m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03013
|
[1] | |||
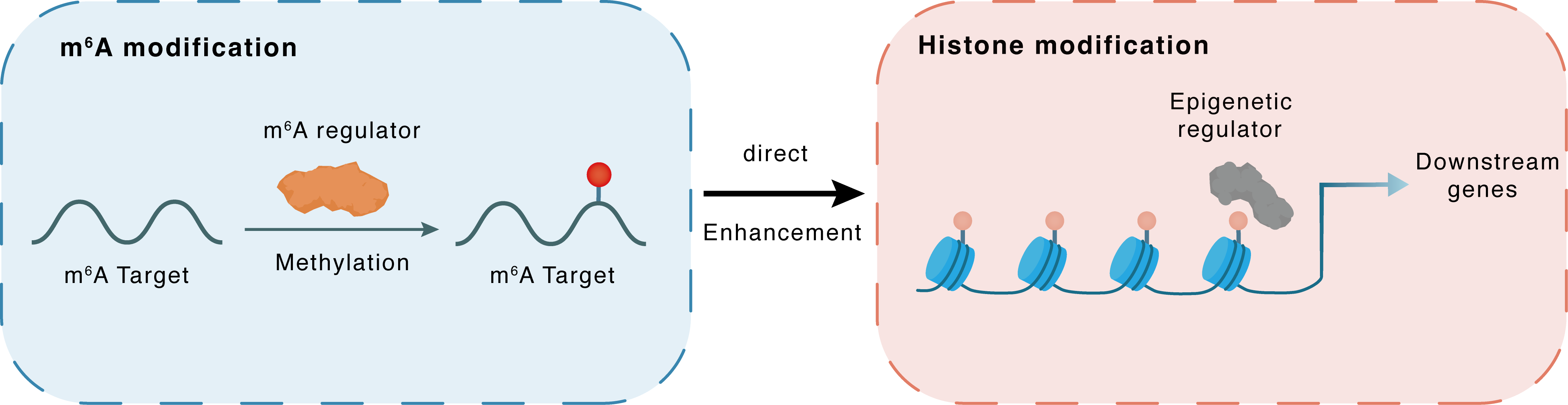 m6A modification
EZH2
EZH2
METTL3
Methylation
m6A modification
EZH2
EZH2
METTL3
Methylation
 : m6A sites
Direct
Enhancement
Histone modification
H3K27me3
Ezh2
Downstream Gene : m6A sites
Direct
Enhancement
Histone modification
H3K27me3
Ezh2
Downstream Gene
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Histone-lysine N-methyltransferase EZH2 (EZH2) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone-lysine N-methyltransferase EZH2 (EZH2) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 27 trimethylation (H3K27me3) | View Details | |||
| Crosstalk Relationship | m6A → Histone modification | Enhancement | |||
| Crosstalk Mechanism | m6A modification impacts directly histone modification through modulating the expression level of histone-associated enzymes | ||||
| Crosstalk Summary | m6A was present on the transcripts of histone methyltransferase Histone-lysine N-methyltransferase EZH2 (EZH2), and its reduction upon METTL3 knockdown decreased both Ezh2 protein expression and consequent Histone H3 lysine 27 trimethylation (H3K27me3) levels. The defects of neurogenesis and neuronal development induced by Mettl3-depletion could be rescued by Ezh2 overexpression. Collectively, our results uncover a crosstalk between RNA and histone modifications and indicate that Mettl3-mediated m6A modification plays an important role in regulating neurogenesis and neuronal development through modulating Ezh2. | ||||
| In-vivo Model | Mice were housed in standard conditions in the Laboratory Animal Center of Zhejiang University on a 12 h light/dark schedule with free access to food and water. The pregnant mice were purchased from Shanghai SLAC Laboratory Animal Company, China. All animal experiments were conducted according to protocols approved by the Zhejiang University Animal Care and Use Committee. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Histone-lysine N-methyltransferase EZH2 (EZH2) | 74 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Tazemetostat | Approved | [2] | ||
| Synonyms |
EPZ-6438; 1403254-99-8; EPZ6438; UNII-Q40W93WPE1; N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(morpholinomethyl)-[1,1'-biphenyl]-3-carboxamide; Q40W93WPE1; EPZ 6438; E7438; (1,1'-Biphenyl)-3-carboxamide, N-((1,2-dihydro-4,6-dimethyl-2-oxo-3-pyridinyl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(4-morpholinylmethyl)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 2.5 nM | |||
| External Link | ||||
| DS-3201b | Phase 2 | [3] | ||
| Synonyms |
Valemetostat; 1809336-39-7; UNII-60RD0234VE; 60RD0234VE; 1809336-39-7 (free base); DS-3201; (2R)-7-chloro-2-[trans-4-(dimethylamino)cyclohexyl]-N-[(4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl]-2,4-dimethyl-1,3-benzodioxole-5-carboxamide; Valemetostat 2HCl; Valemetostat [INN]; Valemetostat (DS-3201); CHEMBL4597193; EZH1/2 inhibitor DS-3201; SCHEMBL18393626; SCHEMBL18393627; SCHEMBL18639210; EX-A3423; DS3201; NSC813381; s8926; NSC-813381; HY-109108; CS-0039740; D11551
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CPI-1205 | Phase 1/2 | [4] | ||
| Synonyms |
HPODOLXTMDHLLC-QGZVFWFLSA-N; 1621862-70-1; UNII-455J2479FY; CPI1205; CPI 1205; 455J2479FY; (R)-N-((4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-methyl-1-(1-(1-(2,2,2-trifluoroethyl)piperidin-4-yl)ethyl)-1H-indole-3-carboxamide; GTPL9115; SCHEMBL17329268; MolPort-044-560-382; KS-000006BA; EX-A1068; s8353; AKOS030628484; ZINC220982768; CS-7648; compound 13 [PMID: 27739677]; HY-100021; J3.556.402K; N-[(4-methoxy-6-methyl-2-oxo-1H-pyridin-3-yl)methyl]-2-methyl-1-[(1R)-1-[1-(2,2,2-trifluoroethyl)piperidin-4-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SHR2554 | Phase 1/2 | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| CPI-0209 | Phase 1/2 | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| GSK2816126 | Phase 1 | [7] | ||
| Synonyms |
GSK 126; GSK-126
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | Ki = 0.5 nM | |||
| External Link | ||||
| PF-06821497 | Phase 1 | [8] | ||
| Synonyms |
UNII-S4L4MM20B6; S4L4MM20B6; CHEMBL4080228; PF06821497; 1844849-10-0; 5,8-dichloro-2-[(4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl]-7-[(R)-methoxy(oxetan-3-yl)methyl]-3,4-dihydroisoquinolin-1(2H)-one; SCHEMBL17330426; GTPL10516; BDBM50246967; NSC800019; DB14799; HY-101571A; NSC-800019; compound 23a [PMID: 29211475]; CS-0092626; Q29209799; 1(2H)-Isoquinolinone, 5,8-dichloro-2-((1,2-dihydro-4-methoxy-6-methyl-2-oxo-3-pyridinyl)methyl)-3,4-dihydro-7-((R)-methoxy-3-oxetanylmethyl)-; 5,8-dichloro-2-[(4-methoxy-6-methyl-2-oxo-1H-pyridin-3-yl)methyl]-7-[(R)-methoxy(oxetan-3-yl)methyl]-3,4-dihydroisoquinolin-1-one; CJD
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| DS-3201 | Phase 1 | [4] | ||
| Synonyms |
QTGYNKYZRZATJB-UHFFFAOYSA-N; 701899-63-0; 2-(2-(2-Methyl-1H-imidazol-5-yl)ethyl)isoindoline-1,3-dione; 2-(2-(2-Methyl-1H-imidazol-5-yl)-ethyl)isoindoline-1,3-dione; SCHEMBL7743461; MolPort-035-945-474; MolPort-035-690-353; ZINC98086069; AKOS024459002; AKOS024262663; MCULE-2708350770; FCH4077443; AK158834; AX8292467; ST2403812; 2-[2-(2-methyl-1H-imidazol-5-yl)ethyl]-2,3-dihydro-1H-isoindole-1,3-dione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| HH2853 | Phase 1 | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-33 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID28394193-Compound-Figure3bI | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-35 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| PMID28394193-Compound-54 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-24 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-27 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-25 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-50 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-47 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2 nM; Ki < 1 nM | |||
| External Link | ||||
| PMID28394193-Compound-21 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-41 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-53 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 80 nM | |||
| External Link | ||||
| PMID28394193-Compound-Figure5aVIII | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-38 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-51 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-31 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 20 nM | |||
| External Link | ||||
| PMID28394193-Compound-42 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-15 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-52 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-32 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| PMID28394193-Compound-23 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-29 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID28394193-Compound-30 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 16 nM | |||
| External Link | ||||
| PMID28394193-Compound-39 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-49 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-43 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-40 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-Figure3bIII | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-36 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 316 nM | |||
| External Link | ||||
| PMID28394193-Compound-28 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 32 nM | |||
| External Link | ||||
| PMID28394193-Compound-22 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-18 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-16 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-44 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-20 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-19 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-37 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-Figure3bII | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-26 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-17 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-34 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID28394193-Compound-56 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.93 nM | |||
| External Link | ||||
| PMID28394193-Compound-46 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| PMID28394193-Compound-57 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6.45 nM | |||
| External Link | ||||
| PMID28394193-Compound-14 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 21900 nM | |||
| External Link | ||||
| PMID28394193-Compound-12 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 10 nM | |||
| External Link | ||||
| PMID26882240-Compound-1 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-55 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 8.13 nM | |||
| External Link | ||||
| PMID28394193-Compound-45 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 168000 nM | |||
| External Link | ||||
| PMID28394193-Compound-13 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1470 nM | |||
| External Link | ||||
| EPZ005687 | Investigative | [12] | ||
| Synonyms |
EPZ-005687; EPZ 005687
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| EI1 | Investigative | [13] | ||
| Synonyms |
KB-145943
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| UNC1999 | Investigative | [14] | ||
| Synonyms |
UNC 1999; UNC-1999
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MS1943 | Investigative | [5] | ||
| Synonyms |
2225938-17-8; SCHEMBL21271666; EX-A3962; s8918; HY-133129; CS-0112146; 6-(6-(4-(2-(2-((3r,5r,7r)-adamantan-1-yl)acetamido)ethyl)piperazin-1-yl)pyridin-3-yl)-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-1-isopropyl-1H-indazole-4-carboxamide; 6-(6-(4-(2-(2-(Adamantan-1-yl)acetamido)ethyl)piperazin-1-yl)pyridin-3-yl)-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-1-isopropyl-1H-indazole-4-carboxamide
Click to Show/Hide
|
|||
| MOA | Degrader | |||
| External Link | ||||
| GSK343 | Investigative | [15] | ||
| Synonyms |
compound 6 [PMID 24900432]; GSK 343
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 174 nM | |||
| External Link | ||||
| PMID28394193-Compound-11 | Patented | [16] | ||
| External Link | ||||
| PMID28394193-Compound-10 | Patented | [16] | ||
| External Link | ||||
| PMID28394193-Compound-14 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 21900 nM | |||
| External Link | ||||
| PMID28394193-Compound-12 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 10 nM | |||
| External Link | ||||
| PMID28394193-Compound-13 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1470 nM | |||
| External Link | ||||
| PMID28394193-Compound-56 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.93 nM | |||
| External Link | ||||
| PMID28394193-Compound-46 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| PMID28394193-Compound-57 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6.45 nM | |||
| External Link | ||||
| PMID28394193-Compound-55 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 8.13 nM | |||
| External Link | ||||
| PMID28394193-Compound-45 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 168000 nM | |||
| External Link | ||||
References