m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03086
|
[1] | |||
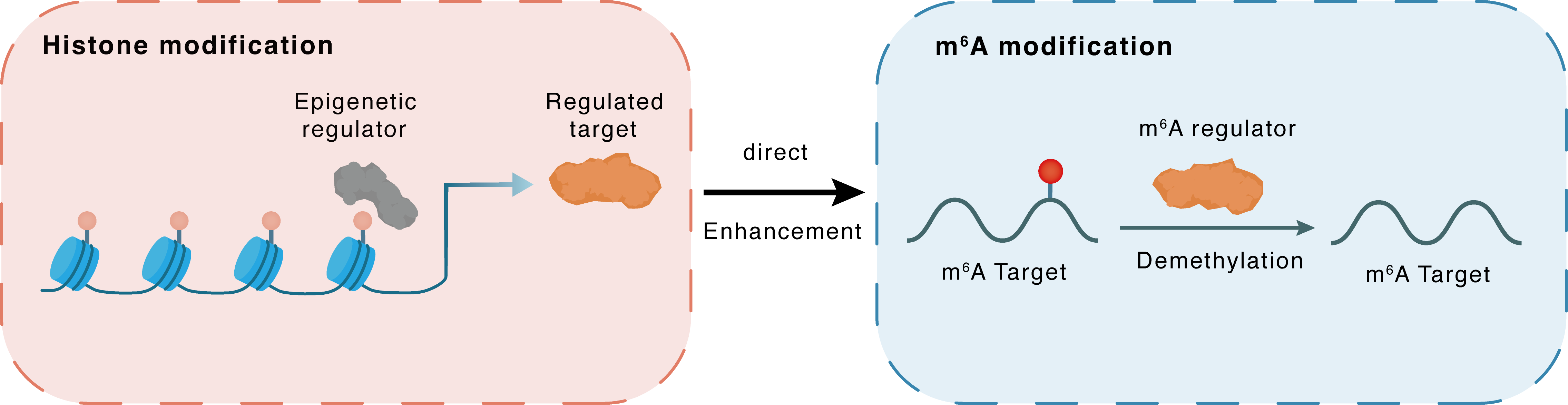 Histone modification
H3K18la
p300
FTO
Direct
Enhancement
m6A modification
CDK2
CDK2
FTO
Demethylation
Histone modification
H3K18la
p300
FTO
Direct
Enhancement
m6A modification
CDK2
CDK2
FTO
Demethylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | |||
| m6A Target | Cyclin-dependent kinase 2 (CDK2) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone acetyltransferase p300 (P300) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 18 lactylation (H3K18la) | View Details | |||
| Downstream Gene | FTO | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification directly impacts m6A modification through modulating the level of m6A regulator | ||||
| Crosstalk Summary | FTO affected EC features via modulating Cyclin-dependent kinase 2 (CDK2) mRNA stability in an m6A-YTHDF2-dependent manner. FTO up-regulation under diabetic conditions was driven by p300-mediated Histone H3 lysine 18 lactylation (H3K18la). FB23-2, an inhibitor to FTO's m6A demethylase activity, suppressed angiogenic phenotypes in vitro. | ||||
| Responsed Disease | Diabetic retinopathy | ICD-11: 9B71.0 | |||
| Responsed Drug | FB23-2 | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Cyclin-dependent kinase 2 (CDK2) | 138 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| PHA848125 | Phase 2 | [2] | ||
| Synonyms |
Milciclib; PHA-848125; 802539-81-7; PHA 848125; Milciclib (PHA-848125); UNII-688000M8S8; MMV676602; 4,5-dihydro-N,1,4,4-tetramethyl-8-[[4-(4-methyl-1-piperazinyl)phenyl]amino]-1H-Pyrazolo[4,3-h]quinazoline-3-carboxamide; 688000M8S8; N,1,4,4-tetramethyl-8-(4-(4-methylpiperazin-1-yl)phenylamino)-4,5-dihydro-1H-pyrazolo[4,3-h]quinazoline-3-carboxamide; Milciclib [INN]; N,1,4,4-Tetramethyl-8-{[4-(4-Methylpiperazin-1-Yl)phenyl]amino}-4,5-Dihydro-1h-Pyrazolo[4,3-H]quinazoline-3-Carboxamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 45 nM | |||
| External Link | ||||
| Ro 31-7453 | Phase 2 | [3] | ||
| Synonyms |
Bisindolylmaleimide deriv. 44; MKC-1; Ro-31-7453; 3-(1-methylindol-3-yl)-4-(1-methyl-6-nitroindol-3-yl)pyrrole-2,5-dione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| R-roscovitine | Phase 2 | [4] | ||
| Synonyms |
Seliciclib; roscovitine; 186692-46-6; (R)-roscovitine; CYC202; CYC-202; CYC 202; 2-(R)-(1-Ethyl-2-hydroxyethylamino)-6-benzylamino-9-isopropylpurine; UNII-0ES1C2KQ94; Roscovitine (Seliciclib,CYC202); NSC 701554; AL-39256; CHEMBL14762; 0ES1C2KQ94; CHEBI:45307; NSC701554; NSC-701554; (2R)-2-[[6-(benzylamino)-9-isopropyl-purin-2-yl]amino]butan-1-ol; (R)-2-((6-(Benzylamino)-9-isopropyl-9H-purin-2-yl)amino)butan-1-ol; (2R)-2-[[6-(benzylamino)-9-propan-2-ylpurin-2-yl]amino]butan-1-ol; RRC; Rosco; M02443; BMK1-E12; CYC202, Seliciclib, R-roscovitine, Roscovitine; (2r)-2-{[6-(benzylamino)-9-isopropyl-9h-purin-2-yl]amino}-1-butanol; 2-(R)-[[9-(1-Methylethyl)-6-[(phenylmethyl)amino]-9H-purin-2-yl]amino]-1-butanol; 2-[[9-(1-Methylethyl)-6-[(phenylmethyl)amino]-9H-purin-2-yl]amino]-(R)-1-butanol; 6-(Benzylamino)-2(R)-[[1-(hydroxymethyl)propyl]amino]-9-isopropylpurine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| TG02 | Phase 1/2 | [5] | ||
| Synonyms |
SB1317; 937270-47-8; TG-02; TG02 (Double bond Z/E); UNII-40D08182TT; CHEMBL1944698; 40D08182TT; SB-1317; 1204918-72-8; C23H24N4O; Tube011; SB-1317 free base; TG02 (Double bond E); TG02 [WHO-DD]; SCHEMBL823947; SCHEMBL2298965; GTPL9095; SCHEMBL17595943; EX-A239; MolPort-039-139-793; AOB87361; BCP07033; ZINC68251500; BDBM50363196; 4029AH; AKOS030527020; AKOS032950000; SB1317(TG-02); SB14606; CS-0884; compound 26h [PMID: 22148278]; KB-80503; HY-15166; W-5884
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 3 nM | |||
| External Link | ||||
| NUV-422 | Phase 1/2 | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| AZD-5438 | Phase 1 | [7] | ||
| Synonyms |
602306-29-6; AZD5438; AZD 5438; 4-(1-isopropyl-2-methyl-1H-imidazol-5-yl)-N-(4-(methylsulfonyl)phenyl)pyrimidin-2-amine; CHEMBL488436; UNII-276Z913G29; MMV676604; 4-[2-METHYL-1-ISOPROPYL-1H-IMIDAZOL-5-YL]-N-[4-(METHYLSULFONYL)PHENYL]-2-PYRIMIDINAMINE; 2-Pyrimidinamine,4-[2-methyl-1-(1-methylethyl)-1H-imidazol-5-yl]-N-[4-(methylsulfonyl)phenyl]-; 276Z913G29; 4-(2-methyl-3-propan-2-ylimidazol-4-yl)-N-(4-methylsulfonylphenyl)pyrimidin-2-amine
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 < 6 nM | |||
| External Link | ||||
| AT7519 | Phase 1 | [8] | ||
| Synonyms |
LZE; AT 7519, AT7519; AT-7519; 4-[(2,6-dichlorobenzoyl)amino]-N-(4-piperidyl)-2H-pyrazole-3-carboxamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 47 nM | |||
| External Link | ||||
| PF-07104091 | Phase 1 | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| CYC065 | Phase 1 | [10] | ||
| Synonyms |
DLPIYBKBHMZCJI-WBVHZDCISA-N; 1070790-89-4; CYC-065; UNII-YET2XNU791; YET2XNU791; SCHEMBL13300946; CHEMBL3655762; BDBM106950; CS-7615; HY-101212; US8592581, 1; 2-Pentanol, 3-((6-(((4,6-dimethyl-3-pyridinyl)methyl)amino)-9-(1-methylethyl)-9H-purin-2-yl)amino)-, (2R,3S)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| AG-024322 | Phase 1 | [4] | ||
| Synonyms |
AG-24322; N-[[5-[(3E)-3-(4,6-difluorobenzimidazol-2-ylidene)-1,2-dihydroindazol-5-yl]-4-methylpyridin-3-yl]methyl]ethanamine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PHA-793887 | Phase 1 | [11] | ||
| Synonyms |
Cyclin-dependent kinase inhibitor (cancer), Nerviano
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SNS-032 | Phase 1 | [12] | ||
| Synonyms |
BMS-387032; BMS-387072; BMS-387032, SNS-032; N-[5-[[[5-(1,1-Dimethylethyl)-2-oxazolyl]methyl]thio]-2-thiazolyl]-4-piperidinecarboxamide L-tartaric acid salt (2:1); N-(5-(((5-(1,1-Dimethylethyl)-2-oxazolyl)methyl)thio)-2-thiazolyl)-4-piperidinecarboxamide; N-[5-[(5-tert-butyl-1,3-oxazol-2-yl)methylsulfanyl]-1,3-thiazol-2-yl]piperidine-4-carboxamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 38 nM | |||
| External Link | ||||
| RGT-419B | Phase 1 | [13] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| FN-1501 | Phase 1 | [14] | ||
| Synonyms |
1429515-59-2; CHEMBL4077071; UNII-6MC966B505; TQR1001; BDBM50270304; NSC781143; 6MC966B505; NSC-781143; HY-111361; CS-0039834; 4((7HPyrrolo[2,3d]pyrimidin-4-yl)amino)N(4-((4-methylpiperazin-1-yl)methyl)phenyl)1Hpyrazole-3-carboxamide; 4-((7H-Pyrrolo (2,3-d)pyrimidin-4-yl)amino)-N-(4-((4-methylpiperazin-1-yl)methyl)phenyl)-1H-pyrazole-3-carboxamide; 4-((7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)-N-(4-((4-methylpiperazin-1-yl)methyl)phenyl)-1H-pyrazole-5-carboxamide; 4a?(7Ha'Pyrrolo[2,3a'd]pyrimidin-4-yl)amino)a'Na?4-((4-methylpiperazin-1-yl)methyl)phenyl)a?Ha'pyrazole-3-carboxamide; N-[4-[(4-methylpiperazin-1-yl)methyl]phenyl]-4-(7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)-1H-pyrazole-5-carboxamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RGB-286638 | Phase 1 | [15] | ||
| Synonyms |
GPC-286199; RGB-286199; RGB-344064; Non-selective CDK inhibitors, Agennix; Non-selective CDK inhibitors, GPC Biotech; Non-selective cyclin dependent kinase inhibitors, Agennix; Non-selective cyclin dependent kinase inhibitors, GPC Biotech
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Fluorinated compound 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-15
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1.7 nM | |||
| External Link | ||||
| 4-amino-3,5-di-substituted-thiazole derivative 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-54
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Indole-based analog 11 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-16
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| N-(pyridin-2-yl)pyridine methylsulfone derivative 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-27
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 170 nM | |||
| External Link | ||||
| Palbociclib/ribociclib analog 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-4
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Alkyl sulfone derivative 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-29
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Aniline derivative 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-33
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 39 nM | |||
| External Link | ||||
| 4-(thiazol-5-yl)-pyrimidine derivative 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-35
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 45 nM | |||
| External Link | ||||
| N-phenyl-pyrimidin-4-amine derivative 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-30
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1300 nM | |||
| External Link | ||||
| PMID26161698-Compound-25 | Patented | [16] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 260 nM | |||
| External Link | ||||
| Diaryl amine derivative 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-23
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2 to 6 nM | |||
| External Link | ||||
| Flavopiridol analog 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-5
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = nM range | |||
| External Link | ||||
| Isosteric imidazolyl pyrimidine derivative 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-37
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2 nM | |||
| External Link | ||||
| N-(pyridin-2-yl)pyrimidin-4-amine derivative 2 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-31
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 63 nM | |||
| External Link | ||||
| Pyrazolo-triazine derivative 2 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-14
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1,5-di-substituted pyridine derivative 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-26
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Kd < 100 nM | |||
| External Link | ||||
| 5-fluoro-N-(pyridin-2-yl)pyridin-2-amine derivative 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-28
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4 nM | |||
| External Link | ||||
| Pyrrolo[2,3-d]pyrimidine derivative 9 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 1 nM | |||
| External Link | ||||
| Naphthyridine and isoquinoline derivative 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-51
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 40 nM | |||
| External Link | ||||
| Nitrogen mustard derivative 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-55
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2.7 nM | |||
| External Link | ||||
| 4-(thiazol-5-yl)-pyrimidine derivative 2 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-36
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = single digit nM | |||
| External Link | ||||
| Diamidothiazole derivative 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-53
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Indole-based analog 13 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-20
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Pyrazolo[1,5-a]-1,3,5-triazine derivative 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-12
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 12 nM | |||
| External Link | ||||
| Isoquinoline 1,3-dione derivative 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-49
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3100 nM | |||
| External Link | ||||
| Roscovitine derivative 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-8
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = nM range | |||
| External Link | ||||
| PMID25991433-Compound-A1 | Patented | [17] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 300 nM | |||
| External Link | ||||
| Oxazolyl methylthiothiazole derivative 1 | Patented | [16] | ||
| Synonyms |
PMID26161698-Compound-52
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100 nM | |||
| External Link | ||||
| SCH 727965 | Discontinued in Phase 3 | [4] | ||
| Synonyms |
Dinaciclib; SCH-727965
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1 nM | |||
| External Link | ||||
| BAY 10-00394 | Discontinued in Phase 2 | [18] | ||
| Synonyms |
roniciclib; BAY 1000394; KB-145902; 1223498-69-8; Tube010; SCHEMBL875845; GTPL7874
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| R547 | Discontinued in Phase 1 | [4] | ||
| Synonyms |
LIA; R-547; [4-amino-2-[(1-methylsulfonylpiperidin-4-yl)amino]pyrimidin-5-yl]-(2,3-difluoro-6-methoxyphenyl)methanone
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 3 nM | |||
| External Link | ||||
| ZK 304709 | Discontinued in Phase 1 | [4] | ||
| Synonyms |
1010440-84-2; ZK CDK; UNII-87GI98VT0I; SCHEMBL955299; 87GI98VT0I; DTXSID20143701
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| INOC-005 | Preclinical | [19] | ||
| Synonyms |
Capridine beta (prostate cancer), Prostagenics
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| L-751250 | Preclinical | [20] | ||
| Synonyms |
Purvalanol B; 212844-54-7; PURVALANOL; Purvalanol B(NG-95); (R)-2-chloro-4-((2-((1-hydroxy-3-methylbutan-2-yl)amino)-9-isopropyl-9H-purin-6-yl)amino)benzoic acid; (2R)-2-[[6-[(3-CHLORO-4-CARBOXYPHENYL)AMINO]-9-(1-METHYLETHYL)-9H-PURIN-2-YL]AMINO]-3-METHYL-1-BUTANOL; CHEMBL23254; CHEBI:49840; NG 95; NG-95; C20H25ClN6O3; 2-chloro-4-[[2-[[(2R)-1-hydroxy-3-methylbutan-2-yl]amino]-9-propan-2-ylpurin-6-yl]amino]benzoic acid; J-502183; PVB
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 9 nM | |||
| External Link | ||||
| Olomoucine | Terminated | [20] | ||
| Synonyms |
101622-51-9; 4erk; UNII-6A839B2HYS; 2-(2-Hydroxyethylamino)-6-benzylamino-9-methylpurine; 6A839B2HYS; CHEMBL280074; 2-{[6-(benzylamino)-9-methyl-9h-purin-2-yl]amino}ethanol; 6-Benzylamino-2-(2-hydroxyethylamino)-9-methylpurine; 6-(Benzylamino)-2-(2-hydroxyethylamino)-9-methylpurine; Ethanol,2-[[9-methyl-6-[(phenylmethyl)amino]-9H-purin-2-yl]amino]-; OLO; 2-(Hydroxyethylamino)-6-benzylamino-9-methylpurine; 6-Benylamino-2-(2-hydroxyethylamino)-9-methylpurine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1200 nM | |||
| External Link | ||||
| PD-0183812 | Terminated | [21] | ||
| Synonyms |
PETCVZZPKYJZAU-UHFFFAOYSA-N; PD183812; AC1NS8PJ; CHEMBL139653; SCHEMBL5268115; BDBM6280; PD 0183812; N8 Pyrido[2,3-d]pyrimidin-7-one deriv 72; 8-{bicyclo[221]heptan-2-yl}-2-({4-[4-(3-hydroxypropyl)piperidin-1-yl]phenyl}amino)-7H,8H-pyrido[2,3-d]pyrimidin-7-one; 8-(3-bicyclo[221]heptanyl)-2-[4-[4-(3-hydroxypropyl)piperidin-1-yl]anilino]pyrido[2,3-d]pyrimidin-7-one; PD0183813
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-[(6-chloropyrazin-2-yl)amino]benzenesulfonamide | Investigative | [22] | ||
| Synonyms |
CHEMBL487738; 4-(6-chloropyrazin-2-ylamino)benzenesulfonamide; 642459-21-0; 2vtj; SCHEMBL6659290; BDBM24631; ZINC16052861; DB08134; FT-0723597; 4-[(6-chloropyrazin-2-yl)amino]benzene-1-sulfonamide; 4-[(6-chloropyrazin-2-yl)amino]benzenesulfonamide, 10
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1900 nM | |||
| External Link | ||||
| PHENYLAMINOIMIDAZO(1,2-ALPHA)PYRIDINE | Investigative | [22] | ||
| Synonyms |
4-{[6-(2,6-Dichlorobenzoyl)imidazo[1,2-A]pyridin-2-Yl]amino}benzenesulfonamide; 1ykr; 4-[[6-(2,6-dichlorobenzoyl)imidazo[1,2-a]pyridin-2-yl]amino]benzenesulfonamide; aminoimidazo[1,2-a]pyridine deriv. 2i; AC1NS9IQ; CHEMBL182260; BDBM6670; DB04607; 4-({6-[(2,6-dichlorophenyl)carbonyl]imidazo[1,2-a]pyridin-2-yl}amino)benzenesulfonamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-CYCLOHEXYLMETHOXY-2-(3'-CHLOROANILINO) PURINE | Investigative | [22] | ||
| Synonyms |
N-(3-chlorophenyl)-6-(cyclohexylmethoxy)-7H-purin-2-amine; 444722-81-0; 1h1r; AC1L1IG7; CHEMBL341273; SCHEMBL6790651; BDBM5531; CTK1D2420; DTXSID10274437; O6-Cyclohexylmethylguanine deriv. 15; DB07203; 2-(3 -Chloroanilino)-6-cyclohexylmethoxypurine; N-(3-chlorophenyl)-6-(cyclohexylmethoxy)-9H-purin-2-amine; N-(3-Chlorophenyl)-6-(cyclohexylmethoxy)-9H-purine-2-amine; 1H-Purin-2-amine, N-(3-chlorophenyl)-6-(cyclohexylmethoxy)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BOHEMINE | Investigative | [23] | ||
| Synonyms |
189232-42-6; 3-((6-(Benzylamino)-9-isopropyl-9H-purin-2-yl)amino)propan-1-ol; 3-{[6-(benzylamino)-9-(propan-2-yl)-9h-purin-2-yl]amino}propan-1-ol; CHEMBL83980; CHEBI:86007; 2-(3-Hydroxypropylamino)-6-benzylamino-9-isopropylpurine; [6-Benzylamino-2-(3-hydroxypropylamino)-9-isopropylpurine; 2-[(3-hydroxypropyl)amino]-6-benzylamino-9-isopropylpurine; 3-{[6-(benzylamino)-9-isopropyl-9H-purin-2-yl]amino}propan-1-ol; purine deriv. 1; AC1L1DN2; SCHEMBL1443403; GTPL5938; BDBM10633; CTK8A4395; DTXSID00274365; EX-A931
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 800 nM | |||
| External Link | ||||
| N-phenyl-1H-pyrazole-3-carboxamide | Investigative | [22] | ||
| Synonyms |
124828-46-2; CHEMBL445420; N-phenyl-1H-pyrazole-5-carboxamide; 1H-Pyrazole-3-carboxylic Acid Phenylamide; MLS000519301; 2vtl; pyrazole amide, 15; pyrazole-5-carboxanilide; ChemDiv2_004267; AC1LE63W; SCHEMBL19748; N-phenylpyrazol-3-ylcarboxamide; SCHEMBL13373589; BDBM24636; MolPort-001-664-649; HMS2155C18; HMS3604B14; HMS1381B21; HMS3323C02; ALBB-018739; ZINC5286379; STK926745; BDBM50109707; AKOS000321787; DB08135; 1H-pyrazole-3-carboxamide, N-phenyl-; MCULE-7079696193; IDI1_002982; ST034406
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 97000 nM | |||
| External Link | ||||
| 5-hydroxynaphthalene-1-sulfonamide | Investigative | [22] | ||
| Synonyms |
17286-26-9; 5-Hydroxynaphthalene-1-sulphonamide; NFVBVKHGDDDCEA-UHFFFAOYSA-N; 5-Hydroxy-1-naphthalenesulfonamide; 5-hydroxy-naphthalene-1-sulfonic acid amide; 2vth; EINECS 241-319-7; AC1L3CSX; AC1Q6ULI; SCHEMBL588923; CHEMBL457047; CTK4D4388; NFVBVKHGDDDCEA-UHFFFAOYSA-; BDBM24628; DTXSID50169414; ZINC2242749; 5-hydroxy-1-naphthalenesulphonamide; 1-Naphthalenesulfonamide,5-hydroxy-; 1-Naphthalenesulfonamide, 5-hydroxy-; 5-hydroxynaphthalene-1-sulfonamide, 7; AKOS030618521; DB08132; TX-017211
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 120000 nM | |||
| External Link | ||||
| N-(4-sulfamoylphenyl)-1H-indazole-3-carboxamide | Investigative | [22] | ||
| Synonyms |
N-(4-Sulphamoylphenyl)-1H-indazole-3-carboxamide; 660822-60-6; 2vti; indazole amide, 14; CHEMBL455946; SCHEMBL4524087; BDBM24635; MNHPHKFLWAPNOV-UHFFFAOYSA-N; ZINC15270554; DB08133; DA-41761; FT-0707176; 1H-Indazole-3-carboxylic acid (4-sulphamoyl-phenyl)-amide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 660 nM | |||
| External Link | ||||
| N-(3-METHYLBUT-2-EN-1-YL)-9H-PURIN-6-AMINE | Investigative | [22] | ||
| Synonyms |
2365-40-4; Isopentenyladenine; N6-Isopentenyladenine; N6-(2-Isopentenyl)adenine; Isopentenyl adenine; 6-(gamma,gamma-Dimethylallylamino)purine; IPADE; Dimethylallyladenine; N6-(2-Isopentenyl)-adenine; N6-Dimethylallyladenine; N6-(delta2-Isopentenyl)adenine; N-(3-Methyl-2-butenyl)adenine; N6-(3-Methyl-2-butenyl)adenine; N6-(delta 2-Isopentenyl)-adenine; N(6)-(delta(2)-Isopentenyl)adenine; 1H-Purin-6-amine, N-(3-methyl-2-butenyl)-; N-(3-methylbut-2-enyl)-7H-purin-6-amine; Adenine, N-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1-Amino-6-Cyclohex-3-Enylmethyloxypurine | Investigative | [24] | ||
| Synonyms |
872556-74-6; 4-(cyclohexylmethoxy)-1H-imidazo[4,5-c]pyridin-6-amine; AC1L9IVC; CTK2I2667; DTXSID90332206; 3h-imidazo[4,5-c]pyridin-6-amine,4-(cyclohexylmethoxy)-; ZINC20149007; AKOS030619175; DB02603; KB-268453; 1H-Imidazo[4,5-c]pyridin-6-amine, 4-(cyclohexylmethoxy)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Benzyl-(9-isopropyl-9H-purin-6-yl)-amine | Investigative | [23] | ||
| Synonyms |
CHEMBL85015; 111853-20-4; 9H-Purin-6-amine, 9-(1-methylethyl)-N-(phenylmethyl)-; purine deriv. 9; ACMC-20mewm; SCHEMBL754120; BDBM10641; CTK0D3360; DTXSID10444118; 6-(benzylamino)-9-isopropylpurine; ZINC13538226; AKOS030562149; 6-(Benzylamino)-9-isopropyl-9H-purine; N-benzyl-9-isopropyl-9H-purin-6-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4500 nM | |||
| External Link | ||||
| N-(5-Cyclopropyl-1h-Pyrazol-3-Yl)Benzamide | Investigative | [24] | ||
| Synonyms |
CHEMBL324942; N-(3-cyclopropyl-1H-pyrazol-5-yl)benzamide; N5B; 1vyz; AC1L9MPJ; SCHEMBL4314346; BDBM7127; LUCORKWTQSQFFU-UHFFFAOYSA-N; 3-Benzamidoaminopyrazole deriv. 4; DB02647
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 289.73 nM | |||
| External Link | ||||
| TRIAZOLOPYRIMIDINE | Investigative | [22] | ||
| Synonyms |
8-Azapurine; 273-40-5; 2H-triazolo[4,5-d]pyrimidine; 179268-22-5; 6H-1,2,3-Triazolo[4,5-d]pyrimidine; 2H-1,2,3-Triazolo[4,5-d]pyrimidine; 3H-1,2,3-Triazolo[4,5-d]pyrimidine; 3H-[1,2,3]triazolo[4,5-d]pyrimidine; triazolo[4,5-d]pyrimidine; triazolo(4,5-d)pyrimidine; 273-39-2; 99331-25-6; 3H-1,2,3-TRIAZOLO(4,5-D)PYRIMIDINE; ACMC-1CNOM; AC1L22AZ; SCHEMBL26606; 179268-21-4; SCHEMBL11026556; CTK1A0899; CTK0H1173; CTK0H1170; CTK3G7531; CTK0H1174; DTXSID80181745; GIIGHSIIKVOWKZ-UHFFFAOYSA-N; ZINC2020258; ACM273405; FCH907879; AKOS006372727
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-ANILINO-6-CYCLOHEXYLMETHOXYPURINE | Investigative | [22] | ||
| Synonyms |
6-(cyclohexylmethoxy)-N-phenyl-7H-purin-2-amine; CHEMBL122264; 444722-80-9; 2A6; NU-6094; 1h1q; AC1L1BOU; SCHEMBL6791643; BDBM5530; CTK1D2421; DTXSID30274313; XWWRLKIBRPJQJX-UHFFFAOYSA-N; O6-Cyclohexylmethylguanine deriv 2; AKOS030619892; DB06948; N-Phenyl-6-(cyclohexylmethoxy)-9H-purine-2-amine; 6-(cyclohexylmethoxy)-N-phenyl-9H-purin-2-amine; 1H-Purin-2-amine,
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2300 nM | |||
| External Link | ||||
| SCH-546909 | Investigative | [25] | ||
| Synonyms |
CDK2 inhibitors (cancer), Merck & Co; CDK2 inhibitors (cancer), Schering-Plough; Cyclindependent kinase 2 inhibitors (cancer), Schering-Plough
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 140 nM | |||
| External Link | ||||
| PHA-690509 | Investigative | [26] | ||
| Synonyms |
PHA-404611; PHA-533533; PNU-252808; PNU-292137; CDK inhibitors (cancer), Nerviano; Cyclin dependent kinase inhibitors (cancer), Nerviano; CDK-2 inhibitors (anticancer), Pfizer; CDK-2 inhibitors (anticancer), Pharmacia
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-(2,4-Dimethyl-Thiazol-5-Yl)-Pyrimidin-2-Ylamine | Investigative | [24] | ||
| Synonyms |
364334-94-1; 4-(2,4-Dimethylthiazol-5-yl)pyrimidin-2-amine; 4-(2,4-DIMETHYL-1,3-THIAZOL-5-YL)PYRIMIDIN-2-AMINE; CTFDMGIBHFQWKB-UHFFFAOYSA-N; 4-(2,4-dimethylthiazol-5-yl)pyrimidin-2-ylamine; CK2; 4-(dimethyl-1,3-thiazol-5-yl)pyrimidin-2-amine; 1pxj; 2c5o; AC1L9LFI; Maybridge3_001247; CHEMBL47302; CS12; SCHEMBL4314069; BDBM8037; CTK4H6460; DTXSID70332264; MolPort-002-896-655; HMS1434I15; ZINC141286; HMS3604E21; ANW-58550; 4623AB; AKOS013063215; MCULE-3137548628; DB02091; CCG-243780
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 396 nM | |||
| External Link | ||||
| VER-54505 | Investigative | [25] | ||
| Synonyms |
Cdk2 inhibitors, Vernalis; RBT-0049985
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-Amino-6-Chloropyrazine | Investigative | [24] | ||
| Synonyms |
33332-28-4; 6-chloropyrazin-2-amine; 2-chloro-6-aminopyrazine; Pyrazinamine, 6-chloro-; 2-Pyrazinamine, 6-chloro-; 6-Chloro-pyrazin-2-ylamine; 6-chloropyrazin-2-ylamine; 2-amino-6-chloro-pyrazine; CHEMBL191632; 6-CHLORO-2-PYRAZINAMINE; CIG; 6-chlorpyrazin-2-amin; 1wcc; PubChem8543; zlchem 1337; AI3-61778; AC1Q3PRB; AC1L3MZH; ACMC-209i0e; 6-chloropyrazine-2-ylamine; SCHEMBL67803; 6-chloro-pyrazin-2-yl-amine; 6-chloropyrazin-2-amine, 5; KSC222G6J; AC1Q52Q1; Jsp006119; DTXSID0067761; CTK1C2364; BDBM24626; ZLE0115
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 350000 nM | |||
| External Link | ||||
| 6-cyclohexylmethyloxy-2-(4'-hydroxyanilino)purine | Investigative | [22] | ||
| Synonyms |
CHEMBL340813; 4-{[6-(cyclohexylmethoxy)-9H-purin-2-yl]amino}phenol; N20; NU-6086; 1oi9; AC1L9L2Q; BDBM5541; SCHEMBL6792176; O6-Cyclohexylmethylguanine deriv 25; DB08233; 6-Cyclohexylmethoxy-2-(4 -hydroxyanilino)purine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1000 nM | |||
| External Link | ||||
| 6-(Cyclohex-3-enylmethoxy)-9H-purin-2-ylamine | Investigative | [27] | ||
| Synonyms |
CHEMBL115498; O6-Substituted Guanine Deriv. 27; AC1NS6ZQ; 9H-Purin-2-amine, 6-(3-cyclohexen-1-ylmethoxy)-; SCHEMBL6267556; BDBM5487; 220035-95-0; 6-(cyclohex-3-en-1-ylmethoxy)-9H-purin-2-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (2'Z,3'E)-5-Chloro-5'-chloro-indirubin-3'-oxime | Investigative | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-Cyclohexylmethoxy-pyrimidine-2,4,5-triamine | Investigative | [29] | ||
| Synonyms |
6-(cyclohexylmethoxy)pyrimidine-2,4,5-triamine; pyrimidine deriv. 16; AC1NS76Q; CHEMBL71036; BDBM5588; SCHEMBL6263731; IMNSQZHRHASLAR-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (2'Z,3'E)-5-Chloro-5'-methyl-indirubin-3'-oxime | Investigative | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-(3-Methyl-benzyloxy)-9H-purin-2-ylamine | Investigative | [27] | ||
| Synonyms |
O6-Substituted Guanine Deriv. 32; CHEMBL405773; BDBM5492; AC1NS702; SCHEMBL12227591; O6-[3-(Methyl)Benzyl]Guanine; ZINC13475147; 2-Amino-6-(3 -methylbenzyl)oxypurine; 6-[(3-methylphenyl)methoxy]-9H-purin-2-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (2'Z,3'E)-5-Fluoro-5'-methoxy-indirubin-3'-oxime | Investigative | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| JNJ-7706621 | Investigative | [30] | ||
| Synonyms |
443797-96-4; JNJ7706621; 4-((5-Amino-1-(2,6-difluorobenzoyl)-1H-1,2,4-triazol-3-yl)amino)benzenesulfonamide; UNII-74GK72DON8; RWJ-387252; 4-[[5-amino-1-(2,6-difluorobenzoyl)-1,2,4-triazol-3-yl]amino]benzenesulfonamide; CHEMBL191003; 74GK72DON8; 4-[[5-Amino-1-(2,6-difluorobenzoyl)-1H-1,2,4-triazol-3-yl]amino]benzenesulfonamide; JNJ 7706621; 4-(5-amino-1-(2,6-difluorobenzoyl)-1H-1,2,4-triazol-3-ylamino)benzenesulfonamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2 nM | |||
| External Link | ||||
| GW-8510 | Investigative | [31] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 9.7 nM | |||
| External Link | ||||
| BMS-265246 | Investigative | [32] | ||
| Synonyms |
BMS 265246; BMS265246
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (2'Z,3'E)-5-Fluoro-5'-fluoro-indirubin-3'-oxime | Investigative | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| (2'Z,3'E)-5-Fluoro-5'-chloro-indirubin-3'-oxime | Investigative | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| RESCOVITINE | Investigative | [33] | ||
| Synonyms |
CHEMBL1087791
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cdk4 inhibitor III | Investigative | [34] | ||
| Synonyms |
Ryuvidine; 265312-55-8; CHEMBL290904; 2-Methyl-5-[(4-methylphenyl)amino]benzothiazole-4,7-dione; 5-(N-(4-Methylphenyl)amino)-2-methyl-4,7-dioxobenzothiazole; 2-methyl-5-(4-methylanilino)-1,3-benzothiazole-4,7-dione; AC1Q6BBC; AC1LA59T; 2-Methyl-5-p-tolylamino-benzothiazole-4,7-dione; SCHEMBL2169284; GTPL5952; CTK4F8075; CHEBI:92119; AOB6479; MolPort-023-276-509; HMS3269F11; HMS3229E08; ZINC5930916; BDBM50086655; 2-METHYL-5-[(4-METHYLPHENYL)AMINO]-4,7-BENZOTHIAZOLEDIONE; AKOS024457195; CCG-206830
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 > 200000 nM | |||
| External Link | ||||
| 6-(3-Amino-benzyloxy)-9H-purin-2-ylamine | Investigative | [27] | ||
| Synonyms |
O6-Substituted Guanine Deriv. 33; BDBM5493; CHEMBL270977; AC1NS705; 2-Amino-6-(3 -aminobenzyl)oxypurine; 6-[(3-aminophenyl)methoxy]-9H-purin-2-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (2'Z,3'E)-5-Chloro-5'-fluoro-indirubin-3'-oxime | Investigative | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-((3,5-diamino-1H-pyrazol-4-yl)diazenyl)phenol | Investigative | [35] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-nitroindirubin-3'-oxime | Investigative | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| (2'Z,3'E)-5-Nitro-5'-methyl-indirubin-3'-oxime | Investigative | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| (2'Z,3'E)-5-Nitro-5'-chloro-indirubin-3'-oxime | Investigative | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| (2'Z,3'E)-5-Fluoro-5'-hydroxy-indirubin-3'-oxime | Investigative | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| (2'Z,3'E)-5-Chloro-5'-hydroxy-indirubin-3'-oxime | Investigative | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-[3-Hydroxyanilino]-6,7-Dimethoxyquinazoline | Investigative | [36] | ||
| Synonyms |
whi-p180; 211555-08-7; 3-[(6,7-dimethoxyquinazolin-4-yl)amino]phenol; WHI-P180, Hydrochloride; JANEX 3; CHEMBL127907; 3-((6,7-Dimethoxyquinazolin-4-yl)amino)phenol; AK341047; DTQ; 4-(3& -Hydroxyphenyl)amino-6,7-dimethoxyquinazoline, HCl; JANEX-3; 4btk; 1di8; AC1L1KWT; AC1Q4XVE; 3-(6,7-dimethoxyquinazolin-4-ylamino)phenol; Anilinoquinazoline deriv 5; WHI-P180(Janex 3); BDBM4622; SCHEMBL1180103; CTK4E5981; AOB5495; MolPort-000-672-017; BNDYIYYKEIXHNK-UHFFFAOYSA-N; HMS3229P15
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1000 nM | |||
| External Link | ||||
| (2'Z,3'E)-5-Nitro-5'-fluoro-indirubin-3'-oxime | Investigative | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| (2'Z,3'E)-5-Nitro-5'-methoxy-indirubin-3'-oxime | Investigative | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Oxindole 95 | Investigative | [20] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| CVT-313 | Investigative | [25] | ||
| Synonyms |
AC1OCFD4; NG 26
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-O-Cyclohexylmethyl Guanine | Investigative | [24] | ||
| Synonyms |
6-(Cyclohexylmethoxy)-9H-purin-2-amine; nu2058; 161058-83-9; 2-amino-6-[(cyclohexylmethyl)oxy]purine; NU 2058; 2-amino-6-cyclohexylmethoxypurine; NU-2058; O6-Cyclohexylmethylguanine; CHEMBL269881; 1e1v; 1h1p; O-Cyclohexylmethylguanine; AC1Q4XUD; 9H-Purin-2-amine, 6-(cyclohexylmethoxy)-; O6-Cyclohexylmethyl guanine; AC1L1IG4; MLS001074898; SCHEMBL3462331; BDBM5485; CTK8D6642; CTK0E6580; EX-A790; AOB6207; MolPort-033-437-778; MolPort-003-958-993; MolPort-044-561-861; HMS3372K06; HMS3262L18; HMS2233F06
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 1200 nM | |||
| External Link | ||||
| NU-6027 | Investigative | [29] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 1300 nM | |||
| External Link | ||||
| NSC-625987 | Investigative | [37] | ||
| Synonyms |
NSC 625987
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (2'Z,3'E)-5-Fluoro-5'-methyl-indirubin-3'-oxime | Investigative | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| (2'Z,3'E)-5-Nitro-5'-hydroxy-indirubin-3'-oxime | Investigative | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| aloisine | Investigative | [38] | ||
| Synonyms |
IN1538; RP106
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MERIOLIN 8 | Investigative | [39] | ||
| Synonyms |
SCHEMBL2991886; CHEMBL406102
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PYRAZOLOPYRIDAZINE 1 | Investigative | [40] | ||
| Synonyms |
551920-54-8; GW810576X; n-(3-methoxyphenyl)-4-pyrazolo[1,5-b]pyridazin-3-yl-2-pyrimidinamine; pyrazolo[1,5-b]pyridazine deriv. 19; AC1O6ZIQ; CHEMBL187081; BDBM8128; SCHEMBL4489357; CTK1F7320; DTXSID60424889; HMS3305F24; HMS3303K24; ZINC13582569; NCGC00242229-01; DA-42106; FT-0707969; AB01092291-01; 2-Pyrimidinamine, N-(3-methoxyphenyl)-4-pyrazolo[1,5-b]pyridazin-3-yl-; N-(3-methoxyphenyl)-4-pyrazolo[1,5-b]pyridazin-3-ylpyrimidin-2-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1.995 nM | |||
| External Link | ||||
| Purvalanol A | Investigative | [41] | ||
| Synonyms |
Purvalanola; AC1L1JD0; IN1131; 2-(1R-Isopropyl-2-hydroxyethylamino)-6-(3-chloroanilino)-9-isopropyl-purine; SCHEMBL3311119; GTPL6030; CTK8F1120; CHEBI:93781; HMS3229M14; KS-00001DB0; HSCI1_000128; AKOS030238850; CCG-206875; RT-015158; K00014; 2-[[6-(3-chloroanilino)-9-propan-2-ylpurin-2-yl]amino]-3-methylbutan-1-ol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 70 nM | |||
| External Link | ||||
| Cdk1/2 inhibitor III | Investigative | [42] | ||
| Synonyms |
443798-55-8; CDK 1/2 INHIBITOR; UNII-LFR1253W75; LFR1253W75; 5-AMINO-3-{[4-(AMINOSULFONYL)PHENYL]AMINO}-N-(2,6-DIFLUOROPHENYL)-1H-1,2,4-TRIAZOLE-1-CARBOTHIOAMIDE; 5-amino-N-(2,6-difluorophenyl)-3-[(4-sulfamoylphenyl)amino]-1H-1,2,4-triazole-1-carbothioamide; 5-Amino-3-((4-(aminosulfonyl)phenyl)amino)-N-(2,6-difluorophenyl)-1H-1,2,4-triazole-1-carbothioamide; 2wu6; AC1NS9OB; CHEMBL261720; GTPL5946; BDBM6878; SCHEMBL1394721; CTK8E9250; DTXSID60416209; MolPort-044-561-528; HMS3229C16; ZINC12355112; DB07664
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MERIOLIN 1 | Investigative | [39] | ||
| Synonyms |
341998-55-8; 4-(1H-PYRROLO[2,3-B]PYRIDIN-3-YL)PYRIMIDIN-2-AMINE; 2-Pyrimidinamine, 4-(1H-pyrrolo[2,3-b]pyridin-3-yl)-; SCHEMBL2241315; CHEMBL405665; HQHSJJZIICGOIX-UHFFFAOYSA-; CTK1B7876; DTXSID60469811; BDBM50371404; ZINC29135178; AKOS027252841; 4-(7-aza-indol-3-yl)-2-amino pyrimidine; 3-(2-Amino-4-pyrimidinyl)-1H-pyrrolo[2,3-b]pyridine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MERIOLIN 4 | Investigative | [39] | ||
| Synonyms |
SCHEMBL3001373; CHEMBL406464
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Lysine Nz-Carboxylic Acid | Investigative | [24] | ||
| Synonyms |
N(6)-carboxylysine; N(6)-carboxy-L-lysine; (2S)-2-amino-6-(carboxyamino)hexanoic acid; N~6~-carboxy-L-lysine; SCHEMBL357483; CHEBI:43575; ZINC6753301
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MERIOLIN 6 | Investigative | [39] | ||
| Synonyms |
CHEMBL406463; SCHEMBL2999009
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| K00024 | Investigative | [43] | ||
| Synonyms |
indolocarbazole deriv. 4(d)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 10Z-Hymenialdisine | Investigative | [20] | ||
| Synonyms |
Hymenialdisine; (Z)-Hymenialdisine; CHEMBL361708; 95569-43-0; 82005-12-7; (4Z)-4-(2-amino-4-oxo-1H-imidazol-5-ylidene)-2-bromo-1,5,6,7-tetrahydropyrrolo[2,3-c]azepin-8-one; Hymenialdisine, 1; 4-(2-Amino-4-oxo-2-imidazolidin-5-ylidene)-2-bromo-4,5,6,7-tetrahydropyrrolo[2,3-c]azepin-8-one; AC1MHZEP; SCHEMBL155899; STO156; BDBM7491; SCHEMBL15426167; MolPort-006-394-608; 4-(2-Amino-5-oxo-3,5-dihydro-imidazol-4-ylidene)-2-bromo-4,5,6,7-tetrahydro-1H-pyrrolo[2,3-c]azepin-8-one; NSC607173
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| aminopurvalanol A | Investigative | [44] | ||
| Synonyms |
NG-97
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 9-Nitropaullone | Investigative | [20] | ||
| Synonyms |
Alsterpaullone; 237430-03-4; NSC 705701; NSC-705701; CHEMBL50894; MLS002702475; 9-Nitro-7,12-dihydroindolo[3,2-d][1]benzazepin-6(5H)-one; 9-nitro-7,12-dihydro-5H-indolo[3,2-d][1]benzazepin-6-one; 9-Nitro-7,12-dihydroindolo-[3,2-d][1]benzazepin-6(5)-one; 9-NITRO-5,12-DIHYDRO-7H-BENZO[2,3]AZEPINO[4,5-B]INDOL-6-ONE; Paullone Analog 1; AC1NMCUD; Kinome_3754; 1q3w; Alsterpaullone derivative, 2; Lopac0_000057; CBiol_001723; GTPL5925; BDBM7262; SCHEMBL2170104; CTK8F0374; ZINC23894; BDBM84528; DTXSID50407444
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 15 nM | |||
| External Link | ||||
| MERIOLIN 5 | Investigative | [39] | ||
| Synonyms |
1011711-76-4; 4-(4-Propoxy-1h-Pyrrolo[2,3-B]pyridin-3-Yl)pyrimidin-2-Amine; 4-{4-propoxy-1H-pyrrolo[2,3-b]pyridin-3-yl}pyrimidin-2-amine; SCHEMBL2997013; CHEMBL270687; ZINC16052674; BDBM50371400; DB08182
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SU9516 | Investigative | [20] | ||
| Synonyms |
377090-84-1; SU 9516; SU-9516; AC1NRD9P; 666837-93-0; (3Z)-3-(1H-IMIDAZOL-5-YLMETHYLENE)-5-METHOXY-1H-INDOL-2(3H)-ONE; (Z)-3-((1H-imidazol-5-yl)methylene)-5-methoxyindolin-2-one; (3Z)-3-(1H-imidazol-5-ylmethylidene)-5-methoxy-2,3-dihydro-1H-indol-2-one; 3-[1-(3H-Imidazol-4-yl)-meth-(Z)-ylidene]-5-methoxy-1,3-dihydro-indol-2-one; SU9; (Z)-1,3-DIHYDRO-3-(1H-IMIDAZOL-4-YLMETHYLENE)-5-METHOXY-2H-INDOL-2-ONE; 3py1; 3py0; imidazole indolinone driv.; MLS001074893; SCHEMBL1170686; BDBM7238; SCHEMBL1170687; CHEMBL258805
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 22 nM | |||
| External Link | ||||
| PYRAZOLOPYRIDAZINE 2 | Investigative | [40] | ||
| Synonyms |
pyrazolo[1,5-b]pyridazine deriv. 25; AC1O6ZJ2; BDBM8134; CHEMBL186054; N-(3,4-dimethoxyphenyl)-4-pyrazolo[1,5-b]pyridazin-3-ylpyrimidin-2-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 50 nM | |||
| External Link | ||||
| MERIOLIN 3 | Investigative | [39] | ||
| Synonyms |
954143-48-7; 4-(4-Methoxy-1h-Pyrrolo[2,3-B]pyridin-3-Yl)pyrimidin-2-Amine; 4-{4-methoxy-1H-pyrrolo[2,3-b]pyridin-3-yl}pyrimidin-2-amine; SCHEMBL2996575; CHEMBL270686; BDBM50371402; ZINC16052675; DB08178; KB-274295
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MERIOLIN 2 | Investigative | [39] | ||
| Synonyms |
SCHEMBL2999865; CHEMBL270897; 1H-Pyrrolo[2,3-b]pyridin-4-ol, 3-(2-amino-4-pyrimidinyl)-; BDBM50371403; 954143-47-6
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID18986805C9b | Investigative | [45] | ||
| Synonyms |
GTPL8160; BDBM50246396; ZINC38224644
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 1 nM | |||
| External Link | ||||
| 3-((3,5-diamino-1H-pyrazol-4-yl)diazenyl)phenol | Investigative | [35] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Indirubin-5-sulfonate | Investigative | [20] | ||
| Synonyms |
indirubin-5-sulphonate; CHEMBL1207227; 2',3-DIOXO-1,1',2',3-TETRAHYDRO-2,3'-BIINDOLE-5'-SULFONIC ACID; INR; AC1NRBUE; AC1NZHHM; 1v0o; AC1O8NUW; Indirubin derivative, 20; SCHEMBL490806; BDBM84534; A05-A11B1-I; NSC717821; BDBM50023871; NSC-717821; DB02519; NCI60_040625; 2-oxo-3-(3-oxo-1H-indol-2-ylidene)-1H-indole-5-sulfonic acid; (3Z)-2-oxo-3-(3-oxoindolin-2-ylidene)indoline-5-sulfonic acid; (3E)-2-oxo-3-(3-oxo-1H-indol-2-ylidene)-1H-indole-5-sulfonic acid; (2Z)-2',3-dioxo-1,1',2',3-tetrahydro-2,3'-biindole-5'-sulfonic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 22 nM | |||
| External Link | ||||
| N-(4-amino-5-cyano-6-phenylpyridin-2-yl)acetamide | Investigative | [46] | ||
| Synonyms |
Kinome_3019; CHEMBL210032; SCHEMBL1145340; BDBM15968; Aminopyridine-Based Inhibitor 24
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 1300 nM | |||
| External Link | ||||
| NU-6102 | Investigative | [47] | ||
| Synonyms |
nu6102; 444722-95-6; NU 6102; O6-CYCLOHEXYLMETHOXY-2-(4'-SULPHAMOYLANILINO) PURINE; Cdk1/2 Inhibitor II, NU6102; 6-Cyclohexylmethoxy-2-(4& -sulfamoylanilino)purine; 4-{[6-(cyclohexylmethoxy)-9H-purin-2-yl]amino}benzenesulfonamide; 4SP; 1h1s; 4-[[6-(cyclohexylmethoxy)-7H-purin-2-yl]amino]benzenesulfonamide; 4-{[6-(cyclohexylmethoxy)-7h-purin-2-yl]amino}benzenesulfonamide; 4-[[6-(cyclohexylmethoxy)-9h-purin-2-yl]amino]benzenesulfonamide; 4eor; 4eok; 2iw9; 2c6o; 2iw8; AC1L1IGA; SCHEMBL2170816; CHEMBL319467
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| PF-228 | Investigative | [48] | ||
| Synonyms |
869288-64-2; PF-573228; PF 573228; PF573228; CHEMBL514554; 3,4-Dihydro-6-[[4-[[[3-(methylsulfonyl)phenyl]methyl]amino]-5-(trifluoromethyl)-2-pyrimidinyl]amino]-2(1H)-quinolinone; 6-((4-((3-(Methylsulfonyl)benzyl)amino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)-3,4-dihydroquinolin-2(1H)-one; 6-(4-(3-(methylsulfonyl)benzylamino)-5-(trifluoromethyl)pyrimidin-2-ylamino)-3,4-dihydroquinolin-2(1H)-one; 6-[4-(3-Methanesulfonyl-benzylamino)-5-trifluoromethyl-pyrimidin-2-ylamino]-3,4-dihydro-1H-quinolin-2-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| aloisine A | Investigative | [49] | ||
| Synonyms |
4-(7-butyl-5H-pyrrolo[2,3-b]pyrazin-6-yl)phenol; 496864-16-5; RP107; 6-PHENYL[5H]PYRROLO[2,3-B]PYRAZINE; CHEMBL75680; 7-n-Butyl-6-(4-hydroxyphenyl)[5H]pyrrolo[2,3-b]pyrazine; RP-107; ALH; 4-{7-butyl-5H-pyrrolo[2,3-b]pyrazin-6-yl}phenol; 1ung; SCHEMBL80147; BDBM7377; GTPL5924; AC1NS169; 4-(7-Butyl-5H-pyrrolo[2,3-b]pyrazin-6-yl)-phenol; CHEBI:93641; CTK8D4068; DTXSID30416115; HMS3229A14; RM-39; BCP26893; ZINC2540737; ACT06534; IN1539; HSCI1_000219; MFCD04973541; AKOS005145972; CCG-206814; DB07364; RTC-063070; AJ-39131
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NU6140 | Investigative | [50] | ||
| Synonyms |
Cdk2 Inhibitor IV, NU6140; 444723-13-1; NU 6140; CHEMBL1802728; 4-(6-Cyclohexylmethoxy-9H-purin-2-ylamino)-N,N-diethylbenzamide; 4-{[6-(cyclohexylmethoxy)-7H-purin-2-yl]amino}-N,N-diethylbenzamide; Cdk2 inhibitor IV; SCHEMBL2169233; GTPL5949; CTK8E7940; DTXSID30436732; MolPort-023-276-742; MolPort-044-561-419; HMS3229E18; IN1369; ZINC22309248; BDBM50347924; AKOS024457537; CCG-206836; NCGC00370819-01; NU6140, > RT-011957; 4-(6-cyclohexylmethoxy-9hpurin-2-ylamino)-N,N-diethyl-benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 400 nM | |||
| External Link | ||||
| BX-912 | Investigative | [51] | ||
| Synonyms |
BX 912; BX912
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BX-795 | Investigative | [51] | ||
| Synonyms |
BX795; BX 795
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID19115845C89S | Investigative | [52] | ||
| Synonyms |
3du8; GTPL8114; BDBM27380; DB07149
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 138 nM | |||
| External Link | ||||
| Indirubin-3'-monoxime | Investigative | [20] | ||
| Synonyms |
indirubin-3'-oxime; 160807-49-8; indirubin-3-oxime; Indirubin-3monoxime; Indirubin-3-monoxime; 3-[3-(Hydroxyamino)-1H-indol-2-yl]indol-2-one; CHEBI:43645; Indirubin 3'-monoxime; indirubin-3'-monooxime; Indirubin-3& CHEMBL216543; CHEMBL126077; (Z)-1H,1'H-[2,3']BIINDOLYLIDENE-3,2'-DIONE-3-OXIME; UNM-0000305771; 3-[1,3-dihydro-3-(hydroxyimino)-2H-indol-2-ylidene]-1,3-dihydro-2H-indol-2-one; 667463-82-3; IXM; SR-01000075929; Indirubin-3; Indirubin 3-oxime; Tocris-1813; BiomolKI_000070
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Double Oxidized Cysteine | Investigative | [24] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-(indole-3-yl)-4-phenyl-1H-pyrrole-2,5-dione | Investigative | [53] | ||
| Synonyms |
CHEMBL380598; SCHEMBL3148490; HVQJGNALTWNDMX-UHFFFAOYSA-N; BDBM50375058; 2-(1H-Indole-3-yl)-3-phenylmaleimide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BMS-536924 | Investigative | [54] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 3,4-di-(4-methoxyphenyl)-1H-pyrrole-2,5-dione | Investigative | [53] | ||
| Synonyms |
1H-Pyrrole-2,5-dione, 3,4-bis(4-methoxyphenyl)-; 108774-82-9; ACMC-20mbs9; CHEMBL381099; CTK0G2626; DTXSID90449388
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3,4-diphenyl-1H-pyrrole-2,5-dione | Investigative | [53] | ||
| Synonyms |
2,3-diphenylmaleimide; 1H-Pyrrole-2,5-dione, 3,4-diphenyl-; 31295-36-0; AC1MBL6S; SCHEMBL114611; CHEMBL201949; CTK1B9880; 3,4-diphenylpyrrole-2,5-dione; DTXSID70372903; ZINC3847556
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-(4-methoxyphenyl)-4-phenyl-1H-pyrrole-2,5-dione | Investigative | [53] | ||
| Synonyms |
CHEMBL372076; SCHEMBL3822337
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-[(3,5-diamino-1H-pyrazol-4-yl)diazenyl]phenol | Investigative | [35] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Histone acetyltransferase p300 (P300) | 2 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| CCS1477 | Phase 1/2 | [55] | ||
| Synonyms |
CCS-1477; CBP-IN-1; 2222941-37-7; (S)-1-(3,4-Difluorophenyl)-6-(5-(3,5-dimethylisoxazol-4-yl)-1-((1r,4S)-4-methoxycyclohexyl)-1H-benzo[d]imidazol-2-yl)piperidin-2-one; SCHEMBL20094038; SCHEMBL21515367; SCHEMBL22134021; EX-A3687; NSC818619; NSC-818619; HY-111784; CS-0091862; (S)-1-(3,4-Difluorophenyl)-6-(5-(3,5-dimethylisoxazol-4-yl)-1-(trans-4-methoxycyclohexyl)-1H-benzo[d]imidazol-2-yl)piperidin-2-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| FT-7051 | Phase 1 | [56] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 9B71: Retinopathy | 12 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| SHP607 | Phase 2 | [57] | ||
| External Link | ||||
| RST-001 | Phase 1/2 | [58] | ||
| Synonyms |
ChR2 gene therapy (retinopathy), RetroSense; AAV-based ChR2 gene therapy (retinopathy), RetroSense; Channelrhodopsin-2 gene therapy (retinopathy), RetroSense
Click to Show/Hide
|
|||
| External Link | ||||
| ReN-003 | Phase 1/2 | [59] | ||
| Synonyms |
Stem cell therapy (retinal diseases), ReNeuron
Click to Show/Hide
|
|||
| External Link | ||||
| PMID28621580-Compound-WO2015089220C70 | Patented | [60] | ||
| External Link | ||||
| SIR-1076 | Preclinical | [61] | ||
| Synonyms |
Antioxidants (topical, retinal oxidative stress), Sirion Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| CytoRet | Investigative | [59] | ||
| Synonyms |
Retinal cell therapy (retinal disease), International Stem Cell; Retinal layers (parthenogenetic stem-cell derived), ISCO
Click to Show/Hide
|
|||
| External Link | ||||
| LPO-1010CSR | Investigative | [59] | ||
| External Link | ||||
| INDUS-815C | Investigative | [62] | ||
| Synonyms |
VEGF modulator (age related macular degeneration/retinopathy), Indus Biotech; INDUS-815C (Huntington's disease), Indus Biotech; INDUS-815C (age related macular degeneration/retinopathy), Indus Biotech; SIRT2 inhibtior (Parkinson's disease/dementia/Huntington's disease), Indus Biotech; NAD-dependent deacetylase sirtuin-2inhibitor (Parkinson's disease/dementia/Huntington's disease), Indus Biotech
Click to Show/Hide
|
|||
| External Link | ||||
| SF-113 | Investigative | [59] | ||
| External Link | ||||
| SF-106 | Investigative | [59] | ||
| External Link | ||||
| Gene therapy, retinopathy, | Investigative | [59] | ||
| Synonyms |
Gene therapy (retinopathy)
Click to Show/Hide
|
|||
| External Link | ||||
| Superoxide dismutase mimetics | Investigative | [59] | ||
| Synonyms |
INO-6001; INO-6002; Superoxide dismutase mimetics (retinal disease); Superoxide dismutase mimetics (retinal disease), Inotek; Superoxide dismutase mimetics (age-related macular degeneration/diabetic retinopathy/diabetic macular edema), Inotek
Click to Show/Hide
|
|||
| External Link | ||||
References