m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03106
|
[1] | |||
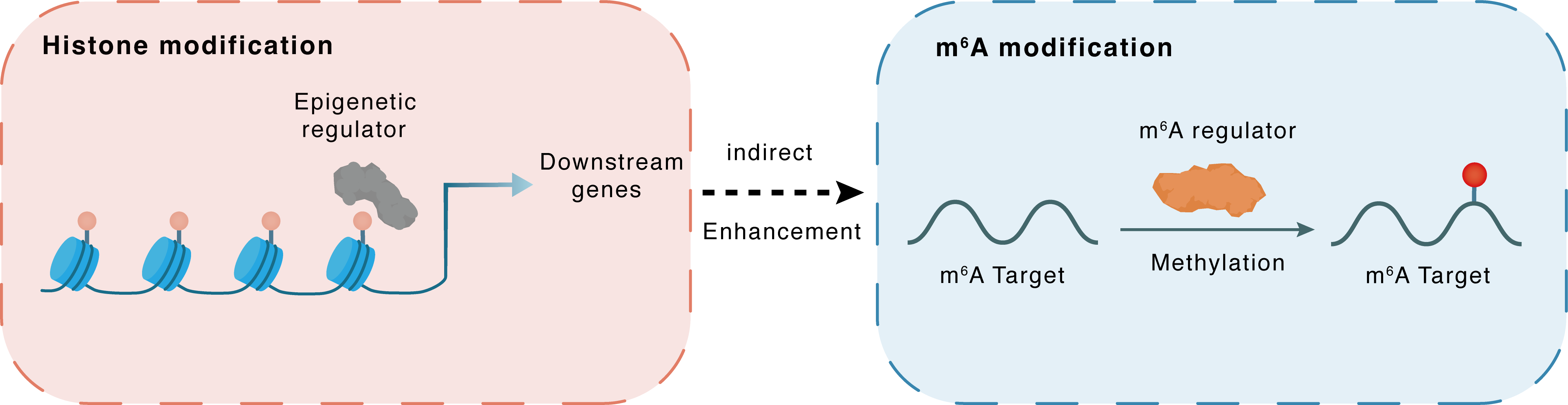 Histone modification
H3K18la
Epigenetic Regulator
AP001885.4
Indirect
Enhancement
m6A modification
MYC
MYC
METTL3
Methylation
Histone modification
H3K18la
Epigenetic Regulator
AP001885.4
Indirect
Enhancement
m6A modification
MYC
MYC
METTL3
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Myc proto-oncogene protein (MYC) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Regulated Target | Histone H3 lysine 18 lactylation (H3K18la) | View Details | |||
| Downstream Gene | AP001885.4 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | Histone modification indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | AP001885.4 was overexpressed in ESCC cells because of a higher Histone H3 lysine 18 lactylation (H3K18la) level in the promoter and amplification, then enhanced histone lactylation- and NF-kappaB (p65)-dependent transcription activation and METTL3-mediated mRNA stability of c-myc eventually upregulated Myc proto-oncogene protein (MYC) and promoted cell proliferation | ||||
| Responsed Disease | Esophageal Squamous Cell Carcinoma | ICD-11: 2B70.1 | |||
| Cell Process | Cell proliferation | ||||
| Cell colony formation | |||||
| mRNA stability | |||||
In-vitro Model |
Eca-109 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_6898 | |
| KYSE-510 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1354 | ||
| KYSE-450 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1353 | ||
| KYSE-410 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1352 | ||
| KYSE-150 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1348 | ||
| KYSE-140 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1347 | ||
| KYSE-70 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1356 | ||
| KYSE-30 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1351 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Myc proto-oncogene protein (MYC) | 3 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| AVI-5126 | Phase 2 | [2] | ||
| Synonyms |
Resten-CP; NeuGene (CABG), AVI
Click to Show/Hide
|
|||
| External Link | ||||
| Resten-NG | Phase 2 | [3] | ||
| Synonyms |
Resten-NG (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| TWS-119 | Investigative | [4] | ||
| Synonyms |
TWS119; 601514-19-6; 3-[[6-(3-Aminophenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl]oxy]phenol; TWS 119; GSK inhibitor XII; GSK-3beta Inhibitor XII, TWS119; Neurogenesis Inducer, TWS119; CHEMBL405759; 3-(6-(3-aminophenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yloxy)phenol; 3-((6-(3-AMINOPHENYL)-7H-PYRROLO[2,3-D]PYRIMIDIN-4-YL)OXY)PHENOL; 3-{[6-(3-aminophenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-yl]oxy}phenol; Phenol, 3-[[6-(3-aminophenyl)-1H-pyrrolo[2,3-d]pyrimidin-4-yl]oxy]-; K00245; MLS006011018; GTPL5980; SCHEMBL5559045; GSK-3BETA INHIB
Click to Show/Hide
|
|||
| External Link | ||||
| 2B70: Esophageal cancer | 15 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Pembrolizumab | Approved | [5] | ||
| External Link | ||||
| Nivolumab | Approved | [5] | ||
| External Link | ||||
| Claudiximab | Phase 3 | [6] | ||
| Synonyms |
IMAB-362; Anti-GC182 mAbs (cancer), Ganymed; Anti-GC182 monoclonal antibodies (cancer), Ganymed; Anti-CLDN18-2 mAbs (cancer), Ganymed; Anti-CLDN18-2 monoclonal antibodies (cancer), Ganymed
Click to Show/Hide
|
|||
| External Link | ||||
| Golnerminogene pradenovac | Phase 3 | [7] | ||
| Synonyms |
TNFerade (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| DKN-01 | Phase 2 | [8] | ||
| External Link | ||||
| Pegamotecan | Phase 2 | [9] | ||
| Synonyms |
Prothecan; EZ-246; PEG-camptothecin; PEG-camptothecin, Enzon; Polyethylene glycol-camptothecin, Enzon
Click to Show/Hide
|
|||
| External Link | ||||
| APR-246 | Phase 2 | [5] | ||
| Synonyms |
Eprenetapopt
Click to Show/Hide
|
|||
| External Link | ||||
| Opdivo + Yervoy | Phase 3 | [5] | ||
| External Link | ||||
| Anti-NY-ESO-1 CAR-T cells | Phase 1/2 | [10] | ||
| External Link | ||||
| Anti-MUC1 CAR-T cells | Phase 1/2 | [11] | ||
| External Link | ||||
| CAR-T Cells targeting EpCAM | Phase 1/2 | [12] | ||
| External Link | ||||
| PCA062 | Phase 1 | [5] | ||
| External Link | ||||
| Cellspan esophageal implant | Clinical trial | [5] | ||
| External Link | ||||
| PKI166 | Discontinued in Phase 2 | [13] | ||
| Synonyms |
PKI-166; CGP-75166; 187724-61-4; NVP-PKI166; CHEMBL1914653; AC1OCFE0; UNII-9RIE5HW38P; 9RIE5HW38P; SCHEMBL177814; GTPL7642; CHEMBL1963502; ZINC23255; AOB1619; PKI-75166; BDBM50358046; NCGC00387215-02; AS-16676; KB-275097; PKI-166, > 4-[4-[[(1R)-1-phenylethyl]amino]-7H-pyrrolo[4,5-e]pyrimidin-6-yl]phenol
Click to Show/Hide
|
|||
| External Link | ||||
| Ramorelix | Discontinued in Phase 1 | [14] | ||
| Synonyms |
Hoe-013
Click to Show/Hide
|
|||
| External Link | ||||
References