m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03063
|
[1] | |||
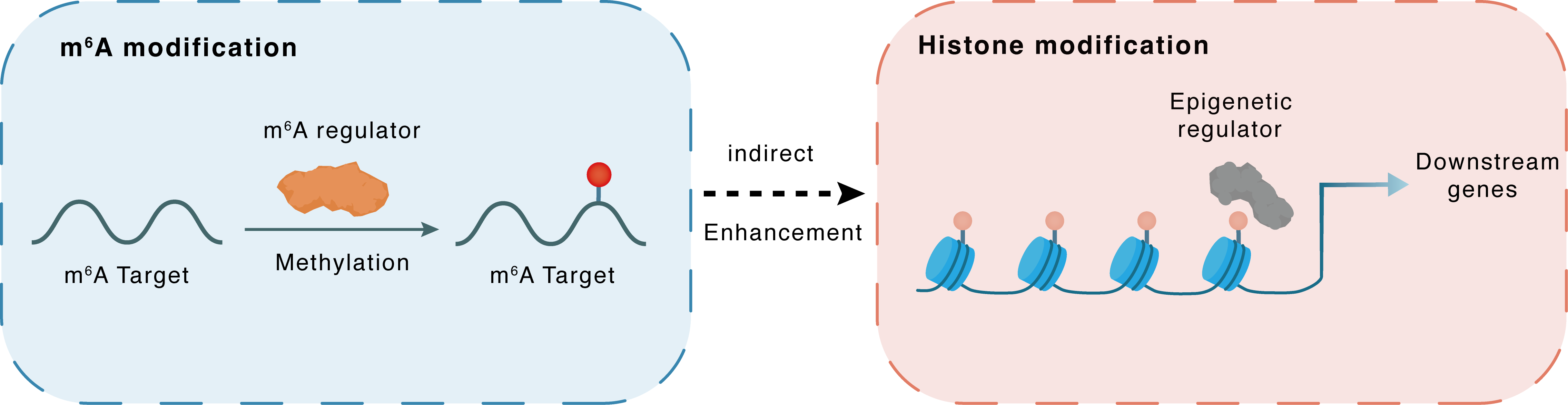 m6A modification
HOTAIR
HOTAIR
METTL14
Methylation
m6A modification
HOTAIR
HOTAIR
METTL14
Methylation
 : m6A sites
Indirect
Enhancement
Histone modification
H3K4me1
LSD1
PPP1CA : m6A sites
Indirect
Enhancement
Histone modification
H3K4me1
LSD1
PPP1CA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 14 (METTL14) | WRITER | |||
| m6A Target | HOX transcript antisense RNA (HOTAIR) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Lysine-specific histone demethylase 1A (KDM1A) | ERASER | View Details | ||
| Regulated Target | Histone H3 lysine 4 monomethylation (H3K4me1) | View Details | |||
| Downstream Gene | PPP1CA | View Details | |||
| Crosstalk Relationship | m6A → Histone modification | Enhancement | |||
| Crosstalk Mechanism | m6A modification indirectly regulates histone modification through downstream signaling pathways | ||||
| Crosstalk Summary | METTL14-mediated upregulation of HOX transcript antisense RNA (HOTAIR) resulted in the repression of PPP1CA, which in turn facilitated the recruitment of KDM1A, thus catalyzing Histone H3 lysine 4 monomethylation (H3K4me1) demethylation and promoting oxycodone addiction. | ||||
| Responsed Disease | Disorders due to use of opioids | ICD-11: 6C43.1 | |||
| Responsed Drug | MM-102 | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Lysine-specific histone demethylase 1A (KDM1A) | 81 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| IMG-7289 | Phase 2 | [2] | ||
| Synonyms |
KQKBMHGOHXOHTD-KKUQBAQOSA-N; UNII-Y2T4ALDEAT; Y2T4ALDEAT; SCHEMBL17984236; Benzamide, N-((1S)-4-(((1R,2S)-2-(4-fluorophenyl)cyclopropyl)amino)-1-((4-methyl-1-piperazinyl)carbonyl)butyl)-4-(1H-1,2,3-triazol-1-yl)-; 1990504-34-1; N-[(2S)-1-(4-(methyl)piperazin-1-yl)-5-[[(1R,2S)-2-(4-fluorophenyl)-cyclopropyl]amino]-1-oxopentan-2-yl]-4-(1H-1,2,3-triazol-1-yl)benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Vafidemstat | Phase 2 | [3] | ||
| Synonyms |
(1R,2S)-2-(4-(Benzyloxy)phenyl)-N-((5-imino-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)cyclopropanamine; 1,3,4-Oxadiazole-2-methanamine, 4,5-dihydro-5-imino-N-((1R,2S)-2-(4-(phenylmethoxy)phenyl)cyclopropyl)-; 1,3,4-Oxadiazole-2-methanamine, 5-amino-N-((1R,2S)-2-(4-(phenylmethoxy)phenyl)cyclopropyl)-; 1357362-02-7; 5-((((1R,2S)-2-(4-(benzyloxy)phenyl)cyclopropyl)amino)methyl)-1,3,4-oxadiazol-2-amine; 5-(((trans)-2-(4-(benzyloxy)phenyl)cyclopropylamino)methyl)-1,3,4-oxadiazol-2-amine; 5-[[[(1R,2S)-2-(4-phenylmethoxyphenyl)cyclopropyl]amino]methyl]-1,3,4-oxadiazol-2-amine; A930244; AKOS040742807; BCP29383; BDBM50594947; CHEMBL4802155; CS-0058593; HY-112623; LZ82JLT4UP; MS-25100; ORY 2001; ORY2001; ORY-2001; ORY-2001; SCHEMBL528204; UNII-LZ82JLT4UP; Vafidemstat; Vafidemstat [INN]; XBBRLCXCBCZIOI-DLBZAZTESA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CC-90011 | Phase 2 | [4] | ||
| Synonyms |
UNII-W6F4FRQ5QC; W6F4FRQ5QC; CC90011; 1821307-10-1; CC-90011 besylate; 4-[2-(4-aminopiperidin-1-yl)-5-(3-fluoro-4-methoxyphenyl)-1-methyl-6-oxo-1,6-dihydropyrimidin-4-yl]-2-fluorobenzonitrile; Pulrodemstat; 4-(2-(4-Aminopiperidin-1-yl)-5-(3-fluoro-4-methoxyphenyl)-1-methyl-6-oxo-1,6-dihydropyrimidin-4-yl)-2-fluorobenzonitrile; Pulrodemstat [INN]; CC-90011 Free base; SCHEMBL17222702; GTPL11284; US10023543, Example 7; BDBM283216; US10023543, Example 85; US10023543, Example 86; NSC822744; NSC-822744; compound 11 [PMID: 33034194]; Q67009340; NC1CCN(CC1)C=1N(C(C(=C(N1)C1=CC(=C(C#N)C=C1)F)C1=CC(=C(C=C1)OC)F)=O)C; 4-(2-(4-Amino-piperidin-1-yl)-5-(3-fluoro-4-methoxyphenyl)-1-methyl-6-oxo-1,6-dihydropyrimidin-4-yl)-2-fluorobenzonitrile; 4-[2-(4-amino-piperidin-1-yl)-5- (3-fluoro-4-methoxy-phenyl)-1- methyl-6-oxo-1,6-dihydro- pyrimidin-4-yl]-2-fluoro- benzonitrile; Benzonitrile, 4-(2-(4-amino-1-piperidinyl)-5-(3-fluoro-4-methoxyphenyl)-1,6-dihydro-1-methyl-6-oxo-4-pyrimidinyl)-2-fluoro-; V0Y
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ORY-2001 | Phase 2 | [5] | ||
| Synonyms |
(1R,2S)-2-(4-(Benzyloxy)phenyl)-N-((5-imino-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)cyclopropanamine; 1,3,4-Oxadiazole-2-methanamine, 4,5-dihydro-5-imino-N-((1R,2S)-2-(4-(phenylmethoxy)phenyl)cyclopropyl)-; 1,3,4-Oxadiazole-2-methanamine, 5-amino-N-((1R,2S)-2-(4-(phenylmethoxy)phenyl)cyclopropyl)-; 1357362-02-7; 5-((((1R,2S)-2-(4-(benzyloxy)phenyl)cyclopropyl)amino)methyl)-1,3,4-oxadiazol-2-amine; 5-(((trans)-2-(4-(benzyloxy)phenyl)cyclopropylamino)methyl)-1,3,4-oxadiazol-2-amine; 5-[[[(1R,2S)-2-(4-phenylmethoxyphenyl)cyclopropyl]amino]methyl]-1,3,4-oxadiazol-2-amine; A930244; AKOS040742807; BCP29383; BDBM50594947; CHEMBL4802155; CS-0058593; HY-112623; LZ82JLT4UP; MS-25100; ORY 2001; ORY2001; ORY-2001; ORY-2001; SCHEMBL528204; UNII-LZ82JLT4UP; Vafidemstat; Vafidemstat [INN]; XBBRLCXCBCZIOI-DLBZAZTESA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| INCB59872 | Phase 1/2 | [2] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| CC-90011 | Phase 1 | [2] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| GSK2879552 | Phase 1 | [6] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 24 nM | |||
| External Link | ||||
| Seclidemstat | Phase 1 | [7] | ||
| Synonyms |
UNII-TYH386V3WJ; SP-2577; TYH386V3WJ; 1423715-37-0; SP2577; CHEMBL4297641; SCHEMBL14697017; SCHEMBL14697019; EX-A3574; s6722; BS-15371; HY-103713; CS-0039281; Benzoic acid, 3-((4-methyl-1-piperazinyl)sulfonyl)-, (2E)-2-(1-(5-chloro-2-hydroxyphenyl)ethylidene)hydrazide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| TAS-1440 | Phase 1 | [8] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27019002-Compound-16 | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2220 nM | |||
| External Link | ||||
| PMID27019002-Compound-41 | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 19 nM | |||
| External Link | ||||
| Benzenamine derivative 2 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-35
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27019002-Compound-28a | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| Aryl cyclopropylamine derivative 5 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-25c
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 352000 nM | |||
| External Link | ||||
| Pyrimidine derivative 17 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-39
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100 nM | |||
| External Link | ||||
| PMID27019002-Compound-31b | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 31380 nM | |||
| External Link | ||||
| Benzenamine derivative 3 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-35a
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclopropylamine derivative 1 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-23
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1 to 100 nM | |||
| External Link | ||||
| N6-cyclopropyllydine derivative 1 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-30
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 to 1000 nM | |||
| External Link | ||||
| Tarnylcypromine derivative 1 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-18
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 1300 nM | |||
| External Link | ||||
| Cyclopropylamine derivative 7 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-29e
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Aryl cyclopropylamine derivative 4 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-25b
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 456000 nM | |||
| External Link | ||||
| PMID27019002-Compound-45 | Patented | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27019002-Compound-13 | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5270 nM | |||
| External Link | ||||
| PMID27019002-Compound-49 | Patented | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclopropylamine derivative 4 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-29b
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27019002-Compound-42b | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 3000 nM | |||
| External Link | ||||
| PMID27019002-Compound-31a | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 39380 nM | |||
| External Link | ||||
| PMID27019002-Compound-43c | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 81 nM | |||
| External Link | ||||
| Aryl cyclopropylamine derivative 3 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-25a
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 173000 nM | |||
| External Link | ||||
| Benzenamine derivative 4 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-36
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclopropylamine derivative 6 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-29d
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Pyrimidine derivative 16 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-38
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27019002-Compound-28 | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| Heteroaryl-cyclopropylamine derivative 2 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-22a
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 1000 nM | |||
| External Link | ||||
| Cyclopropylamine derivative 5 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-29c
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27019002-Compound-43b | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 273 nM | |||
| External Link | ||||
| Pyrimidine derivative 18 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-40
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclopropylamine derivative 3 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-29a
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27019002-Compound-20b | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1 to 100 nM | |||
| External Link | ||||
| PMID27019002-Compound-50 | Patented | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclopropylamine derivative 8 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-32
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 500 nM | |||
| External Link | ||||
| PMID27019002-Compound-43a | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 90 nM | |||
| External Link | ||||
| Benzenamine derivative 1 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-14
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27019002-Compound-7 | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 59 nM | |||
| External Link | ||||
| PMID27019002-Compound-42a | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 3000 nM | |||
| External Link | ||||
| PMID27019002-Compound-37a | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 11160 nM | |||
| External Link | ||||
| PMID27019002-Compound-44 | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1190 nM | |||
| External Link | ||||
| PMID27019002-Compound-21a | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID27019002-Compound-37b | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 10540 nM | |||
| External Link | ||||
| Heteroaryl-cyclopropylamine derivative 4 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-22c
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| Cyclopropylamine derivative 11 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-33c
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 180 nM | |||
| External Link | ||||
| PMID25399762-Compound-Table 6-11 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 200 nM | |||
| External Link | ||||
| PMID27019002-Compound-20a | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1 to 100 nM | |||
| External Link | ||||
| Cyclopropylamine derivative 9 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-33a
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 230 nM | |||
| External Link | ||||
| PMID25399762-Compound-Table 6-13 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 30 nM | |||
| External Link | ||||
| Heteroaryl-cyclopropylamine derivative 3 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-22b
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| Cyclopropylamine derivative 13 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-33e
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100 nM | |||
| External Link | ||||
| PMID27019002-Compound-21b | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1 to 100 nM | |||
| External Link | ||||
| PMID27019002-Compound-48 | Patented | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID25399762-Compound-Table 6-10 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 100 nM | |||
| External Link | ||||
| PMID25399762-Compound-Table 6-12 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 200 nM | |||
| External Link | ||||
| PMID25399762-Compound-Table 6-9 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 20 nM | |||
| External Link | ||||
| PMID25399762-Compound-Table 6-14 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 100 nM | |||
| External Link | ||||
| PMID25399762-Compound-Table 6-15 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 50 nM | |||
| External Link | ||||
| Cyclopropylamine derivative 12 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-33d
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 210 nM | |||
| External Link | ||||
| PMID27019002-Compound-21c | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1 to 100 nM | |||
| External Link | ||||
| Cyclopropylamine derivative 10 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-33b
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 240 nM | |||
| External Link | ||||
| PMID27019002-Compound-47 | Patented | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27019002-Compound-17 | Patented | [9] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 13 nM | |||
| External Link | ||||
| N-(2-phenylcyclopropyl) amino acid derivative 2 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-19a
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 18 nM | |||
| External Link | ||||
| PMID27019002-Compound-46 | Patented | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclic peptide derivative 1 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-compound11
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5000 nM | |||
| External Link | ||||
| Heteroaryl-cyclopropylamine derivative 1 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-22d
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 8 nM | |||
| External Link | ||||
| N-(2-phenylcyclopropyl) amino acid derivative 3 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-19b
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 22 nM | |||
| External Link | ||||
| N-(2-phenylcyclopropyl) amino acid derivative 1 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-19
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki < 1000 nM | |||
| External Link | ||||
| Tarnylcypromine derivative 2 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-26a-h
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1100 to 1900 nM | |||
| External Link | ||||
| Tarnylcypromine derivative 3 | Patented | [9] | ||
| Synonyms |
PMID27019002-Compound-27a-m
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1300 to 40000 nM | |||
| External Link | ||||
| OG-L002 | Investigative | [11] | ||
| Synonyms |
1357302-64-7; 4'-((1R,2S)-2-aminocyclopropyl)-[1,1'-biphenyl]-3-ol; SCHEMBL6837351; GTPL7023; OGL002; AOB2070; MolPort-035-395-885; BDBM179446; BCP12278; EX-A2117; s7237; 2610AH; ZINC114026926; AKOS027422749; SB19352; BC600435; 4'-((1R,2S)-2-aminocyclopropyl)biphenyl-3-ol; 3-{4-[(1R,2S)-2-aminocyclopropyl]phenyl}phenol; J-006764; US9676701, 4 4'-((trans)-2-aminocyclopropyl)biphenyl-3-ol hydrochloride
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 20 nM | |||
| External Link | ||||
| GSK-LSD1 | Investigative | [12] | ||
| Synonyms |
GSK-LSD1 2HCl; 1431368-48-7; N-[(1R,2S)-2-phenylcyclopropyl]piperidin-4-amine; GSK LSD1 Dihydrochloride; GTPL8241; SCHEMBL14880683; BDBM256459; 1431368-48-7(free base); ZINC44675892; AKOS030573682; GSK-LSD1, > NCGC00356416-07; US9487512, 3
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 24 nM | |||
| External Link | ||||
| NCL-1 | Investigative | [13] | ||
| Synonyms |
GTPL7024; ZINC94568752
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6C43: Disorders due to use of opioids | 1 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Lofexidine | Approved | [14] | ||
| Synonyms |
Britlofex; Lofexidina; Lofexidinum; Britlofex (TN); Lofexidina [INN-Spanish]; Lofexidine (INN); Lofexidine [INN:BAN]; Lofexidinum [INN-Latin]; 1H-Imidazole, 2-(1-(2,6-dichlorophenoxy)ethyl)-4,5-dihydro-(9CI); 2-(1-(2,6-Dichlorophenoxy)ethyl)-4,5-dihydro-1H-imidazole; 2-(a-(2,6-dichlorophenoxy)ethyl)2-imidazoline; 2-(alpha-(2,6-Dichlorophenoxy)ethyl)2-imidazoline; 2-(alpha-(2,6-dichlorophenoxy)ethyl) delta-2-imidazoline; 2-[1-(2,6-dichlorophenoxy)ethyl]-4,5-dihydro-1H-imidazole; 2-{1-[(2,6-dichlorophenyl)oxy]ethyl}-4,5-dihydro-1H-imidazole
Click to Show/Hide
|
|||
| External Link | ||||
References