m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03130
|
[1] | |||
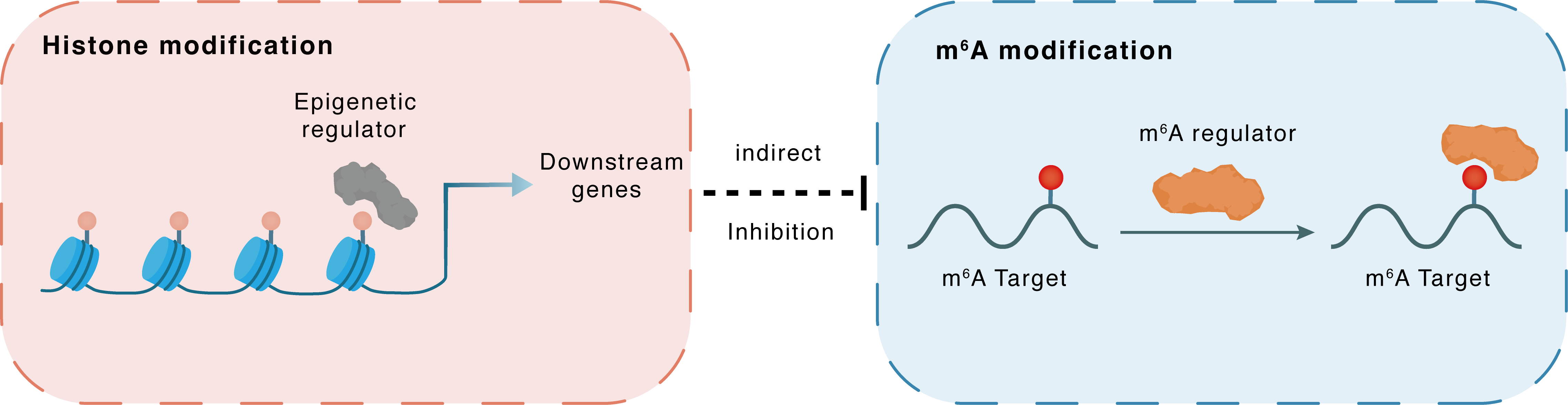 Histone modification
H3K27ac
HDAC11
ALKBH5
Indirect
Inhibition
m6A modification
Htr3a
Htr3a
YTHDF2
Histone modification
H3K27ac
HDAC11
ALKBH5
Indirect
Inhibition
m6A modification
Htr3a
Htr3a
YTHDF2
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | YTH domain-containing family protein 2 (YTHDF2) | READER | |||
| m6A Target | 5-hydroxytryptamine receptor 3A (HTR3A) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone deacetylase 11 (HDAC11) | ERASER | View Details | ||
| Regulated Target | Histone H3 lysine 27 acetylation (H3K27ac) | View Details | |||
| Downstream Gene | ALKBH5 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Inhibition | |||
| Crosstalk Mechanism | histone modification indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | HDAC11 downregulation induced by nerve injury increases Histone H3 lysine 27 acetylation (H3K27ac), facilitating the binding of the transcription factor forkhead box protein D3 (FOXD3) to the ALKBH5 promoter and promoting Alkbh5 transcription. The increased ALKBH5 erases m6A sites in 5-hydroxytryptamine receptor 3A (HTR3A) messenger RNA (mRNA), resulting in an inability of YT521-B homology domain 2 (YTHDF2) to bind to Htr3a mRNA, thus causing an increase in 5-HT3A protein expression and 5-HT3 channel currents. | ||||
| Responsed Disease | Neuropathic pain | ICD-11: 8E43.0 | |||
In-vitro Model |
NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| In-vivo Model | Mice were randomly divided into different groups (at least n = 5 each group). H1299-RR cells or A549 cells (5 × 106) treated in different manners were harvested and inoculated subcutaneously into the left dorsal flank of mice. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| 5-hydroxytryptamine receptor 3A (HTR3A) | 49 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Dolasetron | Approved | [2] | ||
| Synonyms |
Dolasetronum; Dolasteron; Anzemet (TN); Dolasetron (INN); Dolasetron [INN:BAN]; Dolasetronum [INN-Latin]; 3-oxooctahydro-2h-2,6-methanoquinolizin-8-yl 1h-indole-3-carboxylate
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| Palonosetron | Approved | [3] | ||
| Synonyms |
Aloxi; Onicit; Palonosetron [INN]; Aloxi (TN); RS 25233-197; RS 25259-197; RS-25233-197; RS-25259-197; (S-(R*,R*))-2-(1-Azabicyclo(2.2.2)oct-3-yl)-2,3,3a,4,5,6-hexahydro-1H-benz(de)isoquinolin-1-one
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| Activity | Ki = 0.03162 nM | |||
| External Link | ||||
| Alosetron | Approved | [4] | ||
| Synonyms |
Lotronex; Lotrpnex; ALOSETRON HYDROCHLORIDE; Alosetron HCl; Alosetron hydrochloride [USAN]; Alosetron monohydrochloride; GR 68755; GR 68755X; GR 68755c; GR68755; Alosetron (INN); Alosetron [INN:BAN]; Alosetron hydrochloride (USAN); GR-68755C; Lotronex (TN); Lotrpnex (TN); 1H-Pyrido(4,3-b)indol-1-one, 2,3,4,5-tetrahydro-5-methyl-2((5-methyl-1H-imidazol-4-yl)methyl)-, monohydrochloride; 1H-Pyrido(4,3-b)indol-1-one, 2,3,4,5-tetrahydro-5-methyl-2-((5-methyl-1H-imidazol-4-yl)methyl)-, monohydrochloride; 1H-Pyrido[4,3-b]indol-1-one, 2,3,4,5-tetrahydro-5-methyl-2-[(4-methyl-1H-imidazol-5-yl)methyl]-, hydrochloride (1:1); 1H-Pyrido[4,3-b]indol-1-one, 2,3,4,5-tetrahydro-5-methyl-2-[(5-methyl-1H-imidazol; 1H-Pyrido[4,3-b]indol-1-one, 2,3,4,5-tetrahydro-5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-(9CI); 2,3,4,5-Tetrahydro-5-methyl-2-((5-methyl-1H-imidazol-4-yl)methyl)-1H-pyrido(4,3-b)indol-1-one; 2,3,4,5-Tetrahydro-5-methyl-2-((5-methylimidazol-4-yl)methyl)-1H-pyrido(4,3-b)indol-1-one monohydrochloride; 2,3,4,5-tetrahydro-5-methyl-2-((5-methylimidazol-4-yl)methyl)-1H-pyridol(4,3-b)indol-1-one monohydrochloride; 2,3,4,5-tetrahydro-5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-1H-pyrido[4,3-b]indol-1-one, monohydrochloride; 5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indol-1-one; 5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indol-1-one hydrochloride; 5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-3,4-dihydropyrido[4,3-b]indol-1-one; 5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-3,4-dihydropyrido[4,3-b]indol-1-one hydrochloride
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| Activity | Ki = 0.5 nM | |||
| External Link | ||||
| Tropisetron | Approved | [5] | ||
| Synonyms |
Navoban; Novaban; TKT; ICS-205930; Navoban (TN); Tropisetron (INN); [(1R,5S)-8-methyl-8-azabicyclo[3.2.1]octan-3-yl] 1H-indole-3-carboxylate; (3-endo)-8-methyl-8-azabicyclo[3.2.1]oct-3-yl 1H-indole-3-carboxylate
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| Activity | Ki = 2.7 nM | |||
| External Link | ||||
| Procaine | Approved | [6] | ||
| Synonyms |
Allocaine; Anticort; Anuject; Duracaine; Gerokit; Gerovital; Jenacain; Jenacaine; Nissocaine; Norocaine; Novocain; Novocaine; Procain; Procaina; Procainum; Scurocaine; Spinocaine; Solution of novocain; Factor H3; SP01; SP01A; Stoff H3; Vitamin H3; Anticort (TM); Diethylaminoethyl p-aminobenzoate; Gerovital H-3; Novocain (TN); P-Aminobenzoyldiethylaminoethanol; P-Aminobenzyoyldiethylaminoethanol; Procaina [INN-Spanish]; Procaine (INN); Procaine [INN:BAN]; Procaine, base; Procainum [INN-Latin]; SP-01A; Solution of novocain (TN); Beta-Diethylaminoethyl 4-aminobenzoate; P-Aminobenzoic acid 2-diethylaminoethyl ester; Beta-(Diethylamino)ethyl 4-aminobenzoate; Beta-(Diethylamino)ethyl p-aminobenzoate; BENZOIC ACID,4-AMINO,2-DIETHYLAMINOETHYL ESTER PROCAIN BASE; Benzoic acid, 4-amino-, 2-(diethylamino)ethyl ester; Benzoic acid, p-amino-, 2-(diethylamino)ethyl ester; 2-(Diethylamino)ethyl 4-aminobenzoate; 2-(Diethylamino)ethyl p-aminobenzoate; 2-(Diethylamino)ethyl-4-aminobenzoate; 2-Diethylaminoethyl 4-aminobenzoate; 2-Diethylaminoethyl p-aminobenzoate; 2-Diethylaminoethylester kyseliny p-aminobenzoove; 2-Diethylaminoethylester kyseliny p-aminobenzoove [Czech]; 4-Aminobenzoesaeure-beta-diethylaminoethylester; 4-Aminobenzoic acid 2-(diethylamino) ethyl ester; 4-Aminobenzoic acid diethylaminoethyl ester
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| Levetiracetam | Approved | [5] | ||
| Synonyms |
102767-28-2; Keppra; (S)-2-(2-Oxopyrrolidin-1-yl)butanamide; Keppra XR; Levetiracetamum; ucb L059; (2S)-2-(2-oxopyrrolidin-1-yl)butanamide; UCB-L 059; UCB-L059; Spritam; (S)-alpha-Ethyl-2-oxo-1-pyrrolidineacetamide; (-)-(S)-alpha-Ethyl-2-oxo-1-pyrrolidineacetamide; SIB-S1; UNII-44YRR34555; 1-Pyrrolidineacetamide, alpha-ethyl-2-oxo-, (alphaS)-; UCB-22059; Levetiracetamum [INN-Latin]; Levetiractam; CHEBI:6437; ucb L060; Levetiracetam In Sodium Chloride; 44YRR34555; Levroxa; 1-Pyrrolidineacetamide, alpha-ethyl-2-oxo-,; Leviteracetam; Torleva; Levetiracetam [INN]; Etiracetam levo-isomer; Keppra (TN); L-059; Etiracetam, S-isomer; Keppra, Keppra XR),Levetiracetam; Levetriacetam
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| Palonosetron + fosnetupitant | Approved | [7] | ||
| MOA | Antagonist | |||
| External Link | ||||
| Cilansetron | Phase 3 | [8] | ||
| Synonyms |
Calmactin; DU-123265; KC-9946; Cilansetron (USAN/INN)
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| BEMESETRON | Discontinued in Phase 3 | [9] | ||
| Synonyms |
3-Tropanyl-3,5-dichlorobenzoate; MDL 72222; MDL-72222; C15H17Cl2NO2; CHEMBL376379; 40796-97-2; Tropyl 3,5-dichlorobenzoate; 8-Methyl-8-azabicyclo[3.2.1]oct-3-yl 3,5-dichlorobenzoate; Bemesetron [USAN:INN]; Bemesetronum [INN-Latin]; 3,5-Dichloro-benzoic acid 8-methyl-8-aza-bicyclo[3.2.1]oct-3-yl ester; SR-01000075587; Tropanyl 3,5-dichlorobenzoate; 1alphaH,5alphaH-Tropan-3alpha-yl 3,5-dichlorobenzoate; endo-8-Methyl-8-azabicyclo(3.2.1)oct-3-yl 3,5-dichlorobenzoate; (8-methyl-8-azabicyclo[3.2.1]octan-3-yl) 3,5-dichlorobenzo
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 2.5 nM | |||
| External Link | ||||
| YM-114 | Discontinued in Phase 2 | [10] | ||
| Synonyms |
KAE-393; (R)-5-(2,3-Dihydro-1H-indol-1-ylcarbonyl)-4,5,6,7-tetrahydro-1H-benzimidazole hydrochloride
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| Norcisapride | Discontinued in Phase 2 | [11] | ||
| Synonyms |
84946-16-7; CHEMBL1748; 4-Amino-5-chloro-2-methoxy-N-(3-methoxy-4-piperidyl)benzamide; 4-amino-5-chloro-2-methoxy-N-(3-methoxy-4-piperidinyl)benzamide; (4-Amino-5-chloro-2-methoxy)-N-[3-methoxy(4-piperidyl)]benzamide; 4-amino-5-chloro-2-methoxy-N-(3-methoxypiperidin-4-yl)benzamide; Benzamide,4-amino-5-chloro-2-methoxy-N-[(3S,4R)-3-methoxy-4-piperidinyl]-, hydrochloride(1:1); EINECS 284-619-3; AC1MI81F; SCHEMBL593405; CTK4E8653; OMLDMGPCWMBPAN-UHFFFAOYSA-N; BDBM50301927; AKOS030254741; API0006151; DB-076176
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 2300 nM | |||
| External Link | ||||
| ATI-17000 | Preclinical | [12] | ||
| Synonyms |
CompB; J-113397; UNII-00M5444DIY; CHEMBL357076; 00M5444DIY; J113397; 1-[(3R,4R)-1-(cyclooctylmethyl)-3-(hydroxymethyl)piperidin-4-yl]-3-ethylbenzimidazol-2-one; 1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one; AC1NSK6N; J-113,397; 1-(1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl)-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one; 256640-45-6; SCHEMBL875219; J 113397; GTPL1691; (+)-J-113397; ZINC1483900; BDBM50083230; NCGC00344513-02; 2H-Benzimidazol-2-one,
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| AS-8112 | Terminated | [13] | ||
| MOA | Antagonist | |||
| External Link | ||||
| BP4.879a | Terminated | [14] | ||
| MOA | Antagonist | |||
| External Link | ||||
| 5-hydroxyindole | Investigative | [15] | ||
| Synonyms |
1H-Indol-5-ol; 1953-54-4; INDOL-5-OL; 5-Hydroxy-1H-indole; Hydroxy-5 indole; 5-Indolol; UNII-320UN7XZYN; Hydroxy-5-indole; Hydroxy-5 indole [French]; CCRIS 4422; 5-Hydroxyindole, 97%; EINECS 217-782-6; MFCD00005677; NSC 87503; BRN 0112349; 320UN7XZYN; CHEBI:89649; LMIQERWZRIFWNZ-UHFFFAOYSA-N; NSC-87503; 5-hydroxy-indole; Hydroxy-5 indole [French]; 5-hydroxylindole; 5-hydroxy-indol; 5-hydroxy indole; 3fuh; 5H1; zlchem 359; 5Hydroxy-1H-indole; PubChem7263; 1-H-indol-5-ol; 1H-indol-5-ol.; 4b3c; ACMC-1BQT3; WLN: T56 BMJ GQ
Click to Show/Hide
|
|||
| MOA | Modulator (allosteric modulator) | |||
| External Link | ||||
| TMB-8 | Investigative | [16] | ||
| Synonyms |
8-(Diethylamino)octyl 3,4,5-trimethoxybenzoate; TMB 8; 57818-92-5; TM-8; 8-(N,N-Diethylamino)octyl-3,4,5-trimethoxybenzoate; 3,4,5-Trimethoxybenzoic acid, 8-(diethylamino)octyl ester; C22H37NO5; AC1L1KGS; AC1Q67GP; Lopac-861804; Lopac0_000022; BSPBio_001503; GTPL4323; CHEMBL258764; SCHEMBL2173737; CTK5A7496; DTXSID70206532; CHEBI:107633; HMS1989L05; HMS1791L05; HMS3402L05; 53464-72-5 (hydrochloride); ZINC3875139; EI-110; AKOS030559942; MCULE-5343453436; CCG-204118; NCGC00162047-03; NCGC00162047-01; NCGC00014998-06
Click to Show/Hide
|
|||
| MOA | Blocker (channel blocker) | |||
| External Link | ||||
| 1-(biphenyl-4-yl)-3-(4-(piperidin-1-yl)butyl)urea | Investigative | [17] | ||
| Synonyms |
CHEMBL1086332; SCHEMBL13527422
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7900 nM | |||
| External Link | ||||
| 10,11-dihydro-5H-dibenzo[b,f]azepine | Investigative | [18] | ||
| Synonyms |
Iminodibenzyl; 494-19-9; 10,11-Dihydro-5H-dibenz[b,f]azepine; Iminobibenzyl; 2,2'-Iminodibenzyl; 2,2'-Iminobibenzyl; 5H-Dibenz[b,f]azepine, 10,11-dihydro-; RP 23669; UNII-262BX7OE3U; NSC 72110; 10,11-Dihydro-5-dibenz(b,f)azepine; 6,11-dihydro-5H-benzo[b][1]benzazepine; 10,11-Dihydrodibenz(b,f)azepine; EINECS 207-787-1; 10,11-Dihydro-5H-dibenz(b,f)azepine; BRN 0152732; CHEMBL63054; 5H-Dibenz(b,f)azepine, 10,11-dihydro-; AI3-39165; 262BX7OE3U; ZSMRRZONCYIFNB-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Benzoxazo-2-yl-1,4-diazabicyclo[3.2.2]nonane | Investigative | [19] | ||
| Synonyms |
CHEMBL611082; SCHEMBL373021; CXJLWJAYGMWLRR-UHFFFAOYSA-N; BDBM50309862
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 13 nM | |||
| External Link | ||||
| 3alpha-(2'-Indolecarbonyloxy)-nortropane | Investigative | [20] | ||
| Synonyms |
CHEMBL596256; BDBM50304333
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| FLUPENTIXOLE | Investigative | [18] | ||
| Synonyms |
(E)-Flupenthixol; beta-Flupenthixol; trans-flupenthixol; trans-(E)-Flupentixol; trans-Flupentixol; 53772-85-3; EINECS 258-759-0; UNII-895OJP78MJ; Flupentiol; 2709-56-0; 895OJP78MJ; Flupenthixol, Beta; FLUPENTHIXOL, Alpha; 1-Piperazineethanol, 4-(3-(2-(trifluoromethyl)-9H-thioxanthen-9-ylidene)propyl)-, (E)-; (E)-4-(3-(2-(Trifluoromethyl)-9H-thioxanthen-9-ylidene)propyl)-1-piperazineethanol; 2-[4-[(3E)-3-[2-(trifluoromethyl)thioxanthen-9-ylidene]propyl]piperazin-1-yl]ethanol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| [3H]ramosetron | Investigative | [21] | ||
| MOA | Antagonist | |||
| External Link | ||||
| 3alpha-(1'-Methyl-2'-Indolecarbonyloxy)-tropane | Investigative | [20] | ||
| Synonyms |
CHEMBL593963; BDBM50304334
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| [3H]GR65630 | Investigative | [22] | ||
| Synonyms |
[3H]-GR65630
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| [3H](S)-zacopride | Investigative | [23] | ||
| Synonyms |
GTPL4074
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| BRL-24682 | Investigative | [24] | ||
| Synonyms |
Brl 24682; SCHEMBL7292676; CHEMBL301039; BDBM82519; PDSP2_001249; PDSP1_001265; 76272-78-1; CAS_76272-78-1; 4-amino-5-chloro-2-methoxy-N-(8-methyl-8-azabicyclo[3.2.1]octan-3-yl)benzamide; Benzamide, 4-amino-5-chloro-2-methoxy-N-(8-methyl-8-azabicyclo(3.2.1)oct-3-yl)-, endo-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-(4-Methyl-piperazin-1-yl)-phenanthridine | Investigative | [25] | ||
| Synonyms |
CHEMBL43193; 23441-13-6; 6-(4-methylpiperazin-1-yl)phenanthridine; CTK0I7937; DTXSID50433889; ZINC13778637; BDBM50063258; 6-(4-Methylpiperazino)phenanthridine; Phenanthridine, 6-(4-methyl-1-piperazinyl)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| trichloroethanol | Investigative | [26] | ||
| Synonyms |
2,2,2-TRICHLOROETHANOL; 115-20-8; Trichlorethanol; Trichloroethyl alcohol; Ethanol, 2,2,2-trichloro-; 2,2,2-Trichloro-1-ethanol; (Hydroxymethyl)trichloromethane; 2,2,2-Trichloroethyl alcohol; 2,2,2-Trichloroethan-1-ol; C2H3Cl3O; UNII-AW835AJ62N; NSC 66407; beta-trichloroethanol; CCRIS 6763; CCl3CH2OH; EINECS 204-071-0; 2,2,2-trichloro-ethanol; 2,2,2-tris-chloroethanol; BRN 1697495; AW835AJ62N; CHEBI:28094; KPWDGTGXUYRARH-UHFFFAOYSA-N; .beta.,.beta.,.beta-Trichloroethanol; 2,2,2-Trichloroethanol, 99%; 4yas
Click to Show/Hide
|
|||
| MOA | Modulator (allosteric modulator) | |||
| External Link | ||||
| (4-Quinolin-2-ylpiperazin-1-yl)acetic Acid | Investigative | [27] | ||
| Synonyms |
CHEMBL468498; AC1LEONH; SCHEMBL13780710; A3329/0141355; BDBM50258497; AKOS009544966; 2-(4-quinolin-2-ylpiperazin-1-yl)acetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1-phenylbiguanide | Investigative | [28] | ||
| Synonyms |
phenylbiguanide; Phenyl biguanide; 102-02-3; Phenyldiguanide; Phenylguanide; phenyl diguanide; N-Phenyl-N'-guanylguanidine; N-phenylimidodicarbonimidic diamide; Biguanide, phenyl-; Imidodicarbonimidic diamide, N-phenyl-; UNII-W8PKA3T2I3; BIGUANIDE, 1-PHENYL-; C8H11N5; N-Phenyl-imidocarbonimidic diamide; EINECS 202-998-5; W8PKA3T2I3; CHEMBL13791; CHEBI:75377; P 1426; Imidodicarbonimidicdiamide, N-phenyl-; 1-(diaminomethylidene)-2-phenylguanidine; SR-01000075565; N-phenylbiguanide; N'-phenylbiguanide
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| (S)-zacopride | Investigative | [29] | ||
| Synonyms |
CHEMBL28992; Tocris-1795; NCGC00025295-01; AC1O7H1O; SCHEMBL5373467; ZINC3961; GTPL2289; SCHEMBL16233195; PDSP2_001618; PDSP1_001634; BDBM50056419; UNII-9GN3OT4156 component FEROPKNOYKURCJ-CYBMUJFWSA-N; 4-Amino-N-(S)-1-aza-bicyclo[2.2.2]oct-3-yl-5-chloro-2-methoxy-benzamide; 4-amino-N-[(3S)-1-azabicyclo[2.2.2]octan-3-yl]-5-chloro-2-methoxybenzamide; 4-amino-N-[(8S)-1-azabicyclo[2.2.2]octan-8-yl]-5-chloro-2-methoxybenzamide
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| Activity | Ki = 0.182 nM | |||
| External Link | ||||
| PH-709829 | Investigative | [30] | ||
| Synonyms |
PTGWFYYEAUFEAS-ZYHUDNBSSA-N; CHEMBL403858; PHA-709829; SCHEMBL844377; GTPL3997; BDBM50377050
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(4-Benzyl-piperazin-1-yl)-benzothiazole | Investigative | [31] | ||
| Synonyms |
2-(4-benzylpiperazin-1-yl)-1,3-benzothiazole; CHEMBL282234; 2-(4-benzylpiperazino)-1,3-benzothiazole; 35463-75-3; AC1LSALZ; Oprea1_030694; MLS001166194; SCHEMBL7760900; KS-00003DHQ; MolPort-002-878-239; HMS2852D05; ZINC20405012; BDBM50041381; AKOS005101332; MCULE-9582396226; 7P-339S; 1-(2-Benzothiazolyl)-4-benzylpiperazine; SMR000550026; 2-(4-benzylpiperazin-1-yl)benzo[d]thiazole; Z86230191
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| [3H]granisetron | Investigative | [32] | ||
| Synonyms |
[3H]-granisetron; [3H]-BRL-43694
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| Activity | Ki = 1.45 nM | |||
| External Link | ||||
| meta-chlorphenylbiguanide | Investigative | [33] | ||
| Synonyms |
m-Chlorophenylbiguanide; mCPBG; 1-(3-Chlorophenyl)biguanide; 1-(m-Chlorophenyl)biguanide; N-(3-Chlorophenyl)imidodicarbonimidic diamide; 48144-44-1; Imidodicarbonimidic diamide, N-(3-chlorophenyl)-; UNII-910A4X901V; M-Chlorophenylbiguanidine; 3-Chloro-Phenyl biguanide; 2-(3-chlorophenyl)-1-(diaminomethylidene)guanidine; CHEMBL13790; CHEBI:32347; N-(3-chlorophenyl)-N'-(diaminomethylene)guanidine; 910A4X901V; C8H10ClN5; Imidodicarbonimidicdiamide, N-(3-chlorophenyl)-; 1-carbamimidamido-N-(3-chlorophenyl)methanimidamide; 3-chlorophenyl-biguanide; [3H]meta-chlorophenylbiguanide
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 3800 nM | |||
| External Link | ||||
| 2-(1H-Imidazol-4-ylmethyl)-4-phenyl-thiazole | Investigative | [34] | ||
| Synonyms |
CHEMBL289060; BDBM50014174
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CP-810123 | Investigative | [19] | ||
| Synonyms |
UNII-E6G4550EC4; CHEMBL604798; E6G4550EC4; BSNKYWSMUAGMDO-UHFFFAOYSA-N; 439608-12-5; SCHEMBL1459339; BDBM50309861; 1,4-Diazabicyclo(3.2.2)nonane, 4-(5-methyloxazolo(4,5-b)pyridin-2-yl)-; 4-(5-methyloxazolo[4,5-b]pyridin-2-yl)-1,4-diazabicyclo[3.2.2]nonane; 4-(5-Methyloxazolo[4,5-b]pyridin-2-yl)-1,4-diazabicyclo[3.2.2]-nonane; 4-(5-methyloxazolo[4,5-b]pyridin-2-yl)-1,4-diaza-bicyclo[3.2.2]nonane
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| bilobalide | Investigative | [35] | ||
| Synonyms |
33570-04-6; Bilobalid; (-)-Bilobalide; UNII-M81D2O8H7U; CHEBI:3103; M81D2O8H7U; Bilobalide A; 4H,5aH,9H-Furo(2,3-b)furo(3',2':2,3)cyclopenta(1,2-c)furan-2,4,7(3H,8H)-trione, 9-(1,1-dimethylethyl)-10,10a-dihydro-8,9-dihydroxy-, (5aR-(3aS*,5aalpha,8beta,8aS*,9alpha,10aalpha))-; (3aS,8R,8aS,9R,10aS)-9-tert-butyl-8,9-dihydroxydihydro-9H-furo[2,3-b]furo[3',2':2,3]cyclopenta[1,2-c]furan-2,4,7(3H,8H)-trione; tert-butyl(dihydroxy)[ ]trione; C15H18O8; Bilobalide;; Bilobalide A;; ( )-Bilobalide; AC1L2K4G; MLS000563448
Click to Show/Hide
|
|||
| MOA | Blocker (channel blocker) | |||
| External Link | ||||
| 2-(4-Methyl-piperazin-1-yl)-quinoline | Investigative | [25] | ||
| Synonyms |
N-methylquipazine; 2-(4-methylpiperazin-1-yl)quinoline; UNII-0YV1ZIR6S0; 0YV1ZIR6S0; CHEMBL288591; CHEBI:64164; quinoline, 2-(4-methyl-1-piperazinyl)-; Tocris-0566; Lopac-Q-107; Biomol-NT_000084; AC1L1JF3; Oprea1_654246; Lopac0_001000; SCHEMBL606721; BPBio1_001081; DTXSID8043731; CTK6I3065; HOMWNUXPSJQSSU-UHFFFAOYSA-N; MolPort-006-384-975; ZINC403653; 2-(4-Methylpiperazinyl)-quinoline; 1-(2-Quinolyl)-4-methylpiperazine; STK362919; BDBM50053631; AKOS005453926; MCULE-4786527390; CCG-205080
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 0.4571 nM | |||
| External Link | ||||
| 4-((naphthalen-2-yloxy)methyl)piperidine | Investigative | [36] | ||
| Synonyms |
4-[(2-Naphthyloxy)methyl]piperidine; CHEMBL453996; 946680-75-7; 4-[(naphthalen-2-yloxy)methyl]piperidine; CTK7D1529; ZINC14631494; BDBM50278526; AKOS000172158
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 311 nM | |||
| External Link | ||||
| MESULERGINE | Investigative | [37] | ||
| Synonyms |
Mesulerginum; Mesulergina; Mesulergine [INN]; Mesulerginum [INN-Latin]; UNII-SML95FK06I; Mesulergina [INN-Spanish]; 64795-35-3; N'-(1,6-Dimethylergolin-8alpha-yl)-N,N-dimethylsulfamide; SML95FK06I; CQ 32085; CHEMBL12314; C18H26N4O2S; CHEBI:73378; 3-(1,6-Dimethyl-8alpha-ergolinyl)-1,1-dimethylsulfamid; NCGC00163168-01; DSSTox_RID_81540; DSSTox_CID_26324; DSSTox_GSID_46324; CU-32085; N'-[(8alpha)-1,6-dimethylergolin-8-yl]-N,N-dimethylsulfuric diamide; CAS-64795-35-3; AC1L2AKM; Biomol-NT_000077; AC1Q6V4R; GTPL206
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(piperidin-4-ylmethoxy)-2-naphthonitrile | Investigative | [36] | ||
| Synonyms |
CHEMBL444985
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 404 nM | |||
| External Link | ||||
| QUIPAZINE | Investigative | [38] | ||
| Synonyms |
4774-24-7; 2-(piperazin-1-yl)quinoline; 2-Piperazin-1-yl-quinoline; 2-Piperazin-1-ylquinoline; Quinoline, 2-(1-piperazinyl)-; 2-(1-Piperazinyl)quinoline; Quipazine [INN]; 1-(2-Quinolinyl)piperazine; Quipazinum [INN-Latin]; Quipazina [INN-Spanish]; UNII-4WCY05C0SJ; 1-(2-Quinolyl)piperazine; 2-(1-Piperazinyl)chinolin; BRN 0196945; 4WCY05C0SJ; CHEMBL18772; 2-quinolylpiperazine; F3306-0004; Quipazinum; Quipazina; TPC-A004; MA-1291; Spectrum_001733; Tocris-0629; piperazin-1-yl-quinoline; AC1L1JEX; AC1Q4WAV; Spectrum4_001259
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 3.7 nM | |||
| External Link | ||||
| 5-chloro-3,4-dihydroquinazolin-2-amine | Investigative | [39] | ||
| Synonyms |
CHEMBL401541; 2-Amino-5-chlor-3,4-dihydrochinazolin; 109319-86-0
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 1051 nM | |||
| External Link | ||||
| 2-methyl-5-HT | Investigative | [40] | ||
| Synonyms |
2-methyl-5-hydroxytryptamine; 2-Me-5-HT; 2-methylserotonin
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | Ki = 1200 nM | |||
| External Link | ||||
| 5,6-dichloro-3,4-dihydroquinazolin-2-amine | Investigative | [39] | ||
| Synonyms |
Anagrelide impurity 5; 2-Amino-5,6-dichloro-3,4-dihydroquinazoline; 444904-63-6; CHEMBL1548; 2-Quinazolinamine, 5,6-dichloro-1,4-dihydro-; W-202785; SCHEMBL1569300; CTK1D2410; DTXSID80432648; VBKOTIVQMCTTAQ-UHFFFAOYSA-N; ZINC29130869; BDBM50371434; AKOS030254941; 5,6-Dichloro-1,4-dihydro-2-quinazolinamine; 2-amino-5,6-dichloro-3,4dihydroquinazoline; FT-0722369
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 172 nM | |||
| External Link | ||||
| SEROTONIN | Investigative | [25] | ||
| Synonyms |
5-HYDROXYTRYPTAMINE; 3-(2-Aminoethyl)-1H-indol-5-ol; 50-67-9; Enteramine; 5-HT; Serotonine; Thrombotonin; Thrombocytin; Antemovis; Ds substance; Hippophain; Antemoqua; Substance DS; Substanz DS; 1H-Indol-5-ol, 3-(2-aminoethyl)-; 5-Hta; Tryptamine, 5-hydroxy-; 3-(2-Aminoethyl)indol-5-ol; Enteramin; UNII-333DO1RDJY; Indol-5-ol, 3-(2-aminoethyl)-; 5-Hydroxy-3-(beta-aminoethyl)indole; 3-(beta-Aminoethyl)-5-hydroxyindole; EINECS 200-058-9; 3-(2-Amino-ethyl)-1H-indol-5-ol; BRN 0143524; 333DO1RDJY; CHEBI:28790
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 141 nM | |||
| External Link | ||||
| A-987306 | Investigative | [41] | ||
| Synonyms |
SCHEMBL604437; CHEMBL519240; BDBM26226; MolPort-023-276-880; DJKJVWJQAVGLHJ-YPMHNXCESA-N; ZINC42887577; AKOS024457726; NCGC00370852-01; UNII-6BVK16R925 component DJKJVWJQAVGLHJ-YPMHNXCESA-N; (12S,17S)-6-(piperazin-1-yl)-11-oxa-3,5-diazatetracyclo[8.7.0.0^{2,7}.0^{12,17}]heptadeca-1(10),2(7),3,5-tetraen-4-amine; (-)-(7aS*,11aS*)-4-piperazin-1-yl-5,6,7a,8,9,10,11,11a-octahydro[1]benzofuro[2,3h]quinazolin-2-amine; (+/-)-(7aR*,11aR*)-5,6,7a,8,9,10,11,11a-Octahydro-4-(1-piperazinyl)-benzofuran[2,3-h]quinazolin-2-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-(4-butylpiperidin-1-yl)-1-o-tolylbutan-1-one | Investigative | [42] | ||
| Synonyms |
AC-42; CHEMBL1242950; AC42; GTPL289; SCHEMBL4504348; ANTKBACNWQHQJE-UHFFFAOYSA-N; ZINC2022527; BDBM50326219; AKOS030284249; NCGC00485453-01; gamma-(4-Butylpiperidino)-2'-methylbutyrophenone; L019209; 4-(4-butylpiperdin-1-yl)-1-(2-methylphenyl)butan-1-one; 4-(4-butylpiperidin-1-yl)-1-(2-methylphenyl)butan-1-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki >= 1000 nM | |||
| External Link | ||||
| Histone deacetylase 11 (HDAC11) | 7 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| PMID29671355-Compound-36 | Patented | [43] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 110 nM | |||
| External Link | ||||
| PMID29671355-Compound-23 | Patented | [43] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 10500 nM | |||
| External Link | ||||
| PMID29671355-Compound-44 | Patented | [43] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 150 nM | |||
| External Link | ||||
| PMID29671355-Compound-21 | Patented | [43] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 8420 nM | |||
| External Link | ||||
| PMID29671355-Compound-62 | Patented | [43] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 500 nM | |||
| External Link | ||||
| PMID29671355-Compound-43 | Patented | [43] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 12 nM | |||
| External Link | ||||
| PMID29671355-Compound-25 | Patented | [43] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2840 nM | |||
| External Link | ||||
| 8E43: Pain disorders | 1 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| FMX104 | Phase 2 | [44] | ||
| External Link | ||||
References