m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT02276
|
[1], [2] | |||
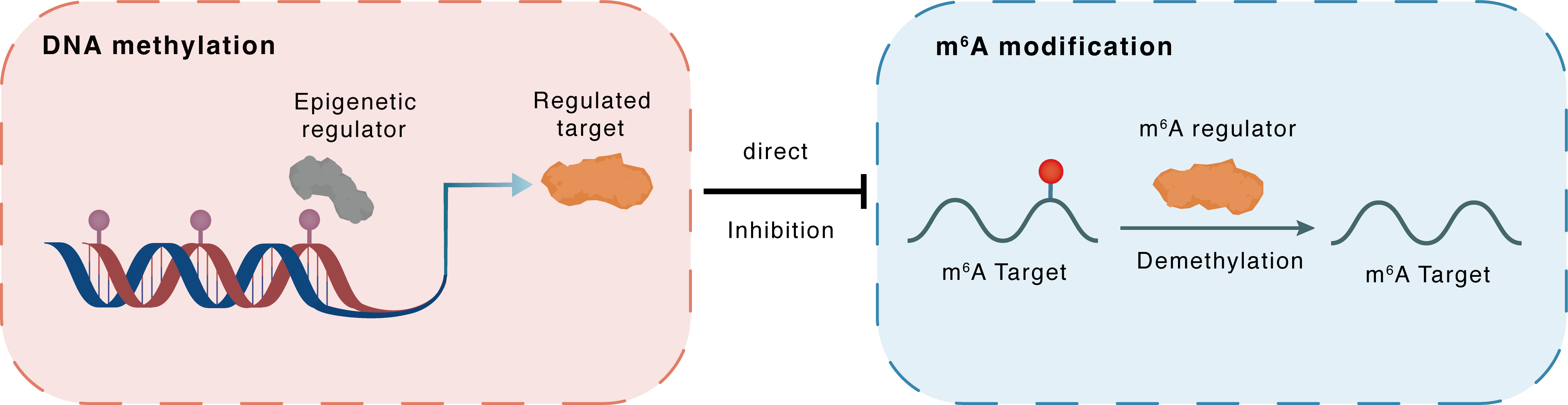 DNA methylation
Epigenetic Regulator
ALKBH5
Direct
Inhibition
m6A modification
AXL
AXL
ALKBH5
Demethylation
DNA methylation
Epigenetic Regulator
ALKBH5
Direct
Inhibition
m6A modification
AXL
AXL
ALKBH5
Demethylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | RNA demethylase ALKBH5 (ALKBH5) | ERASER | |||
| m6A Target | Tyrosine-protein kinase receptor UFO (AXL) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | DNA methylation (DNAMeth) | ||||
| Regulated Target | RNA demethylase ALKBH5 (ALKBH5) | View Details | |||
| Crosstalk Relationship | DNA methylation → m6A | Inhibition | |||
| Crosstalk Mechanism | DNA methylation directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | Folic acid reduces the expression of m6A demethylase AlkB homolog 5 (ALKHB5) via promoter DNA hypermethylation. Decreased ALKBH5 causes increased m6A modification and increased expression of ATG12 in a demethylase activity-dependent manner, thereby promoting autophagy and preventing hepatic steatosis.Chlorogenic acid (CGA) is a naturally occurring plant component with the purpose of alleviating hepatic lipid deposition biological activities.CGA specifically binds to ALKBH5 and inhibits its m6A methylase activity. The inhibition of ALKBH5 activity significantly reduces Tyrosine-protein kinase receptor UFO (AXL) mRNA stability in liver cells. The AXL downregulation resulted in suppressing ERK signaling pathway activation. Overall, this study demonstrates that CGA can alleviate hepatic steatosis by regulating autophagy through the inhibition of ALKBH5 activity inhibition. | ||||
| Responsed Disease | Nonalcoholic fatty liver disease | ICD-11: DB92.Z | |||
| Responsed Drug | Chlorogenic acid (CGA) | ||||
| Cell Process | Cell autophagy | ||||
| Lipid metabolism | |||||
In-vitro Model |
AML12 | Normal | Mus musculus | CVCL_0140 | |
| THLE-2 | Normal | Homo sapiens | CVCL_3803 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Tyrosine-protein kinase receptor UFO (AXL) | 22 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Gilteritinib | Approved | [3] | ||
| Synonyms |
UNII-66D92MGC8M; 66D92MGC8M; Gilteritinib [USAN:INN]; Gilteritinib(ASP2215); Gilteritinib (USAN/INN); Gilteritinib (ASP2215); Gilteritinib (ASP-2215); SCHEMBL282229; GTPL8708; MolPort-038-934-933; BDBM144315; C29H44N8O3; 3694AH; s7754; AKOS030234455; ZINC113476229; DB12141; CS-3885; KS-0000064E; AS-35199
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 1 nM | |||
| External Link | ||||
| Bemcentinib | Phase 2 | [4] | ||
| Synonyms |
R428; 1037624-75-1; BGB324; R-428; BGB-324; UNII-0ICW2LX8AS; (S)-1-(6,7-dihydro-5H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3-yl)-N3-(7-(pyrrolidin-1-yl)-6,7,8,9-tetrahydro-5H-benzo[7]annulen-2-yl)-1H-1,2,4-triazole-3,5-diamine; CHEMBL3809489; SYN1131; CS-1046; HY-15150; QC-11751; R 428; W-5845; Bemcentinib [USAN]; R428 (BGB324); Bemcentinib (USAN/INN); BemcentinibR428BGB324); SCHEMBL1639904; GTPL10478; BGB 324; DTXSID70673109; 1-(6,7-dihydro-5H-benzo[2,3]cyclohepta[2,4-d]pyridazin-3-yl)-3-N-[(7S)-7-pyrrolidin-1-yl-6,7,8,9-tetrahydro-5H-benzo[7]annulen-3-yl]-1,2,4-triazole-3,5-diamine; AMY16774; BCP21180; C30H34N8; EX-A1720; SYN-1131; BDBM50172079; NSC824183; s2841; WHO 10631; ZINC51951669; AKOS032947237; ACN-037541; DB12411; NSC-824183; SB16614; NCGC00386665-07; 1-(6,7-Dihydro-5H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3-yl)-N~3~-[(7S)-7-(pyrrolidin-1-yl)-6,7,8,9-tetrahydro-5H-benzo[7]annulen-2-yl]-1H-1,2,4-triazole-3,5-diamine; 1H-1,2,4-Triazole-3,5-diamine, 1-(6,7-dihydro-5H-benzo(6,7)cyclohepta(1,2-C)pyridazin-3-yl)-N3-((7S)-6,7,8,9-tetrahydro-7-(1-pyrrolidinyl)-5H-benzocyclohepten-2-yl)-; AC-28444; AS-16270; KB-80319; D11438; Q27236818; 1-(6,7-dihydro-5H-benzo[6,7]cyclohepta[1,2-c]pyridazin-3-yl)-N3-(7-(S)-(pyrrolidin-1-yl)-6,7,8,9-tetrahydro-5H-benzo[7]annulene-2-yl)-1H-1,2,4-triazole-3,5-diamine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BGB-324 | Phase 1/2 | [5] | ||
| Synonyms |
BGB-001; SiRNA therapeutic (metastasis cancer), BiobergenBio
Click to Show/Hide
|
|||
| External Link | ||||
| Mecbotamab vedotin | Phase 2 | [6] | ||
| Synonyms |
HTBA3011; HTBA3012
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MGCD265 | Phase 2 | [7] | ||
| Synonyms |
MGCD-265; 875337-44-3; MGCD-265 analog; MGCD 265; N-(3-fluoro-4-(2-(1-methyl-1H-imidazol-4-yl)thieno[3,2-b]pyridin-7-yloxy)phenylcarbamothioyl)-2-phenylacetamide; UNII-93M6577H9D; CHEMBL254760; 93M6577H9D; n-(3-fluoro-4-(2-(1-methyl-1h-imidazol-4-yl)thieno(3,2-b)pyridin-7-yloxy)phenylcarbamothioyl)-2-phenylacetamide; N-[(3-Fluoro-4-{[2-(1-methyl-1H-imidazol-4-yl)thieno[3,2-b]pyridin-7-yl]oxy}phenyl)carbamothioyl]-2-phenylacetamide
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| BI-505 | Phase 2 | [8] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| TP-0903 | Phase 1/2 | [3] | ||
| Synonyms |
YUAALFPUEOYPNX-UHFFFAOYSA-N; 1341200-45-0; TP0903; UNII-14D65TV20J; CHEMBL2022968; compound 13; 14D65TV20J; TP 0903; 2-((5-chloro-2-((4-((4-methylpiperazin-1-yl)methyl)phenyl)amino)pyrimidin-4-yl)amino)-N,N-dimethylbenzenesulfonamide; GTPL8863; SCHEMBL12813478; EX-A609; MolPort-039-193-844; ZINC84617535; BDBM50382425; AKOS026750306; CS-4281; DA-45909; HY-12963; S7846; FT-0700169; B5940; J-690134; 2-{[5-chloro-2-({4-[(4-methylpiperazin-1-yl)methyl]phenyl}amino)pyrimidin-4-yl]amino}-N,N-dimethylbenzene-1-sulfonamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 222 nM | |||
| External Link | ||||
| Enapotamab vedotin | Phase 1/2 | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| ONO-7475 | Phase 1/2 | [10] | ||
| Synonyms |
1646839-59-9; UNII-0VCB95RHRV; N-(5-((6,7-Dimethoxyquinolin-4-yl)oxy)pyridin-2-yl)-2,5-dioxo-1-phenyl-1,2,5,6,7,8-hexahydroquinoline-3-carboxamide; N-[5-[(6,7-Dimethoxy-4-quinolinyl)oxy]-2-pyridinyl]-1,2,5,6,7,8-hexahydro-2,5-dioxo-1-phenyl-3-quinolinecarboxamide; SCHEMBL16426362; ONO7475; BCP33232; MFCD32689448; s8933; ONO 7475; HY-114358; CS-0083699; 3-Quinolinecarboxamide, N-(5-((6,7-dimethoxy-4-quinolinyl)oxy)-2-pyridinyl)-1,2,5,6,7,8-hexahydro-2,5-dioxo-1-phenyl-; N-[5-(6,7-dimethoxyquinolin-4-yl)oxypyridin-2-yl]-2,5-dioxo-1-phenyl-7,8-dihydro-6H-quinoline-3-carboxamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| DS-1205 | Phase 1 | [3] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| AVB-S6-500 | Phase 1 | [3] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| A416 | Phase 1 | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| ONO-7475 | Phase 1 | [3] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| BPI-9016 M | Phase 1 | [12] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PF-07265807 | Phase 1 | [13] | ||
| Synonyms |
PF-5807; ARRY-067
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| INCB81776 | Phase 1 | [14] | ||
| Synonyms |
INCB081776
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RXDX-106 | Phase 1 | [15] | ||
| Synonyms |
CEP-40783; 1437321-24-8; CEP40783; UNII-1969ZJE05Q; 1969ZJE05Q; N-(4-((6,7-dimethoxyquinolin-4-yl)oxy)-3-fluorophenyl)-3-(4-fluorophenyl)-1-isopropyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidine-5-carboxamide; N-[4-(6,7-dimethoxyquinolin-4-yl)oxy-3-fluorophenyl]-3-(4-fluorophenyl)-2,4-dioxo-1-propan-2-ylpyrimidine-5-carboxamide; RXDX-106 (CEP-40783); SCHEMBL16089863; BCP25839; EX-A2540; MFCD28502441; NSC797770; s8570; AKOS032960472; ZINC205893112; CCG-270157; CS-6371; NSC-797770; SB18930; AC-31425; AS-35141; HY-100946; N-(4-((6,7-Dimethoxy-4-quinolinyl)oxy)-3-fluorophenyl)-3-(4-fluorophenyl)-1,2,3,4-tetrahydro-1-(1-methylethyl)-2,4-dioxo-5-pyrimidinecarboxamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cu-anti-hAXL | Preclinical | [16] | ||
| External Link | ||||
| DP-3975 | Preclinical | [17] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| YW327.6S2 | Preclinical | [17] | ||
| External Link | ||||
| GL21.T | Preclinical | [17] | ||
| External Link | ||||
| LDC1267 | Investigative | [18] | ||
| Synonyms |
1361030-48-9; LDC-1267; CHEMBL3808844; SCHEMBL167963; GTPL8247; MolPort-035-944-330; EX-A2265; AOB87188; BCP14023; BDBM50172075; s7638; ZINC113241878; AKOS026750422; CS-3603; SB19391; NCGC00386431-02; HY-12494; N-(4-((6,7-dimethoxyquinolin-4-yl)oxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluoro-2-methylphenyl)-1H-pyrazole-3-carboxamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 19 nM | |||
| External Link | ||||
| DB92: Non-alcoholic fatty liver disease | 12 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Epeleuton | Phase 2 | [19] | ||
| Synonyms |
(S,5Z,8Z,11Z,13E,17Z)-Ethyl 15-hydroxyicosa-5,8,11,13,17-pentaenoate; 15(S)-HEPE-EE; 15(S)-HYDROXY-(5Z,8Z,11Z,13E,17Z)-EICOSAPENTAENOIC ACID ETHYL ESTER; 1667760-39-5; 5,8,11,13,17-Eicosapentaenoic acid, 15-hydroxy-, ethyl ester, (5Z,8Z,11Z,13E,15S,17Z)-; AKOS040748327; CHEMBL5095178; Epeleuton; Epeleuton [INN]; FA9BPX1T6V; UNII-FA9BPX1T6V
Click to Show/Hide
|
|||
| External Link | ||||
| IMM-124E | Phase 2 | [20] | ||
| External Link | ||||
| TVB-2640 | Phase 2 | [21] | ||
| Synonyms |
FASN-IN-2; 1399177-37-7; 4-(1-(4-Cyclobutyl-2-methyl-5-(5-methyl-4H-1,2,4-triazol-3- yl)benzoyl)piperidin-4-yl)benzonitrile; US8871790, 480; CHEMBL3661754; SCHEMBL12488853; BDBM137084; BCP30428; EX-A3643; s9714; ZINC150188638; HY-112829; CS-0066310; TVB2640; TVB 2640;FASN-IN-2; US8871790, 152; 4-(1-(4-Cyclobutyl-2-methyl-5-(3-methyl-1H-1,2,4-triazol-5-yl)benzoyl)piperidin-4-yl)benzonitrile; 4-(1-(4-cyclobutyl-2-methyl-5-(5-methyl-4H-1,2,4-triazol-3-yl)benzoyl)piperidin-4-yl)benzonitrile; 4-[1-[4-cyclobutyl-2-methyl-5-(5-methyl-1H-1,2,4-triazol-3-yl)benzoyl]piperidin-4-yl]benzonitrile
Click to Show/Hide
|
|||
| External Link | ||||
| Vupanorsen | Phase 2 | [22] | ||
| Synonyms |
IONIS-ANGPTL3-LRx; AKCEA-ANGPTL3-LRx
Click to Show/Hide
|
|||
| External Link | ||||
| ZED1227 | Phase 2 | [23] | ||
| Synonyms |
(2E,6S)-7-((1-(2-((2-Ethylbutyl)amino)-2-oxoethyl)-1,2-dihydro-2-oxo-3-pyridinyl)amino)-6-(((1-methyl-1H-imidazol-5-yl)carbonyl)amino)-7-oxo-2-heptenoic acid methyl ester; (2E,6S)-7-[[1-[2-[(2-Ethylbutyl)amino]-2-oxoethyl]-1,2-dihydro-2-oxo-3-pyridinyl]amino]-6-[[(1-methyl-1H-imidazol-5-yl)carbonyl]amino]-7-oxo-2-heptenoic Acid Methyl Ester; 1542132-88-6; 2-Heptenoic acid, 7-((1-(2-((2-ethylbutyl)amino)-2-oxoethyl)-1,2-dihydro-2-oxo-3-pyridinyl)amino)-6-(((1-methyl-1H-imidazol-5-yl)carbonyl)amino)-7-oxo-, methyl ester, (2E,6S)-; 2-Heptenoic acid, 7-[[1-[2-[(2-ethylbutyl)amino]-2-oxoethyl]-1,2-dihydro-2-oxo-3-pyridinyl]amino]-6-[[(1-methyl-1H-imidazol-5-yl)carbonyl]amino]-7-oxo-, methyl ester, (2E,6S)-; AKOS040742843; BDBM50245478; CHEMBL4081588; CS-0015432; EX-A7845R; GLUTAMINASE; GTPL12802; HY-19359; Methyl (2E,6S)-7-((1-(2-((2-ethylbutyl)amino)-2-oxoethyl)-1,2-dihydro-2-oxo-3-pyridinyl)amino)-6-(((1-methyl-1H-imidazol-5-yl)carbonyl)amino)-7-oxo-2-heptenoate; Methyl (2E,6S)-7-[[1-[2-[(2-ethylbutyl)amino]-2-oxoethyl]-1,2-dihydro-2-oxo-3-pyridinyl]amino]-6-[[(1-methyl-1H-imidazol-5-yl)carbonyl]amino]-7-oxo-2-heptenoate; Methyl (E,6S)-7-((1-(2-(2-ethylbutylamino)-2-oxo-ethyl)-2-oxo-3-pyridyl)amino)-6-((3-methylimidazole-4-carbonyl)amino)-7-oxo-hept-2-enoate; methyl (E,6S)-7-[[1-[2-(2-ethylbutylamino)-2-oxoethyl]-2-oxopyridin-3-yl]amino]-6-[(3-methylimidazole-4-carbonyl)amino]-7-oxohept-2-enoate; methyl (S,E)-7-((1-(2-((2-ethylbutyl)amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-yl)amino)-6-(1-methyl-1H-imidazole-5-carboxamido)-7-oxohept-2-enoate; MS-29784; SCHEMBL16735736; SCHEMBL16751074; T4SR539YKF; TAK-227; UNII-T4SR539YKF; ZED1227; ZED-1227
Click to Show/Hide
|
|||
| External Link | ||||
| PXL-770 | Phase 2 | [24] | ||
| External Link | ||||
| ASP9831 | Phase 2 | [25] | ||
| External Link | ||||
| Netoglitazone | Phase 2 | [26] | ||
| Synonyms |
Isaglitazone; Netoglitazone [USAN]; MCC 555; MCC-555; RWJ-241947; Netoglitazone (USAN/INN); 5-((6-((2-fluorophenyl)methoxy)-2-naphthalenyl)methyl)-2,4-thiazolidinedione; 5-({6-[(2-fluorobenzyl)oxy]naphthalen-2-yl}methyl)-1,3-thiazolidine-2,4-dione; 5-[[6-[(2-fluorophenyl)methoxy]naphthalen-2-yl]methyl]-1,3-thiazolidine-2,4-dione
Click to Show/Hide
|
|||
| External Link | ||||
| RG-125 | Phase 1 | [27] | ||
| Synonyms |
AZD4076
Click to Show/Hide
|
|||
| External Link | ||||
| GSK-677954 | Discontinued in Phase 2 | [28] | ||
| Synonyms |
SCHEMBL2065429
Click to Show/Hide
|
|||
| External Link | ||||
| KD-3020 | Preclinical | [28] | ||
| External Link | ||||
| RIPA-56 | Investigative | [29] | ||
| Synonyms |
1956370-21-0; N-benzyl-N-hydroxy-2,2-dimethylbutanamide; CHEMBL4092421; GTPL9643; SCHEMBL17874088; EX-A4338; BDBM50229025; MFCD30738006; s6511; ZINC616570725; CS-6266; compound 92 [WO2016101885]; compound 56 [PMID: 27992216]; HY-101032; C(C1=CC=CC=C1)N(C(C(CC)(C)C)=O)O; A1-28956
Click to Show/Hide
|
|||
| External Link | ||||
References