m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03082
|
[1] | |||
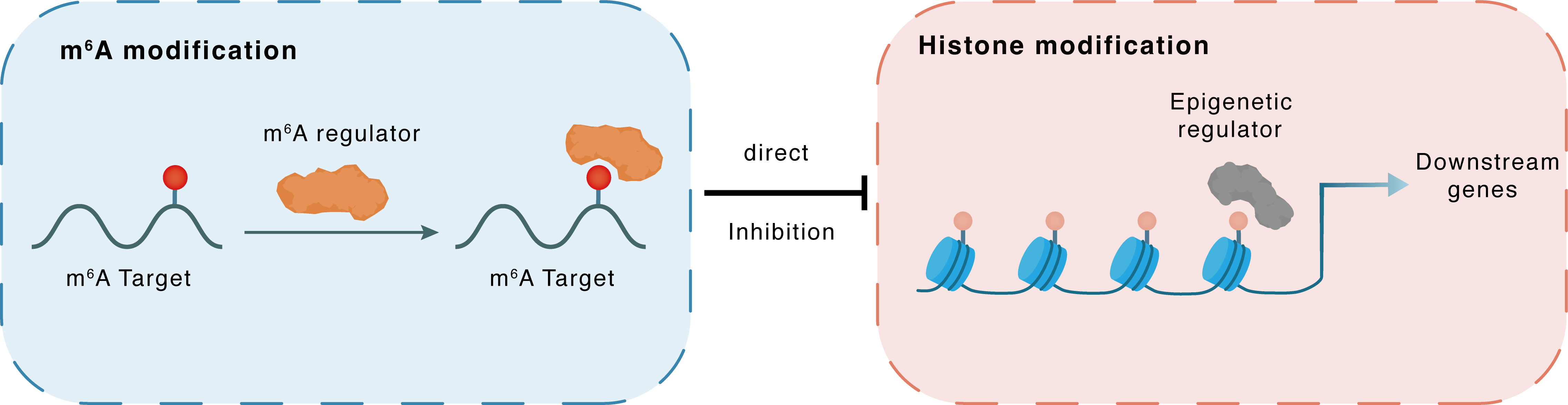 m6A modification
EZH2
EZH2
YTHDF2
m6A modification
EZH2
EZH2
YTHDF2
 : m6A sites
Direct
Inhibition
Histone modification
H3K27me3
EZH2
Downstream Gene : m6A sites
Direct
Inhibition
Histone modification
H3K27me3
EZH2
Downstream Gene
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | YTH domain-containing family protein 2 (YTHDF2) | READER | |||
| m6A Target | Histone-lysine N-methyltransferase EZH2 (EZH2) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone-lysine N-methyltransferase EZH2 (EZH2) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 27 trimethylation (H3K27me3) | View Details | |||
| Crosstalk Relationship | m6A → Histone modification | Inhibition | |||
| Crosstalk Mechanism | m6A modification impacts directly histone modification through modulating the expression level of histone-associated enzymes | ||||
| Crosstalk Summary | Increased Histone-lysine N-methyltransferase EZH2 (EZH2) expression was due to the increased expression of m6A demethylase ALKBH5 and decreased expression of the m6A reader protein YTHDF2. YTHDF2 directly bind to the m6A modification site of EZH2 to promote EZH2 mRNA degradation in ESCs. Enhancer of zeste homology 2 (EZH2) is a methyltransferase which catalyses Histone H3 lysine 27 trimethylation (H3K27me3), leading to decreased expression levels of target genes. | ||||
| Responsed Disease | Endometriosis | ICD-11: GA10 | |||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Histone-lysine N-methyltransferase EZH2 (EZH2) | 74 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Tazemetostat | Approved | [2] | ||
| Synonyms |
EPZ-6438; 1403254-99-8; EPZ6438; UNII-Q40W93WPE1; N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(morpholinomethyl)-[1,1'-biphenyl]-3-carboxamide; Q40W93WPE1; EPZ 6438; E7438; (1,1'-Biphenyl)-3-carboxamide, N-((1,2-dihydro-4,6-dimethyl-2-oxo-3-pyridinyl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(4-morpholinylmethyl)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 2.5 nM | |||
| External Link | ||||
| DS-3201b | Phase 2 | [3] | ||
| Synonyms |
Valemetostat; 1809336-39-7; UNII-60RD0234VE; 60RD0234VE; 1809336-39-7 (free base); DS-3201; (2R)-7-chloro-2-[trans-4-(dimethylamino)cyclohexyl]-N-[(4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl]-2,4-dimethyl-1,3-benzodioxole-5-carboxamide; Valemetostat 2HCl; Valemetostat [INN]; Valemetostat (DS-3201); CHEMBL4597193; EZH1/2 inhibitor DS-3201; SCHEMBL18393626; SCHEMBL18393627; SCHEMBL18639210; EX-A3423; DS3201; NSC813381; s8926; NSC-813381; HY-109108; CS-0039740; D11551
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CPI-1205 | Phase 1/2 | [4] | ||
| Synonyms |
HPODOLXTMDHLLC-QGZVFWFLSA-N; 1621862-70-1; UNII-455J2479FY; CPI1205; CPI 1205; 455J2479FY; (R)-N-((4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-methyl-1-(1-(1-(2,2,2-trifluoroethyl)piperidin-4-yl)ethyl)-1H-indole-3-carboxamide; GTPL9115; SCHEMBL17329268; MolPort-044-560-382; KS-000006BA; EX-A1068; s8353; AKOS030628484; ZINC220982768; CS-7648; compound 13 [PMID: 27739677]; HY-100021; J3.556.402K; N-[(4-methoxy-6-methyl-2-oxo-1H-pyridin-3-yl)methyl]-2-methyl-1-[(1R)-1-[1-(2,2,2-trifluoroethyl)piperidin-4-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SHR2554 | Phase 1/2 | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| CPI-0209 | Phase 1/2 | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| GSK2816126 | Phase 1 | [7] | ||
| Synonyms |
GSK 126; GSK-126
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | Ki = 0.5 nM | |||
| External Link | ||||
| PF-06821497 | Phase 1 | [8] | ||
| Synonyms |
UNII-S4L4MM20B6; S4L4MM20B6; CHEMBL4080228; PF06821497; 1844849-10-0; 5,8-dichloro-2-[(4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl]-7-[(R)-methoxy(oxetan-3-yl)methyl]-3,4-dihydroisoquinolin-1(2H)-one; SCHEMBL17330426; GTPL10516; BDBM50246967; NSC800019; DB14799; HY-101571A; NSC-800019; compound 23a [PMID: 29211475]; CS-0092626; Q29209799; 1(2H)-Isoquinolinone, 5,8-dichloro-2-((1,2-dihydro-4-methoxy-6-methyl-2-oxo-3-pyridinyl)methyl)-3,4-dihydro-7-((R)-methoxy-3-oxetanylmethyl)-; 5,8-dichloro-2-[(4-methoxy-6-methyl-2-oxo-1H-pyridin-3-yl)methyl]-7-[(R)-methoxy(oxetan-3-yl)methyl]-3,4-dihydroisoquinolin-1-one; CJD
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| DS-3201 | Phase 1 | [4] | ||
| Synonyms |
QTGYNKYZRZATJB-UHFFFAOYSA-N; 701899-63-0; 2-(2-(2-Methyl-1H-imidazol-5-yl)ethyl)isoindoline-1,3-dione; 2-(2-(2-Methyl-1H-imidazol-5-yl)-ethyl)isoindoline-1,3-dione; SCHEMBL7743461; MolPort-035-945-474; MolPort-035-690-353; ZINC98086069; AKOS024459002; AKOS024262663; MCULE-2708350770; FCH4077443; AK158834; AX8292467; ST2403812; 2-[2-(2-methyl-1H-imidazol-5-yl)ethyl]-2,3-dihydro-1H-isoindole-1,3-dione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| HH2853 | Phase 1 | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-33 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID28394193-Compound-Figure3bI | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-35 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| PMID28394193-Compound-54 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-24 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-27 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-25 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-50 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-47 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2 nM; Ki < 1 nM | |||
| External Link | ||||
| PMID28394193-Compound-21 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-41 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-53 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 80 nM | |||
| External Link | ||||
| PMID28394193-Compound-Figure5aVIII | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-38 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-51 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-31 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 20 nM | |||
| External Link | ||||
| PMID28394193-Compound-42 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-15 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-52 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-32 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| PMID28394193-Compound-23 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-29 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID28394193-Compound-30 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 16 nM | |||
| External Link | ||||
| PMID28394193-Compound-39 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-49 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-43 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-40 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-Figure3bIII | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-36 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 316 nM | |||
| External Link | ||||
| PMID28394193-Compound-28 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 32 nM | |||
| External Link | ||||
| PMID28394193-Compound-22 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-18 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-16 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-44 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-20 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-19 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-37 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-Figure3bII | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-26 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-17 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-34 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID28394193-Compound-56 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.93 nM | |||
| External Link | ||||
| PMID28394193-Compound-46 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| PMID28394193-Compound-57 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6.45 nM | |||
| External Link | ||||
| PMID28394193-Compound-14 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 21900 nM | |||
| External Link | ||||
| PMID28394193-Compound-12 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 10 nM | |||
| External Link | ||||
| PMID26882240-Compound-1 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-55 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 8.13 nM | |||
| External Link | ||||
| PMID28394193-Compound-45 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 168000 nM | |||
| External Link | ||||
| PMID28394193-Compound-13 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1470 nM | |||
| External Link | ||||
| EPZ005687 | Investigative | [12] | ||
| Synonyms |
EPZ-005687; EPZ 005687
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| EI1 | Investigative | [13] | ||
| Synonyms |
KB-145943
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| UNC1999 | Investigative | [14] | ||
| Synonyms |
UNC 1999; UNC-1999
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MS1943 | Investigative | [5] | ||
| Synonyms |
2225938-17-8; SCHEMBL21271666; EX-A3962; s8918; HY-133129; CS-0112146; 6-(6-(4-(2-(2-((3r,5r,7r)-adamantan-1-yl)acetamido)ethyl)piperazin-1-yl)pyridin-3-yl)-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-1-isopropyl-1H-indazole-4-carboxamide; 6-(6-(4-(2-(2-(Adamantan-1-yl)acetamido)ethyl)piperazin-1-yl)pyridin-3-yl)-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-1-isopropyl-1H-indazole-4-carboxamide
Click to Show/Hide
|
|||
| MOA | Degrader | |||
| External Link | ||||
| GSK343 | Investigative | [15] | ||
| Synonyms |
compound 6 [PMID 24900432]; GSK 343
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 174 nM | |||
| External Link | ||||
| PMID28394193-Compound-11 | Patented | [16] | ||
| External Link | ||||
| PMID28394193-Compound-10 | Patented | [16] | ||
| External Link | ||||
| PMID28394193-Compound-14 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 21900 nM | |||
| External Link | ||||
| PMID28394193-Compound-12 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 10 nM | |||
| External Link | ||||
| PMID28394193-Compound-13 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1470 nM | |||
| External Link | ||||
| PMID28394193-Compound-56 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.93 nM | |||
| External Link | ||||
| PMID28394193-Compound-46 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| PMID28394193-Compound-57 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6.45 nM | |||
| External Link | ||||
| PMID28394193-Compound-55 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 8.13 nM | |||
| External Link | ||||
| PMID28394193-Compound-45 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 168000 nM | |||
| External Link | ||||
| GA10: Endometriosis | 32 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Elagolix sodium | Approved | [17] | ||
| Synonyms |
Elagolix sodium salt; UNII-5948VUI423; Elagolix sodium [USAN]; 5948VUI423; Elagolix sodium (USAN); SCHEMBL1641994; NBI 56418NA; NBI-56418-NA; MolPort-003-984-965; NBI-56418 NA; DQYGXRQUFSRDCH-UQIIZPHYSA-M; AKOS030524154; VA12044; KS-0000063K; KB-76766; HY-14369; AC-29671; CS-0003317; D09336
Click to Show/Hide
|
|||
| External Link | ||||
| Histrelin | Approved | [18] | ||
| Synonyms |
Supprelin (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Relugolix | Phase 3 | [19] | ||
| Synonyms |
737789-87-6; TAK-385; TAK 385; UNII-P76B05O5V6; CHEMBL1800159; TAK-385/TAK385; P76B05O5V6; 1-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)methyl)-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl)phenyl)-3-methoxyurea; Relugolix [USAN:INN]; TAK385; Relugolix (JAN/INN); SCHEMBL778416; GTPL5586; DTXSID40224167; MolPort-044-567-649; AOMXMOCNKJTRQP-UHFFFAOYSA-N; EX-A1083; BCP21587; ZINC43206033; BDBM50347982; AKOS027440398; SB16721; DB11853; CS-5917
Click to Show/Hide
|
|||
| External Link | ||||
| Naproxen | Approved | [20] | ||
| Synonyms |
22204-53-1; (S)-Naproxen; Naproxene; Naprosyn; (+)-Naproxen; Equiproxen; Laraflex; Naproxenum; Naproxeno; d-Naproxen; (S)-(+)-2-(6-Methoxy-2-naphthyl)propionic acid; (S)-(+)-Naproxen; Calosen; Nycopren; Naprosyne; Bonyl; Reuxen; Naixan; Axer; (+)-(S)-Naproxen; Ec-Naprosyn; (S)-2-(6-methoxynaphthalen-2-yl)propanoic acid; Flexipen; Clinosyn; Artrixen; Anexopen; Acusprain; Novonaprox; Arthrisil; Leniartil; Danaprox; Bipronyl; Artroxen; Napren; Naposin; Napflam; Genoxen; Daprox; Atiflan; Artagen; Apronax; Naprius; Nalyxan; Lefaine; Congex
Click to Show/Hide
|
|||
| External Link | ||||
| Nafarelin | Approved | [21] | ||
| Synonyms |
Nafarelina; Nafareline; Nafarelinum; Synarel; NAFARELIN ACETATE; Nafarelina [Spanish]; Nafareline [French]; Nafarelinum [Latin]; HS-2018; Nafarelin (INN); Nafarelin Acetate, Hydrate; Nafarelin [INN:BAN]; Synarel (TN); RS-94991-298; 6-(3-(2-Naphthalenyl)-D-alanine)luteinizing hormone-releasing factor (pig)
Click to Show/Hide
|
|||
| External Link | ||||
| Oestradiol valerate and dienogest | Approved | [22] | ||
| Synonyms |
Dienogest; 65928-58-7; Dienogestrel; Dienogestum; Dienogestril; Dinagest; Natazia; Endometrion; Visanne; STS-557; STS 557; Dienogestum [Latin]; UNII-46M3EV8HHE; MJR-35; 17alpha-17-Hydroxy-3-oxo-19-norpregna-4,9-diene-21-nitrile; BAY86-5258; BAY 86-5258; 46M3EV8HHE; CHEBI:70708; 17-alpha-Cyanomethyl-17-beta-hydroxy-estra-4,9(10)-dien-3-one; 2-[(8S,13S,14S,17R)-17-hydroxy-13-methyl-3-oxo-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-17-yl]acetonitrile; 17-alpha-Cyanomethyl-17-beta-hydroxyestra-4,9(10)-diene-3-one; Natazia (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| TZTX-001 | Phase 3 | [23] | ||
| Synonyms |
Teverelix LA; TX 12-001-HR
Click to Show/Hide
|
|||
| External Link | ||||
| Nestorone | Phase 3 | [24] | ||
| Synonyms |
Nestorone MDTS; Nestorone metered dose transdermal system; Nestorone transdermal spray, Acrux; ST-1435; Nestorone transdermal spray, Population Council/Acrux
Click to Show/Hide
|
|||
| External Link | ||||
| Telapristone | Phase 2 | [25] | ||
| Synonyms |
Telapristone acetate; Proellex; 198414-31-2; UNII-1K9EYK92PQ; CDB-4124; 1K9EYK92PQ; Telapristone acetate [USAN]; Telapristone acetate (USAN); CCRIS 9331; CDB 4124; SCHEMBL374762; CHEMBL2105694; DTXSID60173587; RU-44675; 17alpha-Acetoxy-11beta-(4-(dimethylamino)phenyl)-21-methoxy-19-norpregna-4,9-dien-3,20-dione; D09972; 19-Norpregna-4,9-diene-3,20-dione, 17-(acetyloxy)-11-(4-(dimethylamino)phenyl)-21-methoxy-, (11beta)-; A-Acetoxy-21-methoxy-11
Click to Show/Hide
|
|||
| External Link | ||||
| F-8-IL-10 fusion protein | Phase 2 | [26] | ||
| Synonyms |
Dekavil; F-8-IL-10; F-8-IL-10 fusion protein (rheumatoid arthritis/endometriosis); F-8-IL-10 fusion protein (rheumatoid arthritis/endometriosis), Philogen
Click to Show/Hide
|
|||
| External Link | ||||
| PGL-2 | Phase 2 | [27] | ||
| External Link | ||||
| BAY 98-7196 | Phase 2 | [28] | ||
| External Link | ||||
| KLH-2109 | Phase 2 | [29] | ||
| External Link | ||||
| Vilaprisan | Phase 2 | [30] | ||
| Synonyms |
UNII-IN59K53GI9; BAY1002670; IN59K53GI9; 1262108-14-4; Vilaprisan [INN]; BAY 1002670; Vilaprisan [USAN:INN]; Vilaprisan (USAN/INN); SCHEMBL2121854; CHEMBL3989936; BCP24069; DB11971; 20,20,21,21,21-Pentafluoro-17-hydroxy-11beta-(4-(methanesulfonyl)phenyl)-19-nor-17alpha-pregna-4,9-dien-3-one; 19-Norpregna-4,9-dien-3-one, 20,20,21,21,21-pentafluoro-17-hydroxy-11-(4-(methylsulfonyl)phenyl)-, (11beta,17alpha)-
Click to Show/Hide
|
|||
| External Link | ||||
| PGL-2001 | Phase 2 | [31] | ||
| Synonyms |
Steroid sulfatase inhibitor (endometriosis), PregLem
Click to Show/Hide
|
|||
| External Link | ||||
| BGS-649 | Phase 2 | [32] | ||
| External Link | ||||
| ASP-1707 | Phase 2 | [33] | ||
| External Link | ||||
| BAY 1817080 | Phase 1 | [34] | ||
| Synonyms |
Eliapixant
Click to Show/Hide
|
|||
| External Link | ||||
| Alfa-interferon | Phase 1 | [35] | ||
| External Link | ||||
| PF-4418948 | Phase 1 | [36] | ||
| Synonyms |
PF-04418948; Prostaglandin E2 EP2 subtype antagonist (endometriosis), Pfizer
Click to Show/Hide
|
|||
| External Link | ||||
| BAY-1817080 | Phase 1 | [37] | ||
| External Link | ||||
| PF-2413873 | Phase 1 | [38] | ||
| Synonyms |
PF-02413873
Click to Show/Hide
|
|||
| External Link | ||||
| BAY 1026153 | Phase 1 | [39] | ||
| External Link | ||||
| PMID27215781-Compound-28 | Patented | [40] | ||
| External Link | ||||
| Asoprisnil | Discontinued in Phase 3 | [41] | ||
| Synonyms |
J867; J-867; Asoprisnil (USAN/INN); 11beta-(4-((E)-(Hydroxyimino)methyl)phenyl)-17beta-methoxy-17-(methoxymethyl)estra-4,9-dien-3-one
Click to Show/Hide
|
|||
| External Link | ||||
| FP-1096 | Discontinued in Phase 3 | [42] | ||
| External Link | ||||
| ASP-0265 | Terminated | [43] | ||
| External Link | ||||
| NS398 | Terminated | [44] | ||
| Synonyms |
ns-398; 123653-11-2; NS 398; N-(2-Cyclohexyloxy-4-nitrophenyl)methanesulfonamide; N-[2-(Cyclohexyloxy)-4-nitrophenyl]methanesulfonamide; CHEMBL7162; CHEBI:73458; Methanesulfonamide, N-(2-(cyclohexyloxy)-4-nitrophenyl)-; n-(2-cyclohexyloxy-4-nitrophenyl)methane sulfonamide; N-(2-Cyclohexyloxy-4-nitro-phenyl)-methanesulfonamide; Taisho NS 398; SR-01000597479; N-[2-Cyclohexyloxy-4-nitrophenyl]methanesulfonamide; N-(2-(cyclohexyloxy)-4-nitrophenyl)methanesulfonamide; CCRIS 8523; KTDZCOWXCWUPEO-UHFFFAOYSA-N; Tocris-0942
Click to Show/Hide
|
|||
| External Link | ||||
| LHRH | Investigative | [45] | ||
| Synonyms |
SCHEMBL7378993
Click to Show/Hide
|
|||
| External Link | ||||
| PF-02367982 | Investigative | [46] | ||
| Synonyms |
CHEMBL1083754; BDBM50318934
Click to Show/Hide
|
|||
| External Link | ||||
| Recombinant human CC10 | Investigative | [47] | ||
| Synonyms |
Claragen-WH; Recombinant human CC10 (intravaginal, endometriosis/infertility); Recombinant human CC10 (intravaginal, endometriosis/infertility), Clarassance; Uteroglobin (intravaginal, endometriosis/infertility), Clarassance
Click to Show/Hide
|
|||
| External Link | ||||
| DXL-1215 | Investigative | [47] | ||
| Synonyms |
Monoclonal antibody (endometriosis), InNexus
Click to Show/Hide
|
|||
| External Link | ||||
References