~-(1-methylpiperidin-4-yl)quinazoline-2,4-diamine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BIX-01294 | Preclinical | [42] | ||
| Synonyms |
BIX01294; BIX 01294
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 67 nM | |||
| External Link | ||||
| A-366 | Preclinical | [43] | ||
| Synonyms |
A 366; A366
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3 nM | |||
| External Link | ||||
| UNC0321 | Investigative | [44] | ||
| Synonyms |
UNC-0321; UNC 0321
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 9000 nM | |||
| External Link | ||||
| BRD9539 | Investigative | [45] | ||
| Synonyms |
BRD-9539; BRD 9539
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1500 nM | |||
| External Link | ||||
| UNC0642 | Investigative | [46] | ||
| Synonyms |
1481677-78-4; UNC 0642; UNC-0642; CHEMBL2441082; 2-(4,4-Difluoro-1-piperidinyl)-6-methoxy-N-[1-(1-methylethyl)-4-piperidinyl]-7-[3-(1-pyrrolidinyl)propoxy]-4-quinazolinamine; Barrett; GTPL7017; SCHEMBL17372593; AOB2595; MolPort-035-765-953; EX-A2241; BCP08266; ZINC96285772; BDBM50442103; AKOS024458509; SB19046; CS-5269; NCGC00189140-01; NCGC00189140-02; AS-16721; HY-13980; BC600721; AK547424; UNC0642, > KB-146019; J-008448
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 2.5 nM | |||
| External Link | ||||
| UNC0638 | Investigative | [47] | ||
| Synonyms |
1255580-76-7; UNC-0638; UNC 0638; UNII-26A103L2FO; 2-Cyclohexyl-N-(1-isopropylpiperidin-4-yl)-6-methoxy-7-(3-(pyrrolidin-1-yl)propoxy)quinazolin-4-amine; CHEMBL1231795; 26A103L2FO; 2-Cyclohexyl-6-methoxy-N-[1-(1-methylethyl)-4-piperidinyl]-7-[3-(1-pyrrolidinyl)propoxy]-4-quinazolinamine; 1255517-77-1; 2-cyclohexyl-6-methoxy-N-[1-(propan-2-yl)piperidin-4-yl]-7-[3-(pyrrolidin-1-yl)propoxy]quinazolin-4-amine; 2-Cyclohexyl-N-(1-isopropyl-4-piperidinyl)-6-methoxy-7-[3-(1-pyrrolidinyl)propoxy]-4-quinazolinamine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 3.7 nM | |||
| External Link | ||||
References