m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03071
|
[1] | |||
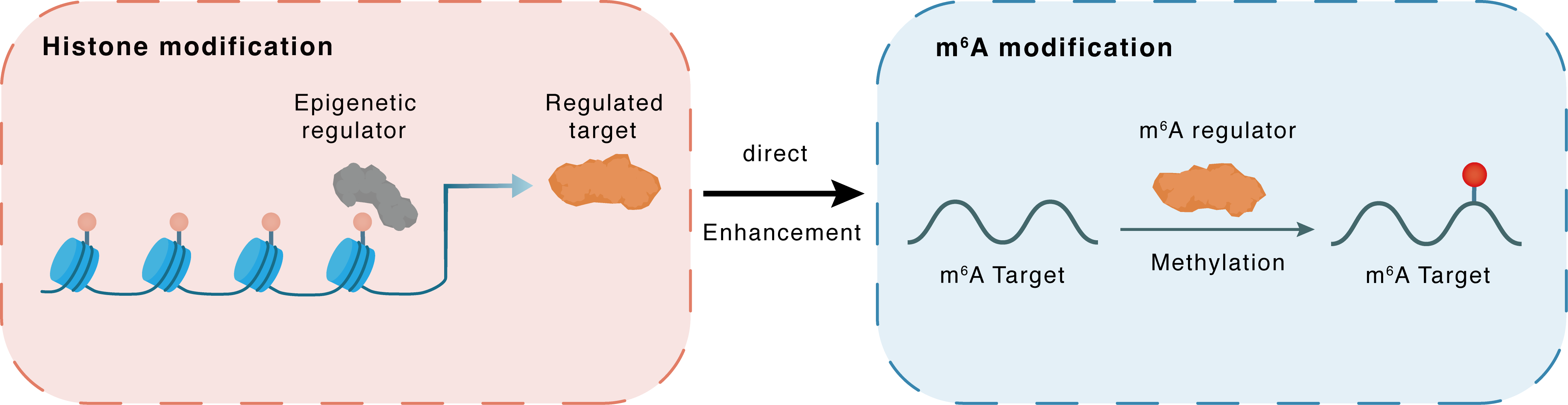 Histone modification
H3K4me3
MLL1
METTL3
Direct
Enhancement
m6A modification
MIR503
MIR503
METTL3
Methylation
Histone modification
H3K4me3
MLL1
METTL3
Direct
Enhancement
m6A modification
MIR503
MIR503
METTL3
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | miR-503 | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone-lysine N-methyltransferase 2A (KMT2A) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 4 trimethylation (H3K4me3) | View Details | |||
| Downstream Gene | METTL3 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification directly impacts m6A modification through modulating the level of m6A regulator | ||||
| Crosstalk Summary | KMT2A induced rapid Histone H3 lysine 4 trimethylation (H3K4me3) of the promoter of the methyltransferase-like 3 gene (METTL3) and resulted in its overexpression. METTL3 overexpression evokes N6-methyladenosine (m6A)-dependent miR-503 biogenesis in endothelial cells. | ||||
| Responsed Disease | Myocardial injury | ICD-11: NB31.Z | |||
| Responsed Drug | C646 | ||||
| Cell Process | Mitochondrial metabolic dysfunction | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| NB31: Injury of heart | 6 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| MyoCell | Phase 2/3 | [2] | ||
| Synonyms |
MyoCell (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Diannexin | Phase 2 | [3] | ||
| External Link | ||||
| CMX-2043 | Phase 2 | [4] | ||
| External Link | ||||
| ED1 | Investigative | [5] | ||
| Synonyms |
ethylenediamine scaffold, 10; BDBM31426; 3-{2'-[{[1-(tert-butoxycarbonyl)piperidin-4-yl]methyl}(2-{(4-cyanophenyl)[(1-methyl-1H-imidazol-5-yl)methyl]amino}ethyl)sulfamoyl]biphenyl-3-yl}propanoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| ED45 | Investigative | [5] | ||
| External Link | ||||
| DD7 | Investigative | [5] | ||
| External Link | ||||
References