m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT02089
|
[1] | |||
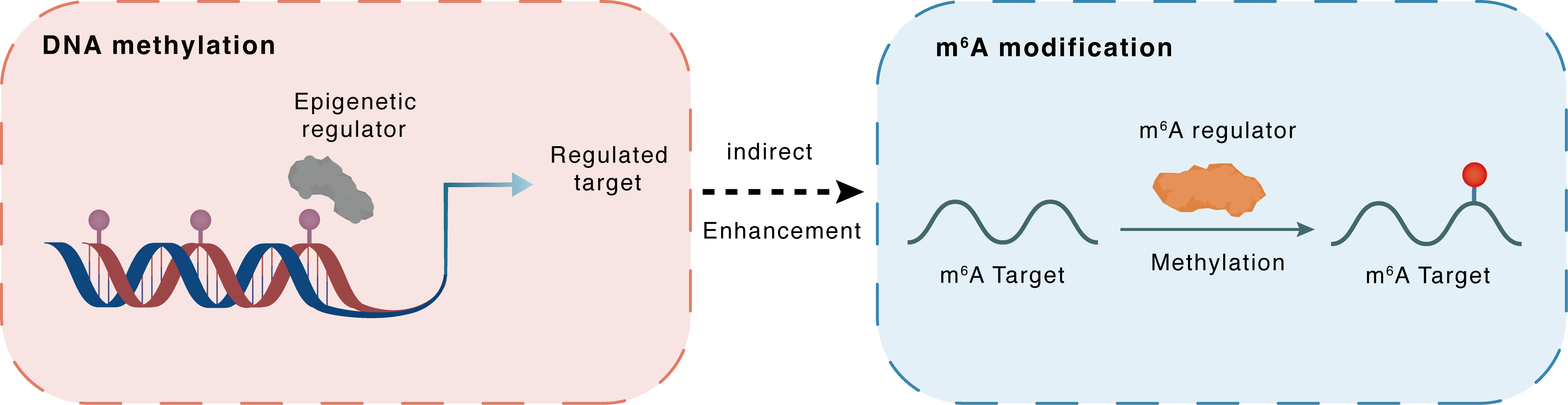 DNA methylation
DNMT1
FTO
Indirect
Enhancement
m6A modification
BDNF
BDNF
METTL3
Methylation
DNA methylation
DNMT1
FTO
Indirect
Enhancement
m6A modification
BDNF
BDNF
METTL3
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Neurotrophic factor BDNF precursor form (BDNF) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | DNA methylation (DNAMeth) | ||||
| Epigenetic Regulator | DNA (cytosine-5)-methyltransferase 1 (DNMT1) | WRITER | View Details | ||
| Regulated Target | FTO alpha-ketoglutarate dependent dioxygenase (FTO) | View Details | |||
| Crosstalk Relationship | DNA methylation → m6A | Enhancement | |||
| Crosstalk Mechanism | DNA methylation indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | A downregulation in demethylating enzyme Fto and upregulation in methylating enzyme Mettl3 were also noted. The FTO alpha-ketoglutarate dependent dioxygenase (FTO) promoter was hypomethylated due to the lower expression of DNMT1 and DNMT3A and Nr3c1, Creb1, Ntrk2, Neurotrophic factor BDNF precursor form (BDNF) are downstream target genes of both METTL3 and FTO. | ||||
| Responsed Disease | Major depressive disorder | ICD-11: 6A70.3 | |||
In-vitro Model |
SH-SY5Y | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In-vivo Model | Rats were housed at 23 ° C and 55% humidity and were given ad libitum food and water. During acclimatization (1 week), rats were placed randomly (3/cage); however, after initial behavioral testing, they were grouped according to their behavioral phenotypes. All experiments were performed under a light cycle (8:00 AM and 10:00 AM). The protocol to induce LH behavior was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. The animal study also adhered to the international guidelines for the use and care of laboratory animals. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| DNA (cytosine-5)-methyltransferase 1 (DNMT1) | 27 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| SGI110 | Phase 3 | [2] | ||
| MOA | Modulator | |||
| External Link | ||||
| Guadecitabine | Phase 3 | [3] | ||
| Synonyms |
UNII-2KT4YN1DP7; 929901-49-5; 2KT4YN1DP7; SGI-110 free acid; Guadecitabine [USAN:INN]; GuadecitabineSGI-110; Guadecitabine (USAN/INN); CHEMBL3544916; Guanosine, 2'-deoxy-5-azacytidylyl-(3'-5')-2'-deoxy-; ZINC43203165; AKOS027321496; AKOS030238181; DB11918; CS-3089; HY-13542; D10877; 2'-deoxy-5-azacytidylyl-(3'-5')-2'-deoxyguanosine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CC-486 | Phase 3 | [4] | ||
| Synonyms |
AG-14361; AG14361; 328543-09-5; UNII-48N0U0K50I; AG 14361; CHEMBL65892; 48N0U0K50I; Imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one, 2-[4-[(dimethylamino)methyl]phenyl]-5,6-dihydro-; AG-014361; 1-(4-((dimethylamino)methyl)phenyl)-8,9-dihydro-2,7,9a-triazabenzo[cd]azulen-6(7H)-one; Imidazo(4,5,1-jk)(1,4)benzodiazepin-7(4H)-one, 2-(4-((dimethylamino)methyl)phenyl)-5,6-dihydro-; 2-[4-[(Dimethylamino)methyl]phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one; SMR000486393; MLS006011157; MLS001065917; Nucleoside analogue
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| S-110 | Phase 3 | [5] | ||
| Synonyms |
DNA demethylating agent (myelodysplastic syndrome), Supergen
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Palifosfamide | Phase 2 | [6] | ||
| Synonyms |
ZIO-201
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RX-3117 | Phase 2 | [7] | ||
| Synonyms |
Antimetabolite (cancer), Rexahn; Antimetabolite (cancer), Rexahn/ Teva
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Antroquinonol | Phase 2 | [8] | ||
| Synonyms |
Hocena; Fungal extract (cancer), Golden Biotechnology
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| GSK4172239 | Phase 1 | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27376512-Compound-miR-155-5p | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 27.88 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example11 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 39440 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example16 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 22520 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example5 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50(DNMT1) = 3530 nM | |||
| External Link | ||||
| PMID27376512-Compound-asCEBP-2HPE | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 135.2 nM | |||
| External Link | ||||
| PMID27376512-Compound-asCEBP-1 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 2003 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example4 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 13810 nM | |||
| External Link | ||||
| PMID27376512-Compound-asCEBP-2 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 434.1 nM | |||
| External Link | ||||
| PMID27376512-Compound-asCEBP-1HPE | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 917.5 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example8 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6850 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-433 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 4.22 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example30 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27376512-Compound-MTC-424 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1940 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-427 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 295 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-422 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1430 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-423 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 363 nM | |||
| External Link | ||||
| PMX-700 | Investigative | [11] | ||
| Synonyms |
SJ-005019; SJ-005059; DC-010-116; Temozolomide analogs (cancer), Pharminox
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| XB-05 | Investigative | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| CP-4200 | Investigative | [11] | ||
| Synonyms |
Lipidated azacitidine (cancer, Lipid Vector), Clavis Pharma; 5-azacytidine-5'-elaidate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Neurotrophic factor BDNF precursor form (BDNF) | 1 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| PYM-50028 | Phase 2 | [12] | ||
| Synonyms |
Cogane; Smilagenin; P-58; P-63
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
References