m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT02071
|
[1] | |||
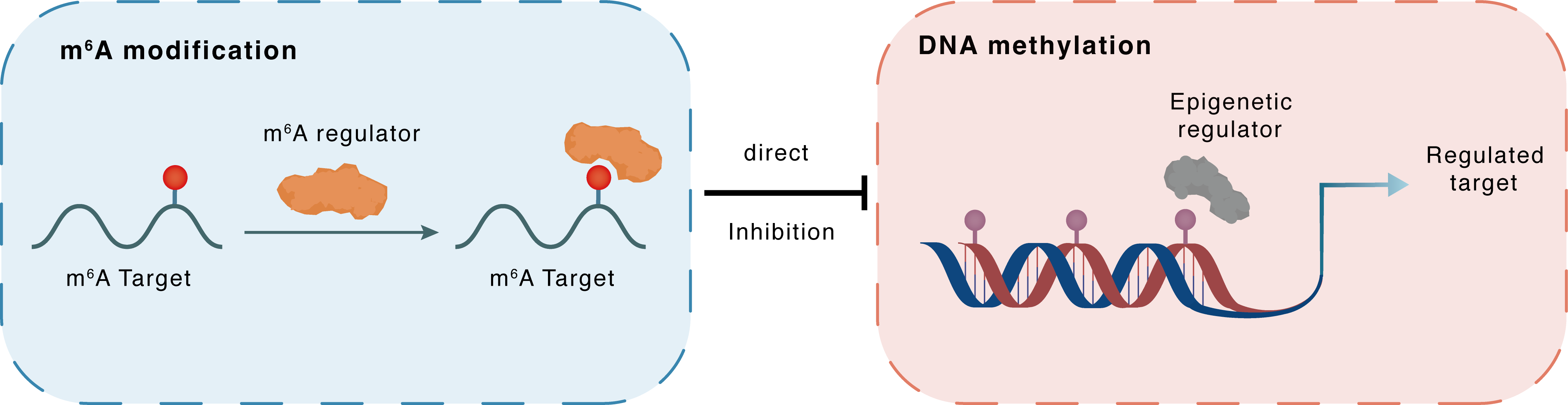 m6A modification
Dnmt3a
Dnmt3a
YTHDF2
m6A modification
Dnmt3a
Dnmt3a
YTHDF2
 : m6A sites
Direct
Inhibition
DNA methylation
DNMT3A
DACH1 : m6A sites
Direct
Inhibition
DNA methylation
DNMT3A
DACH1
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | YTH domain-containing family protein 2 (YTHDF2) | READER | |||
| m6A Target | Cysteine methyltransferase DNMT3A (DNMT3A) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | DNA methylation (DNAMeth) | ||||
| Epigenetic Regulator | Cysteine methyltransferase DNMT3A (DNMT3A) | WRITER | View Details | ||
| Regulated Target | Dachshund family transcription factor 1 (DACH1) | View Details | |||
| Crosstalk Relationship | m6A → DNA methylation | Inhibition | |||
| Crosstalk Mechanism | m6A modification directly impacts DNA methylation through modulating the expression level of DNA methyltransferases or demethylases. | ||||
| Crosstalk Summary | This study suggests that YTHDF2 regulates Cysteine methyltransferase DNMT3A (DNMT3A) expression and nuclear translocation to modulate dendritic cell function and Th17/Treg balance through Dachshund family transcription factor 1 (DACH1)/c-Jun pathway in COPD. | ||||
| Responsed Disease | Chronic obstructive pulmonary disease | ICD-11: CA22 | |||
| Cell Process | Differentiation | ||||
| balance | |||||
| In-vivo Model | Male BALB/c mice (SJA Laboratory Animal Company, Hunan, China) with age of 6-8 weeks were used in this study to establish COPD model. Mice were housed in individually ventilated cages under a pathogen-free condition, with ad libitum access to food and water. Animal welfare was monitored daily, and all efforts were made to minimize suffering. All animal procedures were conducted in accordance with the guidelines for use of laboratory animals, with approval from the Institutional Animal Care and Use Committee at Jiangxi Provincial People's Hospital (The First Affiliated Hospital of Nanchang Medical College). | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Cysteine methyltransferase DNMT3A (DNMT3A) | 8 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| PMID27376512-Compound-Figure3CN | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | EC50 = 1100 nM | |||
| External Link | ||||
| PMID27376512-Compound-Figure3CG | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | EC50 = 2400 nM | |||
| External Link | ||||
| PMID27376512-Compound-Figure3CM | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | EC50 = 1100 nM | |||
| External Link | ||||
| PMID27376512-Compound-Figure2aExample1 | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 3000 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-424 | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1940 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-427 | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 295 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-422 | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1430 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-423 | Patented | [2] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 363 nM | |||
| External Link | ||||
| CA22: Chronic obstructive pulmonary disease | 207 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Carbocisteine | Approved | [3] | ||
| Synonyms |
Carbocysteine; Siroxyl; Carbocisteine, Sopar
Click to Show/Hide
|
|||
| External Link | ||||
| Aclidinium | Approved | [4] | ||
| Synonyms |
UNII-K17VY42F6C; K17VY42F6C; CHEBI:65346; 727649-81-2; CHEMBL551466; (3R)-3-[2-hydroxy(di-2-thienyl)acetoxy]-1-(3-phenoxypropyl)-1-azoniabicyclo[2.2.2]octane; (3R)-3-{[Hydroxy(di-2-thienyl)acetyl]oxy}-1-(3-phenoxypropyl)-1-azoniabicyclo[2.2.2]octane bromide; SCHEMBL72141; GTPL7449; SCHEMBL15096073; CHEMBL1194325; DTXSID00223070; ZINC30691727; BDBM50296331; DB08897; AJ-84146; AB01565828_02; 1-(3-Phenoxypropyl)-3alpha-[hydroxybis(2-thienyl)acetyloxy]-1-azoniabicyclo[2.2.2]octane
Click to Show/Hide
|
|||
| External Link | ||||
| Roflumilast | Approved | [5] | ||
| Synonyms |
162401-32-3; DAXAS; Daliresp; 3-(CYCLOPROPYLMETHOXY)-N-(3,5-DICHLOROPYRIDIN-4-YL)-4-(DIFLUOROMETHOXY)BENZAMIDE; BY217; BYK20869; UNII-0P6C6ZOP5U; BY-217; Roflumilast (Daxas); B9302-107; 0P6C6ZOP5U; 3-(Cyclopropylmethoxy)-N-(3,5-dichloro-4-pyridinyl)-4-(difluoromethoxy)benzamide; Benzamide, 3-(cyclopropylmethoxy)-N-(3,5-dichloro-4-pyridinyl)-4-(difluoromethoxy)-; CHEMBL193240; CHEBI:47657; BYK-20869; ROF; Libertek; AK110425; 3-Cyclopropylmethoxy-N-(3,5-dichloropyridin-4-yl)-4-(difluoromethoxy)benzamide; Roflumilast [USAN]; APTA-2217; Roflumilast (JAN/USAN/INN); 3-cyclopropylmethoxy-4-difluoromethoxy-N-(3,5-di-chloropyrid-4-yl)benzamide; Alogliptin/roflumilast
Click to Show/Hide
|
|||
| External Link | ||||
| Tiotropium | Approved | [6] | ||
| Synonyms |
186691-13-4; UNII-0EB439235F; 0EB439235F; [3H]tiotropium; (1r,2r,4s,5s,7s)-7-{[hydroxy(Dithiophen-2-Yl)acetyl]oxy}-9,9-Dimethyl-3-Oxa-9-Azoniatricyclo[3310~2,4~]nonane; NCGC00167971-01; 0HK; (1R,2R,4S,5S)-7-{[2-hydroxy-2,2-bis(thiophen-2-yl)acetyl]oxy}-9,9-dimethyl-3-oxa-9-azatricyclo[3310^{2,4}]nonan-9-ium; GTPL367; SCHEMBL4662461; GTPL8592; DTXSID5044281; CHEMBL3305968; Spiriva (TN); Tiotropium bromide [USAN:INN]; (1A,2A,4A,5A,7A)-7-[(Hydroxydi-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatri-cyclo[331024]nonane bromide; (1a,2,4,5a,7)-7-[(2-Hydroxy-2,2-di-2-thienylacetyl)oxy]-9,9-dimethyl-3-oxa-9-azoniatricyclo[33102,4]nonane bromide; 7-{[hydroxy(dithiophen-2-yl)acetyl]oxy}-9,9-dimethyl-3-oxa-9-azoniatricyclo[33102,4]nonane bromide
Click to Show/Hide
|
|||
| External Link | ||||
| Fluorometholone | Approved | [3] | ||
| Synonyms |
Cortilet; Cortisdin; Delmeson; Efflumidex; FML; Fluaton; Flucon; Flumetholon; Fluormetholon; Fluormetholone; Fluormetholonum; Fluoromethalone; Fluorometholonum; Fluorometolona; Fluorometolone; Fluoropos; Loticort; Oxylone; Trilcin; Ursnon; Alcon Brand of Fluorometholone; FML Forte; FML Liquifilm; Fluor Op; Fluoro Ophtal; Fluorometolone [DCIT]; Isdin Brand of Fluorometholone; Isopto Flucon; Novartis Brand of Fluorometholone; PMS Fluorometholone; Pharm Allergan Brand of Fluorometholone; Pharmascience Brand of Fluorometholone; Ursapharm Brand of Fluorometholone; Winzer Brand of Fluorometholone; Allergan Brand 1 of Fluorometholone; Allergan Brand 2 of Fluorometholone; Allergan Brand 3 of Fluorometholone; F0414; U 8614; Component of Neo-Oxylone; FML (TN); FML-S Liquifilm; Flarex (TN); Flucon, Isopto; Fluor-Op; Fluoro-Ophtal; Fluorometholonum [INN-Latin]; Fluorometolona [INN-Spanish]; Neo-Oxylone; Oxylone (TN); PMS-Fluorometholone; Pharm-Allergan Brand of Fluorometholone; FML S.O.P; Fluor-op (TN); Fluorometholone [INN:BAN:JAN]; Fluorometholone (JP15/USP/INN); Pregna-1,4-diene-3,20-dione, 9-fluoro-11.beta.,17; Pregna-1,4-diene-3,20-dione, 9-fluoro-11beta,17-dihydroxy-6alpha-methyl-(8CI); (6S,8S,9R,10S,11S,13S,14S,17R)-17-acetyl-9-fluoro-11,17-dihydroxy-6,10,13-trimethyl-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthren-3-one; 11beta,17alpha-Dihydroxy-9-fluoro-6-methyl-1,4-pregnadiene-3,20-dione; 9-Fluoro-11,17-dihydroxy-6-methylpregna-1,4-diene-3,20-dione; 9-Fluoro-11-beta,17-dihydroxy-6-alpha-methylpregna-1,4-diene-3,20-dione; 9-Fluoro-11beta,17-dihydroxy-6alpha-methylpregna-1,4-diene-3,20-dione
Click to Show/Hide
|
|||
| External Link | ||||
| Bamifylline | Approved | [3] | ||
| Synonyms |
Bamifylline hydrochloride; Trentadil; Pulmac; Bamifylline HCl; Bamiphylline hydrochloride; 20684-06-4; mono-HCl; Benzetamophylline hydrochloride; UNII-66466QLM3S; 8102 CB; BAX 2793Z; Bamifylline hydrochloride [USAN]; EINECS 243-967-6; AC 3810; CB 8102; 66466QLM3S; 8-Benzyl-7-(2-(ethyl(2-hydroxyethyl)amino)ethyl)theophylline monohydrochloride; 8'-Benzyl-7-(2-(ethyl(2-hydroxyethyl)amino)ethyl)theophylline hydrochloride; 8-Benzyl-7-(N-ethyl-N-(beta-hydroxyethyl)aminoethyl)theophylline hydrochloride
Click to Show/Hide
|
|||
| External Link | ||||
| Maraviroc | Phase 3 | [7] | ||
| Synonyms |
376348-65-1; Selzentry; Celsentri; UK-427857; UK-427,857; UK 427857; UNII-MD6P741W8A; MD6P741W8A; CHEMBL256907; MVC; CHEMBL1201187; CHEBI:63608; 4,4-difluoro-N-[(1S)-3-[(1R,5S)-3-(3-methyl-5-propan-2-yl-1,2,4-triazol-4-yl)-8-azabicyclo[321]octan-8-yl]-1-phenylpropyl]cyclohexane-1-carboxamide; Isopropyl, 4,4-difluoro-N-((1S)-3-{(1R,3s,5S)-3-(3-methyl-5-(propan-2-yl)-4H-1,2,4-triazol-4-yl)-8-azabicyclo(321)octan-8-yl}-1-phenylpropyl)cyclohexanecarboxamide; Maraviroc [USAN]; Celsentri (TN); Celsentri(TM); PRO 140 & Maraviroc; Selzentry (TN); Selzentry(TM); UK-427,857 maraviroc (MVC); Exo-4,4-Difluoro-N-[3-[3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[321]oct-8-yl]-1(S)-phenylpropyl]cyclohexanecarboxamide; PRO 140 (Anti-CCR5 monoclonal antibody) & exo-4,4-Difluoro-N-[3-[3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo[321]oct-8-yl]-1(S)-phenylpropyl]cyclohexanecarboxamide; 4,4-Difluoro-N-((1S)-3-(exo-3-(3-isopropyl-5-methyl-4H-1,2,4-triazol-4-yl)-8-azabicyclo(321)oct-8-yl)-1-phenylpropyl)cyclohexanecarboxamide; [3H]maraviroc
Click to Show/Hide
|
|||
| External Link | ||||
| Glycopyrrolate | Approved | [8] | ||
| Synonyms |
596-51-0; Glycopyrrolate bromide; Robinul; Gastrodyn; Tarodyl; Nodapton; Tarodyn; Asecryl; Copyrrolate; Cuvposa; Glycopyrronii bromidum; AHR-504; ROBINUL FORTE; Robinal; Robanul; Bromuro de glicopirronio; Bromure de glycopyrronium; NVA-237; AHR 504; Glycopyrronii bromidum [INN-Latin]; EINECS 209-887-0; Bromure de glycopyrronium [INN-French]; Bromuro de glicopirronio [INN-Spanish]; 3-Hydroxy-1,1-dimethylpyrrolidinium bromide
Click to Show/Hide
|
|||
| External Link | ||||
| Salmeterol | Approved | [9] | ||
| Synonyms |
Aeromax; Astmerole; SALMATEROL; Salmeterolum; Serevent; Glaxo Wellcome brand of salmeterol xinafoate; Salmeterolum [Latin]; GR 33343X; S 2692; GR-33343X; Serevent (TN); Salmeterol (USAN/INN); Salmeterol [USAN:BAN:INN]; (+-)-4-Hydroxy-alpha'-(((6-(4-phenylbutoxy)hexyl)amino)methyl)-m-xylene-alpha,alpha'-diol; (+-)-4-Hydroxy-alpha1-(((6-(4-phenylbutoxy)hexyl)amino)methyl)-1,3-benzenedimethanol; (inverted question mark) 4-Hydroxy-a1-[[[6-(4-phenylbutoxy)hexyl]amino]m-ethyl]-1,3-benzenedimethanol; 2-(hydroxymethyl)-4-(1-hydroxy-2-{[6-(4-phenylbutoxy)hexyl]amino}ethyl)phenol; 2-(hydroxymethyl)-4-[1-hydroxy-2-({6-[(4-phenylbutyl)oxy]hexyl}amino)ethyl]phenol; 2-(hydroxymethyl)-4-[1-hydroxy-2-[6-(4-phenylbutoxy)hexylamino]ethyl]phenol
Click to Show/Hide
|
|||
| External Link | ||||
| Vilanterol | Approved | [10] | ||
| Synonyms |
503068-34-6; GW642444x; UNII-028LZY775B; GW-642444x; CHEBI:75037; 028LZY775B; Vilanterol (GW642444; GW 642444X); 4-((1R)-2-((6-(2-((2,6-dichlorophenyl)methoxy)ethoxy)hexyl)amino)-1-hydroxyethyl)-2-(hydroxymethyl)phenol; 4-[(1R)-2-[(6-{2-[(2,6-dichlorophenyl)methoxy]ethoxy}hexyl)amino]-1-hydroxyethyl]-2-(hydroxymethyl)phenol; Vilanterol [USAN:INN]; vilanterolum; Vilanterol base; Vilanterol (USAN); SCHEMBL142630; GTPL7353; CHEMBL1198857; DTXSID80198318; DAFYYTQWSAWIGS-DEOSSOPVSA-N; MolPort-044-560-195; Relovair
Click to Show/Hide
|
|||
| External Link | ||||
| MK-08887A | Approved | [3] | ||
| Synonyms |
DULERA
Click to Show/Hide
|
|||
| External Link | ||||
| Revefenacin | Approved | [11] | ||
| Synonyms |
UNII-G2AE2VE07O; G2AE2VE07O; Revefenacin [INN]; Revefenacin [WHO-DD]; Revefenacin [USAN:INN]; Revefenacin (USAN/INN); SCHEMBL356480; EX-A1722; DB11855; GSK1160724; CS-7743; GSK-1160724; HY-15851; D10978; 1211931-83-7
Click to Show/Hide
|
|||
| External Link | ||||
| Prasterone | Approved | [12] | ||
| Synonyms |
Dehydroepiandrosterone; DHEA; 53-43-0; Dehydroisoandrosterone; Androstenolone; trans-Dehydroandrosterone; Psicosterone; Diandrone; Diandron; 3beta-hydroxyandrost-5-en-17-one; 17-Hormoforin; Prestara; 17-Chetovis; Andrestenol; 5-Dehydroepiandrosterone; Intrarosa; Siscelar plus; 5,6-Didehydroisoandrosterone; Dehydro-epi-androsterone; Prasteronum; 5,6-Dehydroisoandrosterone; Prasterona; Epiandrosterone, 5-dehydro-; Caswell No 051F; 5,6-Dehydroisoandrostorone
Click to Show/Hide
|
|||
| External Link | ||||
| Meprednisone | Approved | [3] | ||
| Synonyms |
Betapar
Click to Show/Hide
|
|||
| External Link | ||||
| Theophylline | Approved | [13] | ||
| Synonyms |
Bronchoretard; Cetraphylline; Chronophyllin; Dimethylxanthine; Elixophylline; Liquophylline; Pseudotheophylline; Afonilum Retard; Bronchodid Duracap; Elixophyllin SR; Somophyllin CRT; Spophyllin retard; Telbans DrySyrup; Teofilina [Polish]; Theal tablets; Theobid Duracap; Theodur Dry Syrup; Uniphyllin continus; Elixophyllin (TN); Elixophyllin(e); Pulmo-Timelets; Somophyllin-CRT; Somophyllin-DF; Theo-Organidin; Theoclear-200; Theodur G (TN); Theolair (TN); Theophylline (JP15); Theophylline-SR; Quibron-t (TN); Theo-Dur-Sprinkle; Theophylline-[8-3H; Theoclear L.A.-130; 1,3 Dimethylxanthine; 1,3-Dimethylxanthine; 1H-purine-2,6-dione
Click to Show/Hide
|
|||
| External Link | ||||
| Almitrine | Approved | [14] | ||
| Synonyms |
Almitrin; Almitrina; Almitrinum; Vectarion; Almitrine bismesylate; Almitrine dimesylate; S 2620; Almitrina [INN-Spanish]; Almitrine (INN); Almitrine Bis(methanesulfonate); Almitrine [BAN:INN]; Almitrine [INN:BAN]; Almitrinum [INN-Latin]; N,N'-diallyl-6-{4-[bis(4-fluorophenyl)methyl]piperazin-1-yl}-1,3,5-triazine-2,4-diamine; 2,4-Bis(allylamino)-6-(4-(bis(p-fluorophenyl)methyl)-1-piperazinyl)-s-triazine; 2,4-bis[allylamino]-6-[4-[bis(p-fluorophenyl)methyl]-1-piperazinyl]-s-triazine; 6-(4-(Bis(4-fluorphenyl)methyl)-1-piperazinyl)-N,N'-di-2-propenyl-1,3,5-triazin-2,4-diamin; 6-[4-[bis(4-fluorophenyl)methyl]piperazin-1-yl]-2-N,4-N-bis(prop-2-enyl)-1,3,5-triazine-2,4-diamine
Click to Show/Hide
|
|||
| External Link | ||||
| Arformoterol | Approved | [15] | ||
| Synonyms |
67346-49-0; Brovana; UNII-F91H02EBWT; Arformoterol tartrate; CHEMBL1363; F91H02EBWT; CHEBI:408174; Brovana (TN); Formoterol/fluticasone propionate
Click to Show/Hide
|
|||
| External Link | ||||
| Incruse Ellipta | Approved | [3] | ||
| Synonyms |
Umeclidinium bromide; 869113-09-7; Umeclidinium (bromide); GSK573719A; UNII-7AN603V4JV; 7AN603V4JV; GSK-573719; 1-[2-(Benzyloxy)ethyl]-4-(hydroxydiphenylmethyl)-1-quinuclidinium Bromide; 1-(2-(benzyloxy)ethyl)-4-(hydroxydiphenylmethyl)quinuclidin-1-ium bromide; CHEMBL523299; Umeclidinium brom; Umeclidinium bromide [USAN:INN]; umeclidinii bromidum; 1-Azoniabicyclo[2.2.2]octane, 4-(hydroxydiphenylmethyl)-1-[2-(phenylmethoxy)ethyl]-, bromide (1:1); 1-Azoniabicyclo(2.2.2)octane, 4-(hydroxydiphenylmethyl)-1-(2
Click to Show/Hide
|
|||
| External Link | ||||
| LAS-34273 | Approved | [16] | ||
| Synonyms |
Bretaris; Eklira; Aclidinium bromide; LAS-W-330; [1-(3-phenoxypropyl)-1-azoniabicyclo[2.2.2]oct-8-yl] 2-hydroxy-2,2-dithiophen-2-yl-acetate Bromide
Click to Show/Hide
|
|||
| External Link | ||||
| Bitolterol | Approved | [3] | ||
| Synonyms |
Tornalate
Click to Show/Hide
|
|||
| External Link | ||||
| Olodaterol | Approved | [17] | ||
| Synonyms |
BI-1744
Click to Show/Hide
|
|||
| External Link | ||||
| Symbicort | Approved | [18] | ||
| Synonyms |
(22R)-Budesonide; UNII-2HI1006KPH; 51333-22-3; 51372-29-3; 2HI1006KPH; DSSTox_CID_202; DSSTox_RID_75430; DSSTox_GSID_20202; (1~{s},2~{s},4~{r},6~{r},8~{s},9~{s},11~{s},12~{s},13~{r})-9,13-Dimethyl-11-Oxidanyl-8-(2-Oxidanylethanoyl)-6-Propyl-5,7-Dioxapentacyclo[10.8.0.0^{2,9}.0^{4,8}.0^{13,18}]icosa-14,17-Dien-16-One; R-Budesonide; Budesonide-22R; NCGC00016862-01; EINECS 257-161-7; CAS-51333-22-3; BUDESONIDE (11beta,16alpha(R)); SCHEMBL4095; AC1L22VC; CHEMBL2110662
Click to Show/Hide
|
|||
| External Link | ||||
| Indacaterol | Approved | [19] | ||
| Synonyms |
312753-06-3; QAB149; Arcapta; Onbrez; QAB-149; QAB 149; UNII-8OR09251MQ; 753498-25-8; CHEMBL1095777; CHEBI:68575; 8OR09251MQ; (R)-5-(2-(5,6-diethyl-2,3-dihydro-1H-inden-2-ylamino)-1-hydroxyethyl)-8-hydroxyquinolin-2(1H)-one; 5-[(1R)-2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl]-8-hydroxy-1H-quinolin-2-one; Indacaterol Maleic Acid Salt; Indacaterol (USAN/INN); Indacaterol [USAN:INN:BAN]; 5-{(1R)-2-[(5,6-diethyl-2,3-dihydro-1H-inden-2-yl)amino]-1-hydroxyethyl}-8-hydroxyquinolin-2(1H)-one; 5-(2-(5,6-Diethylindan-2-ylamino)-1-hydroxyethyl)-8-hydroxy-1H-quinolin-2-one; Indacaterol/mometasone
Click to Show/Hide
|
|||
| External Link | ||||
| Benralizumab | Approved | [20] | ||
| External Link | ||||
| Anoro | Approved | [3] | ||
| Synonyms |
Umeclidinium + vilanterol
Click to Show/Hide
|
|||
| External Link | ||||
| Pregabalin | Approved | [21] | ||
| Synonyms |
Pregabalin CR; Pregabalin (controlled-release, oral); Pregabalin (controlled-release, oral), Pfizer
Click to Show/Hide
|
|||
| External Link | ||||
| Relvar/Breo | Approved | [22] | ||
| Synonyms |
Vilanterol + fluticasone furoate
Click to Show/Hide
|
|||
| External Link | ||||
| Umeclidinium | Approved | [23] | ||
| External Link | ||||
| Aclidinium/formoterol | Phase 4 | [24] | ||
| Synonyms |
(S,S)-Formoterol; CHEBI:63081; (S,S)-N-[2-hydroxy-5-[1-hydroxy-2-[[2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]formaldehyde; NCGC00025167-01; 67346-48-9; Tocris-1448; AC1MHY5S; ZINC856
Click to Show/Hide
|
|||
| External Link | ||||
| Ozagrel | Phase 4 | [25] | ||
| Synonyms |
Ozagrel (ophthalmic, eye disorders); Ozagrel (ophthalmic, eye disorders), Kissei/ Teika
Click to Show/Hide
|
|||
| External Link | ||||
| Levosalbutamol/ipratropium | Phase 4 | [26] | ||
| Synonyms |
8-Azoniabicyclo(3.2.1)octane, 3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-(1-methylethyl)-, (endo,syn)-; 3-(3-Hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-(1-methylethyl)-8-azoniabicyclo(3.2.1)octane (3-endo,8-syn)-
Click to Show/Hide
|
|||
| External Link | ||||
| Itepekimab | Phase 3 | [27] | ||
| External Link | ||||
| MEDI3506 | Phase 3 | [28] | ||
| Synonyms |
Tozorakimab
Click to Show/Hide
|
|||
| External Link | ||||
| Astegolimab | Phase 3 | [29] | ||
| Synonyms |
MSTT1041A; RG6149
Click to Show/Hide
|
|||
| External Link | ||||
| SAR440340 | Phase 3 | [27] | ||
| Synonyms |
Itepekimab
Click to Show/Hide
|
|||
| External Link | ||||
| Olodaterol/tiotropium bromide | Phase 3 | [30] | ||
| Synonyms |
136310-93-5; UNII-XX112XZP0J; XX112XZP0J; (1R,2R,4S,5S,7s)-7-(2-Hydroxy-2,2-di(thiophen-2-yl)acetoxy)-9,9-dimethyl-3-oxa-9-azatricyclo[3.3.1.02,4]nonan-9-ium bromide; AK-72842; (1R,2R,4S,5S,7S)-7-(2-Hydroxy-2,2-di(thiophen-2-yl)acetoxy)-9,9-dimethyl-3-oxa-9-azatricyclo[3.3.1.0
Click to Show/Hide
|
|||
| External Link | ||||
| PT005 | Phase 3 | [31] | ||
| Synonyms |
Perforomist; Atock; Foradil Aerolizer; Foradil Certihaler; YM-08316; Broncoral; Eolus; Oxis Turbuhaler; BD-40A; Eformoterol fumarate; Formoterol Easyhaler; CGP-25827A; AC1OCEVQ; PT-005
Click to Show/Hide
|
|||
| External Link | ||||
| Tozorakimab | Phase 3 | [28] | ||
| External Link | ||||
| GSK642444 | Phase 3 | [32] | ||
| Synonyms |
threo isoleucyl thiazolidide, 1; BDBM17299
Click to Show/Hide
|
|||
| External Link | ||||
| Arofylline | Phase 3 | [33] | ||
| Synonyms |
Arofyllin; KM09088; Arofylline (USAN/INN); 3-(4-chlorophenyl)-1-propyl-3,7-dihydro-1H-purine-2,6-dione; 3-(4-chlorophenyl)-1-propyl-7H-purine-2,6-dione
Click to Show/Hide
|
|||
| External Link | ||||
| Lebrikizumab | Phase 3 | [34] | ||
| Synonyms |
RG3637
Click to Show/Hide
|
|||
| External Link | ||||
| PT003 | Phase 3 | [31] | ||
| External Link | ||||
| PT001 GP | Phase 3 | [35] | ||
| External Link | ||||
| QVA-149 | Phase 3 | [36] | ||
| Synonyms |
Glycopyrrolate mixture with indacaterol; 1262431-94-6; Utibron; Utibron Neohaler; Ultibro Breezhaler; Glycopyrrolate / indacaterol; Indacaterol / glycopyrronium; Indacaterol / glycopyrrolate; QVA149; QVA 149; Glycopyrronium bromide / indacaterol
Click to Show/Hide
|
|||
| External Link | ||||
| Darotropium + 642444 | Phase 3 | [37] | ||
| External Link | ||||
| QMF149 | Phase 3 | [38] | ||
| Synonyms |
Indacaterol, mometasone furoate
Click to Show/Hide
|
|||
| External Link | ||||
| SUN-101 | Phase 3 | [39] | ||
| External Link | ||||
| Bococizumab | Phase 3 | [40] | ||
| External Link | ||||
| Mitiperstat | Phase 2 | [41] | ||
| Synonyms |
1-((2-((1R)-1-Aminoethyl)-4-chloro-phenyl)methyl)-2-thioxo-5hpyrrolo(3,2-d)pyrimidin-4-one; 1-({2-[(1R)-1-aminoethyl]-4-chlorophenyl}methyl)-2-sulfanylidene-1,2,3,5-tetrahydro-4H-pyrrolo[3,2-d]pyrimidin-4-one; 1-({2-[(1R)-1-aminoethyl]-4-chlorophenyl}methyl)-2-sulfanylidene-1H,2H,3H,4H,5H-pyrrolo[3,2-d]pyrimidin-4-one; 1-[[2-[(1R)-1-aminoethyl]-4-chlorophenyl]methyl]-2-sulfanylidene-5H-pyrrolo[3,2-d]pyrimidin-4-one; 1-{2-[(1R)-1-Aminoethyl]-4-chlorobenzyl}-2-thioxo-1,2,3,5-tetrahydro-4H-pyrrolo[3,2-d]pyrimidin-4-one; 1933460-19-5; 4H-Pyrrolo(3,2-d)pyrimidin-4-one, 1-((2-((1R)-1-aminoethyl)-4-chlorophenyl)methyl)-1,2,3,5-tetrahydro-2-thioxo-; 4H-Pyrrolo[3,2-d]pyrimidin-4-one, 1-[[2-[(1R)-1-aminoethyl]-4-chlorophenyl]methyl]-1,2,3,5-tetrahydro-2-thioxo-; Alternative Preparation; Azd 4831; AZD 4831 [WHO-DD]; AZD4831; AZD-4831; AZD-4831 [WHO-DD]; BDBM312172; BHKKSKOHRFHHIN-MRVPVSSYSA-N; CHEMBL5095218; compound 16 [PMID: 36005476]; CS-0376445; EX-A7129; GLXC-26157; GTPL12154; HY-145581; Mitiperstat; Mitiperstat [INN]; MITIPERSTAT [USAN]; S6GYK3X4QQ; SCHEMBL17782047; UNII-S6GYK3X4QQ; US9616063, 3
Click to Show/Hide
|
|||
| External Link | ||||
| OrM3 | Phase 2b | [32] | ||
| External Link | ||||
| Bimosiamose | Phase 2a | [42] | ||
| Synonyms |
TBC 1269; TBC-1269
Click to Show/Hide
|
|||
| External Link | ||||
| CSJ117 | Phase 2 | [43] | ||
| Synonyms |
Ecleralimab
Click to Show/Hide
|
|||
| External Link | ||||
| AZD8871 | Phase 2 | [44] | ||
| Synonyms |
1435519-06-4; 2-THIOPHENEACETIC ACID, .ALPHA.-HYDROXY-.ALPHA.-2-THIENYL-, TRANS-4-((3-(5-((((2R)-2-(1,2-DIHYDRO-8-HYDROXY-2-OXO-5-QUINOLINYL)-2-HYDROXYETHYL)AMINO)METHYL)-1H-BENZOTRIAZOL-1-YL)PROPYL)METHYLAMINO)CYCLOHEXYL ESTER; 2-Thiopheneacetic acid, alpha-hydroxy-alpha-2-thienyl-, trans-4-((3-(5-((((2R)-2-(1,2-dihydro-8-hydroxy-2-oxo-5-quinolinyl)-2-hydroxyethyl)amino)methyl)-1H-benzotriazol-1-yl)propyl)methylamino)cyclohexyl ester; AKOS040750679; Azd 8871; AZD 8871 [WHO-DD]; AZD8871; AZD-8871; BDBM50528210; CHEMBL4297483; CS-0079218; EX-A7799; HY-120802; LAS191351; LAS-191351; Navafenterol; NAVAFENTEROL [INN]; Navafenterol [USAN]; SCHEMBL16429536; SCHEMBL22766780; TRANS-4-((3-(5-((((2R)-2-HYDROXY-2-(8-HYDROXY-2-OXO-1,2-DIHYDRO-5- QUINOLINYL)ETHYL)AMINO)METHYL)-1H-BENZOTRIAZOL-1-YL)PROPYL)(METHYL)AMINO)CYCLOHEXYL HYDROXY(DI-2-THIENYL)ACETATE; U29GY32XJ4; UNII-U29GY32XJ4; WHO 11100
Click to Show/Hide
|
|||
| External Link | ||||
| YPL-001 | Phase 2 | [45] | ||
| External Link | ||||
| DS102 | Phase 2 | [46] | ||
| External Link | ||||
| QBW251 | Phase 2 | [47] | ||
| Synonyms |
(S)-3-Amino-6-methoxy-N-(3,3,3-trifluoro-2-hydroxy-2-methylpropyl)-5-(trifluoromethyl)picolinamide; 1334546-77-8; 2-Pyridinecarboxamide, 3-amino-6-methoxy-N-((2S)-3,3,3-trifluoro-2-hydroxy-2-methylpropyl)-5-(trifluoromethyl)-; 2-pyridinecarboxamide, 3-amino-6-methoxy-N-[(2S)-3,3,3-trifluoro-2-hydroxy-2-methylpropyl]-5-(trifluoromethyl)-; 3-amino-6-methoxy-5-trifluoromethyl-pyridine-2-carboxylic acid ((S)-3,3,3-trifluoro-2-hydroxy-2-methyl-propyl)-amide; 3-Amino-6-methoxy-N-[(2S)-3,3,3-trifluoro-2-hydroxy-2-methylpropyl]-5-(trifluoromethyl)-2-pyridinecarboxamide; 3-amino-6-methoxy-N-[(2S)-3,3,3-trifluoro-2-hydroxy-2-methylpropyl]-5-(trifluoromethyl)pyridine-2-carboxamide; AKOS040759841; AMB0BO0WFH; BDBM297402; CHEMBL4650318; compound 33 [PMID: 34028270]; CS-0116371; DTXSID601336884; GTPL11547; HY-109177; Icenticaftor; Icenticaftor [INN]; Icenticaftor [USAN:INN]; Icenticaftor [USAN]; MS-25717; Qbw251; QBW-251; SCHEMBL2372751; UNII-AMB0BO0WFH; US10117858, Example 5; WHO 11246
Click to Show/Hide
|
|||
| External Link | ||||
| SelK2 | Phase 2 | [48] | ||
| External Link | ||||
| GSP 304 | Phase 2 | [49] | ||
| External Link | ||||
| AZD7594 | Phase 2 | [50] | ||
| External Link | ||||
| TA-2005 | Phase 2 | [33] | ||
| Synonyms |
Carmoterol hydrochloride; CHF 4226.01; 8-Hydroxy-5-((1R)-1-hydroxy-2-(N-(1R)-2-(p-methoxyphenyl)isopropylamino)ethyl)carbostyril HCl; 8-Hydroxy-5-(1-hydroxy-2-(N-(2-(4-methoxyphenyl)-1-methylethyl)amino)ethyl)carbostyril hydrochloride; 8-hydroxy-5-[(1R)-1-hydroxy-2-[[(2R)-1-(4-methoxyphenyl)propan-2-yl]amino]ethyl]-1H-quinolin-2-one hydrochloride; 8-hydroxy-5-[(1r)-1-hydroxy-2-{[(2r)-1-(4-methoxyphenyl)propan-2-yl]amino}ethyl]quinolin-2(1h)-one hydrochloride(1:1)
Click to Show/Hide
|
|||
| External Link | ||||
| Interferon-alpha lozenge | Phase 2 | [51] | ||
| Synonyms |
Veldona (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Tetomilast | Phase 2 | [52] | ||
| Synonyms |
145739-56-6; 6-(2-(3,4-Diethoxyphenyl)thiazol-4-yl)picolinic acid; OPC-6535; UNII-S6RXB5KF56; S6RXB5KF56; 2-(3,4-Diethoxyphenyl)-4-(2-carboxy-6-pyridyl)thiazole; 2-Pyridinecarboxylicacid, 6-[2-(3,4-diethoxyphenyl)-4-thiazolyl]-; 6-[2-(3,4-diethoxyphenyl)-1,3-thiazol-4-yl]pyridine-2-carboxylic acid; Tetomilast [INN]; 6-(2-(3,4-Diethoxyphenyl)-1,3-thiazol-4-yl)pyridine-2-carboxylic acid; OPC 6535; ACMC-1BW9F; AC1MHG07; SCHEMBL436663; GTPL9877; CHEMBL332750; CTK0H7037; OPC6535; DTXSID00163149
Click to Show/Hide
|
|||
| External Link | ||||
| TPI-1020 | Phase 2 | [53] | ||
| Synonyms |
Nitrobudesonide; NCX-1020; NO-budesonide; Budesonide-21-nitrooxymethylbenzoate
Click to Show/Hide
|
|||
| External Link | ||||
| Vasoactive intestinal peptide | Phase 2 | [54] | ||
| Synonyms |
CCRIS 7231; Vasoactive intestinal polypeptide; Vasointestinal Peptide; Vasoactive intestinal peptide, synthetic porcine; SCHEMBL3041046; GTPL1152; VIP (Vasoactive Intestinal Peptide); LS-174653; VIP
Click to Show/Hide
|
|||
| External Link | ||||
| AZD1981 | Phase 2 | [55] | ||
| Synonyms |
802904-66-1; AZD-1981; UNII-2AD53WQ2CX; AZD 1981; 2AD53WQ2CX; CHEMBL1914489; 2-[4-acetamido-3-(4-chlorophenyl)sulfanyl-2-methylindol-1-yl]acetic acid; 1H-Indole-1-acetic acid, 4-(acetylamino)-3-((4-chlorophenyl)thio)-2-methyl-; 1H-Indole-1-acetic acid, 4-(acetylamino)-3-[(4-chlorophenyl)thio]-2-methyl-; JWYIGNODXSRKGP-UHFFFAOYSA-N; GTPL7680; SCHEMBL1053662; EX-A662; MolPort-035-395-811; HMS3653A06; BCP20957; ZINC73196066; s7263; BDBM50357102; AKOS027263775; SB16902; DB11946; CS-4189; NCGC00386290-04; HY-15950; SC-94603
Click to Show/Hide
|
|||
| External Link | ||||
| SB-656933 | Phase 2 | [56] | ||
| Synonyms |
Elubrixin; UNII-MW2AIJ8USP; MW2AIJ8USP; SB-656933-AAF; 688763-64-6; CHEMBL2178579; Elubrixin [USAN:INN]; SB656933; elubirixin; Elubrixin (USAN); SCHEMBL1562280; GTPL8499; DTXSID60218962; BDBM50398333; DB12135; SB 656933; D10332
Click to Show/Hide
|
|||
| External Link | ||||
| GSK1325756 | Phase 2 | [57] | ||
| Synonyms |
Danirixin; 954126-98-8; UNII-R318PGH5VP; GSK-1325756B; R318PGH5VP; GSK1325756B; Danirixin [USAN:INN]; GSK-1325756; GSK 1325756; Danirixin (USAN/INN); Danirixin(GSK1325756); GTPL8500; SCHEMBL1198688; CHEMBL3039531; MolPort-044-561-791; EX-A1178; ZINC95627831; AKOS030527036; DB11922; CS-5465; 1-(4-Chloro-2-hydroxy-3-(((3S)-piperidine-3-sulfonyl)phenyl)-3-(3-fluoro-2-methylphenyl)urea; HY-19768; D10387; (S)-1-(4-chloro-2-hydroxy-3-(piperidin-3-ylsulfonyl)phenyl)-3-(3-fluoro-2-methylphenyl)urea
Click to Show/Hide
|
|||
| External Link | ||||
| Igmesine | Phase 1 | [33] | ||
| Synonyms |
Igmesine hydrochloride; JO-1784; UNII-JV6M14TY35; Igmesine Hydrochloride [USAN]; 130152-35-1; Cinnamyl-1-phenyl-1-N-methyl-N-cyclopropylene; JO 1784; CI 1019; CI-1019; JV6M14TY35; (+)-alpha-(E)-Cinnamyl)-N-(cyclopropylmethyl)-alpha-ethyl-N-methylbenzylamine; (+)-Igmesine hydrochloride; (+)-(E)-N-(Cyclopropylmethyl)-alpha-ethyl-N-methyl-alpha-(3-phenyl-2-propenyl)benzenemethanamine hydrochloride; Igmesine hydrochloride (USAN); (+)-alpha-((E)-Cinnamyl)-N-(cyclopropylmethyl)-alpha-ethyl-N-methylbenzylamine; Alphagen
Click to Show/Hide
|
|||
| External Link | ||||
| PF-489791 | Phase 2 | [58] | ||
| Synonyms |
UNII-2S27T3DSZ3; PF-00489791; 853003-48-2; 2S27T3DSZ3; PF-489,791; ZUHZNKJIJDAJFD-UHFFFAOYSA-N; UK-489,791; SCHEMBL331279; SCHEMBL8042129; GTPL8377; DB11736; SB16722; 1H-Pyrazolo(4,3-d)pyrimidine-3-carboxamide, 1-(2-ethoxyethyl)-5-(ethylmethylamino)-7-((4-methyl-2-pyridinyl)amino)-N-(methylsulfonyl); n-[1-(2-ethoxyethyl)-5-(n-ethyl-n-methylamino)-7-(4-methylpyridin-2-yl-amino)-1h-pyrazolo[4,3-d]pyrimidine-3-carbonyl]methanesulfonamide
Click to Show/Hide
|
|||
| External Link | ||||
| BCT-197 | Phase 2 | [59] | ||
| Synonyms |
BCT-197-A2201
Click to Show/Hide
|
|||
| External Link | ||||
| ONO-6126 | Phase 2 | [33] | ||
| Synonyms |
21-Cyanosaframycin-B; NSC 325663; 66082-27-7; C29H30N4O8; 21-cyanosaframycin B; AC1L2P2O; SCHEMBL637953; CHEMBL452709
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-2115 | Phase 2 | [60] | ||
| Synonyms |
Dual action MABA (COPD), AstraZeneca; Dual action muscarinic acetylcholine receptor antagonist/beta 2 adrenoceptor agonist (COPD), AstraZeneca
Click to Show/Hide
|
|||
| External Link | ||||
| EP-101 | Phase 2 | [61] | ||
| Synonyms |
106-86-5; 1,2-Epoxy-4-vinylcyclohexane; 3-Vinyl-7-oxabicyclo[4.1.0]heptane; Epoxide 101; 4-Vinylcyclohexene oxide; Unoxat epoxide 101; Vinylcyclohexane monoxide; 4-Vinyl-1,2-epoxycyclohexane; 1-Vinyl-3,4-epoxycyclohexane; 4-Vinylcyclohexene monoxide; 3,4-Epoxycyclohexylethylene; 7-Oxabicyclo[4.1.0]heptane, 3-ethenyl-; 4-Vinylcyclohexane monoepoxide; Vinylcyclohexene monoxide; 4-Vinylcyclohexene-1,2-epoxide; 4-Vinylcyclohexane, 1,2-epoxide; EINECS 203-436-1; 4-Vinyl-1-Cyclohexene 1,2-Epoxide; NSC 35409
Click to Show/Hide
|
|||
| External Link | ||||
| PH-797804 | Phase 2 | [62] | ||
| Synonyms |
586379-66-0; PH797804; PH 797804; 3-(3-Bromo-4-((2,4-difluorobenzyl)oxy)-6-methyl-2-oxopyridin-1(2H)-yl)-N,4-dimethylbenzamide; UNII-SI09I1V827; UNII-GEL7GRJ3R6; GEL7GRJ3R6; CHEMBL1088751; CHEBI:82715; SI09I1V827; 3-{3-Bromo-4-[(2,4-Difluorobenzyl)oxy]-6-Methyl-2-Oxopyridin-1(2h)-Yl}-N,4-Dimethylbenzamide; PHA-797804; 1358027-80-1; 3-Bromo-4-((2,4-difluorobenzyl)oxy)-1-(5-((methylamino)carbonyl)-2-methylphenyl)-6-methylpyridin-2(1H)-one; 3hll; KCAJXIDMCNPGHZ-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| External Link | ||||
| LAS 100977 | Phase 2 | [63] | ||
| Synonyms |
LABA
Click to Show/Hide
|
|||
| External Link | ||||
| PF-3635659 | Phase 2 | [64] | ||
| Synonyms |
PF-03635659
Click to Show/Hide
|
|||
| External Link | ||||
| Lancovutide | Phase 2 | [65] | ||
| Synonyms |
Duramycin; Cystic fibrosis treatment, Molichem; Moli-1901; Moli-901; Duramycin(ophthalmic, dry eye), Lantibio; Lancovutide (ophthalmic, dry eye), Lantibio; Duramycin (inhaled, cystic fibrosis), Lantibio/AOP Orphan; Duramycin (inhaled, cystic fibrosis), Molichem/AOP Orphan; Lancovutide (inhaled, cystic fibrosis), Lantibio/AOP Orphan; Moli-1901 (ophthalmic, dry eye), Lantibio
Click to Show/Hide
|
|||
| External Link | ||||
| PS-938285 | Phase 2 | [66] | ||
| External Link | ||||
| GSK961081 | Phase 2 | [67] | ||
| Synonyms |
CHEMBL1683934; SCHEMBL524583; NVEMUJANQDPDSC-DHUJRADRSA-N; BDBM50337878; Biphenyl-2-ylcarbamic acid 1-{9-[(R)-2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydroquinolin-5-yl)ethylamino]nonyl}piperidin-4-yl ester; (R)-1-(9-(2-hydroxy-2-(8-hydroxy-2-oxo-1,2-dihydroquinolin-5-yl)ethylamino)nonyl)piperidin-4-yl biphenyl-2-ylcarbamate
Click to Show/Hide
|
|||
| External Link | ||||
| Haemophilus recombinant vaccine | Phase 2 | [68] | ||
| External Link | ||||
| Carmoterol | Phase 2 | [69] | ||
| Synonyms |
147568-66-9; 8-Hydroxy-5-((R)-1-hydroxy-2-(((R)-1-(4-methoxyphenyl)propan-2-yl)amino)ethyl)quinolin-2(1H)-one; UNII-9810NUL4D1; CHEMBL1094785; 9810NUL4D1; 2(1h)-quinolinone, 8-hydroxy-5-[(1r)-1-hydroxy-2-[[(1r)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]-; 8-hydroxy-5-[(1R)-1-hydroxy-2-{[(2R)-1-(4-methoxyphenyl)propan-2-yl]amino}ethyl]-1,2-dihydroquinolin-2-one; Carmoterol [INN]; 8-hydroxy-5-[(1R)-1-hydroxy-2-[[(2R)-1-(4-methoxyphenyl)propan-2-yl]amino]ethyl]-1H-quinolin-2-one; AC1Q6MT1
Click to Show/Hide
|
|||
| External Link | ||||
| LIRIMILAST | Phase 2 | [70] | ||
| Synonyms |
Methanesulfonic acid 2-(2,4-dichlorophenylcarbonyl)-3-ureidobenzofuran-6-ylester
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-2423 | Phase 2 | [71] | ||
| Synonyms |
CCR2b antagonist (pain, COPD), AstraZeneca
Click to Show/Hide
|
|||
| External Link | ||||
| UK-432097 | Phase 2 | [72] | ||
| Synonyms |
Adenosine A2a agonist (asthma), Pfizer
Click to Show/Hide
|
|||
| External Link | ||||
| AZD9056 | Phase 2 | [73] | ||
| Synonyms |
AZD-9056; 345304-65-6; UNII-F13K378W4L; N-(1-adamantylmethyl)-2-chloro-5-[3-(3-hydroxypropylamino)propyl]benzamide; F13K378W4L; AZD 9056; GTPL7826; SCHEMBL4126642; CHEMBL3545108; HSQAARMBHJCUOK-UHFFFAOYSA-N; MolPort-044-723-510; KS-000000WO; BCP25185; ZINC34356159; AKOS030228502; DB12594; Benzamide, 2-chloro-5-(3-((3-hydroxypropyl)amino)propyl)-N-(tricyclo(3.3.1.13,7)dec-1-ylmethyl)-; 2-Chloro-5-[3-[(3-hydroxypropyl)amino]propyl]-N-(tricyclo[3.3.1.13,7]dec-1-ylmethyl)-benzamide
Click to Show/Hide
|
|||
| External Link | ||||
| BIO-11006 | Phase 2 | [74] | ||
| External Link | ||||
| PF-03715455 | Phase 2 | [75] | ||
| Synonyms |
UNII-0TJ631J0KP; 1056164-52-3; 0TJ631J0KP; CHEMBL1938400; PF-3715455; 2yis; SCHEMBL981777; GTPL8179; DTXSID70147214; BDBM50361467; SB16724; DB12138
Click to Show/Hide
|
|||
| External Link | ||||
| IC-485 | Phase 2 | [33] | ||
| External Link | ||||
| TOFIMILAST | Phase 2 | [76] | ||
| Synonyms |
CP-325366; Tofimilast < Prop INN; 9-Cyclopentyl-7-ethyl-3-(2-thienyl)-5,6-dihydro-9H-pyrazolo[3,4-c][1,2,4]triazolo[4,3-a]pyridine
Click to Show/Hide
|
|||
| External Link | ||||
| ODSH | Phase 2 | [77] | ||
| Synonyms |
Cardiac ischemia reperfusion injury therapy, ParinGenix; ODSH (iv); PGX-100; PGX-200; ODSH (inhaled formulation), ParinGenix; ODSH (iv), ParinGenix; 2-O, 3-O desulfated heparin (inhaled), ParinGenix; 2-O, 3-O heparin (intravenous formulation), ParinGenix
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-8683 | Phase 2 | [78] | ||
| External Link | ||||
| AER-002 | Phase 2 | [79] | ||
| Synonyms |
Aerolytic; Bikunin; Pulmolytic; Bikunin, Aerovance; Bikunin, Bayer
Click to Show/Hide
|
|||
| External Link | ||||
| QBM076 | Phase 2 | [80] | ||
| External Link | ||||
| GSK233705 | Phase 2 | [32] | ||
| External Link | ||||
| AZD-9164 | Phase 2 | [81] | ||
| Synonyms |
IDDBCP239717
Click to Show/Hide
|
|||
| External Link | ||||
| QAX-028 | Phase 2 | [82] | ||
| Synonyms |
Bronchodilatory agent (inhaled, COPD), Novartis
Click to Show/Hide
|
|||
| External Link | ||||
| AZD7624 | Phase 2 | [83] | ||
| External Link | ||||
| GSK2245840 | Phase 2 | [84] | ||
| Synonyms |
Gepirone hydrochloride; Gepirone HCl; UNII-80C9L8EP6V; Gepirone hydrochloride [USAN]; 80C9L8EP6V; 83928-66-9; CHEMBL1204187; Gepirone hydrochloride (USAN); BMY 138951; AC1Q3ELB; AC1L1IK3; SCHEMBL318838; DTXSID30232812; AOB5299; 83928-76-1 (Parent); ORG-33062; SB19633; BMY-13805-1; BMY 13805-1; 3,3-Dimethyl-1-(4-(4-(2-pyrimidinyl)-1-piperazinyl)butyl)glutarimide monohydrochloride; D04314; 4,4-dimethyl-1-[4-(4-pyrimidin-2-ylpiperazin-1-yl)butyl]piperidine-2,6-dione hydrochloride; 2,6-Piperidinedione,
Click to Show/Hide
|
|||
| External Link | ||||
| THRX-198321 | Phase 2 | [85] | ||
| Synonyms |
Beta 2 adrenoreceptor agonist/muscarinic receptor antagonist (asthma), Theravance
Click to Show/Hide
|
|||
| External Link | ||||
| TRN-157 | Phase 2 | [86] | ||
| Synonyms |
LAMA antagonist (inhalant, COPD/asthma), Theron Pharmaceuticals; Long acting muscarinic M3 antagonist (inhalant, COPD/asthma), Theron Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| AZD9668 | Phase 2 | [87] | ||
| Synonyms |
Alvelestat; 848141-11-7; Alvelestat (AZD9668); AZD 9668; CHEMBL3617964; AZD-9668; KB-105160; Avelestat; 6-Methyl-5-(1-methyl-1H-pyrazol-5-yl)-N-{[5-(methylsulfonyl)pyridin-2-yl]methyl}-2-oxo-1-[3-(trifluoromethyl)phenyl]-1,2-dihydropyridine-3-carboxamide; 6-methyl-5-(1-methyl-1H-pyrazol-5-yl)-N-((5-(methylsulfonyl)pyridin-2-yl)methyl)-2-oxo-1-(3-(trifluoromethyl)phenyl)-1,2-dihydropyridine-3-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| SB-265610 | Phase 2 | [33] | ||
| Synonyms |
SB 265610; 211096-49-0; CHEMBL38182; UNII-9P785F0579; N-(2-BROMOPHENYL)-N'-(7-CYANO-1H-BENZOTRIAZOL-4-YL)UREA; 9P785F0579; 1-(2-Bromophenyl)-3-(4-cyano-1H-benzo[d][1,2,3]triazol-7-yl)urea; sb265610; SCHEMBL1535925; CTK8E9340; DTXSID10175339; MolPort-019-939-274; MolPort-044-561-465; ZINC603064; BCP28242; BDBM50102222; AKOS024457259; NCGC00242491-01; NCGC00242491-02; KB-80496; RT-015648; SB265610, > J-013849; 1-(2-Bromo-phenyl)-3-(7-cyano-3H-benzotriazol-4-yl)-urea; Urea, N-(2-bromophenyl)-N'-(4-cyano-1H-be
Click to Show/Hide
|
|||
| External Link | ||||
| AQX-1125 | Phase 2 | [88] | ||
| Synonyms |
AQX-108; AQX-131; AQX-132; AQX-133; AQX-134; AQX-135; AQX-140; AQX-150; SHIP agonists (cancer/inflammation), Aquinox
Click to Show/Hide
|
|||
| External Link | ||||
| JNJ-10311795 | Phase 2 | [89] | ||
| Synonyms |
CHEMBL374027; 518062-14-1; SCHEMBL1260969; GTPL6563; BDBM50208224; Phosphonic acid, (2-(3-((methyl(1-(2-naphthalenylcarbonyl)-4-piperidinyl)amino)carbonyl)-2-naphthalenyl)-1-(1-naphthalenyl)-2-oxoethyl)-; Phosphonic acid, P-(2-(3-((methyl(1-(2-naphthalenylcarbonyl)-4-piperidinyl)amino)carbonyl)-2-naphthalenyl)-1-(1-naphthalenyl)-2-oxoethyl)-; [2-[3-[methyl-[1-(naphthalene-2-carbonyl)piperidin-4-yl]carbamoyl]naphthalen-2-yl]-1-naphthalen-1-yl-2-oxoethyl]phosphonic acid
Click to Show/Hide
|
|||
| External Link | ||||
| SCH-527123 | Phase 2 | [90] | ||
| Synonyms |
Navarixin; 473727-83-2; Sch527123; SCH 527123; (R)-2-Hydroxy-N,N-dimethyl-3-((2-((1-(5-methylfuran-2-yl)propyl)amino)-3,4-dioxocyclobut-1-en-1-yl)amino)benzamide; MK-7123; CHEMBL216981; (R)-2-hydroxy-N,N-dimethyl-3-(2-(1-(5-methylfuran-2-yl)propylamino)-3,4-dioxocyclobut-1-enylamino)benzamide; SCHEMBL184744; GTPL8497; KS-00001CQK; CTK8B8735; MolPort-023-331-228; BDBM50200880; MK7123; ANW-61143; ZINC100033051; AKOS016003539; CS-0609; NCGC00390675-01; HY-10198; AX8217127; TC-149888; W-5650; SCH 527123,CAS:473727-83-2; PF-00547659
Click to Show/Hide
|
|||
| External Link | ||||
| O-desulfated heparin | Phase 2 | [91] | ||
| Synonyms |
Intravenous
Click to Show/Hide
|
|||
| External Link | ||||
| Zofin | Phase 1/2 | [92] | ||
| External Link | ||||
| CCI 15106 | Phase 1 | [93] | ||
| External Link | ||||
| PUR1800 | Phase 1 | [94] | ||
| External Link | ||||
| BI 1323495 | Phase 1 | [95] | ||
| External Link | ||||
| GSK3923868 | Phase 1 | [96] | ||
| External Link | ||||
| CHF 5407 | Phase 1 | [32] | ||
| External Link | ||||
| AZD-9819 | Phase 1 | [97] | ||
| Synonyms |
Neutrophil elastase inhibitor (inhaled, COPD), AstraZeneca
Click to Show/Hide
|
|||
| External Link | ||||
| RG-7103 | Phase 1 | [98] | ||
| Synonyms |
R-7103; RO-5024118; VPAC2 agonist (COPD), Roche
Click to Show/Hide
|
|||
| External Link | ||||
| AZD4721 | Phase 1 | [99] | ||
| External Link | ||||
| Ronomilast | Phase 1 | [100] | ||
| Synonyms |
ELB-353; PDE4 inhibitor, BioTie; PDE4 inhibitor, elbion; AWD-12-353
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-5122 | Phase 1 | [101] | ||
| External Link | ||||
| Dexpirronium | Phase 1 | [102] | ||
| Synonyms |
Anticholinergic compound (COPD), Meda
Click to Show/Hide
|
|||
| External Link | ||||
| MEDI7814 | Phase 1 | [103] | ||
| External Link | ||||
| AZD-2551 | Phase 1 | [104] | ||
| External Link | ||||
| BI-137882 | Phase 1 | [105] | ||
| External Link | ||||
| GSK2256294 | Phase 1 | [106] | ||
| Synonyms |
GSK-2256294
Click to Show/Hide
|
|||
| External Link | ||||
| PUR0200 | Phase 1 | [107] | ||
| External Link | ||||
| MEDI-2338 | Phase 1 | [108] | ||
| External Link | ||||
| QAK-423 | Phase 1 | [109] | ||
| External Link | ||||
| POL-6014 | Investigative | [110] | ||
| Synonyms |
Serine protease inhibitors (inhaled, PEM, asthma/COPD), Polyphor
Click to Show/Hide
|
|||
| External Link | ||||
| Quinazoline derivative 12 | Patented | [111] | ||
| Synonyms |
PMID26936077-Compound-23
Click to Show/Hide
|
|||
| External Link | ||||
| Quinazoline derivative 10 | Patented | [111] | ||
| Synonyms |
PMID26936077-Compound-21
Click to Show/Hide
|
|||
| External Link | ||||
| Quinazoline derivative 11 | Patented | [111] | ||
| Synonyms |
PMID26936077-Compound-22
Click to Show/Hide
|
|||
| External Link | ||||
| PMID27998201-Compound-5 | Patented | [112] | ||
| External Link | ||||
| Telenzepine | Discontinued in Preregistration | [113] | ||
| Synonyms |
Telenzepine [INN]; Telenzepinum [Latin]; Telenzepino [Spanish]; 80880-90-6; UNII-0990EG3K10; 0990EG3K10; NCGC00015987-05; DSSTox_RID_80752; DSSTox_CID_25209; 1-methyl-10-[2-(4-methylpiperazin-1-yl)acetyl]-5H-thieno[3,4-b][1,5]benzodiazepin-4-one; DSSTox_GSID_45209; 4,9-Dihydro-3-methyl-4-((4-methyl-1-piperazinyl)acetyl)-10H-thieno(3,4-b)(1,5)benzodiazepin-10-one; Telenzepine hydrochloride; Telenzepine dihydrochloide; C19H22N4O2S; Telenzepino; Telenzepinum; Telenzepine dihydrochloride hydrate; BY-802; [3H](+)telenzepine
Click to Show/Hide
|
|||
| External Link | ||||
| Levcromakalim | Discontinued in Preregistration | [114] | ||
| Synonyms |
Lemakalim; Cromakalim; (-)-Cromakalim; 94535-50-9; Levcromakelim; BRL 38227; BRL-38227; UNII-RW7PN4BLDJ; RW7PN4BLDJ; CHEMBL100; BRN 3622889; MLS000069770; CHEBI:6436; Cromakalime [French]; Cromakalimum [Latin]; Cromakalim, (3S-trans)-Isomer; SMR000058880; (3S,4R)-3-Hydroxy-2,2-dimethyl-4-(2-oxo-1-pyrrolidinyl)-6-chromancarbonitrile; DSSTox_RID_81051; DSSTox_CID_25677; DSSTox_GSID_45677; 94470-67-4; Cromakalimum; Cromakalime; BRL-34915; BRL 34915; 2H-1-Benzopyran-6-carbonitrile, 3,4-dihydro-3-hydroxy-2,2-dimethyl-4-(2-o
Click to Show/Hide
|
|||
| External Link | ||||
| Sibenadet | Discontinued in Phase 3 | [115] | ||
| Synonyms |
UNII-N32934RHGW; 154189-40-9; CHEMBL82663; N32934RHGW; Sibenadet [INN:BAN]; AC1L4DOB; SCHEMBL48983; DTXSID50165552; AR-C 68397XX; ZINC36268680; ARC-68397; BDBM50128690; AR-C 68397; 4-Hydroxy-7-(2-((2-((3-(2-phenylethoxy)propyl)sulfonyl)ethyl)amino)ethyl)-1,3-benzothiazol-2(3H)-one; SB-07499; 2(3H)-Benzothiazolone, 4-hydroxy-7-(2-((2-((3-(2-phenylethoxy)propyl)sulfonyl)ethyl)amino)ethyl)-; FT-0674579; L001485; 4-Hydroxy-7-{2-[2-(3-phenethyloxy-propane-1-sulfonyl)-ethylamino]-ethyl}-3H-benzothiazol-2-one
Click to Show/Hide
|
|||
| External Link | ||||
| Cilomilast | Discontinued in Phase 3 | [116] | ||
| Synonyms |
Ariflo; CIO; SB 207499; SB207499; Ariflo (TN); Cilomilast [USAN:INN]; SB-207499; Ariflo, SB-207499,Cilomilast; Cilomilast (JAN/USAN/INN); CIS-4-CYANO-4-[3-(CYCLOPENTYLOXY)-4-METHOXYPHENYL]CYCLOHEXANECARBOXYLIC ACID; Cis-4-Cyano-4-(3-cyclopentyloxy-4-methoxyphenyl)cyclohexanecarboxylic acid; Cis-4-Cyano-4-(3-(cyclopentyloxy)-4-methoxyphenyl)cyclohexanecarboxylic acid; Cis-4-(3-(Cyclopentyloxy)-4-methoxyphenyl)-4-cyanocyclohexane-1-carboxylic acid; 4-cyano-4-(3-cyclopentoxy-4-methoxy-phenyl)-cyclohexane-1-carboxylic acid; 4-cyano-4-(3-cyclopentyloxy-4-methoxyphenyl)cyclohexane-1-carboxylic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Rispenzepine | Discontinued in Phase 2 | [117] | ||
| Synonyms |
Nuvenzepine; DF-545
Click to Show/Hide
|
|||
| External Link | ||||
| CDP840 | Discontinued in Phase 2 | [118] | ||
| Synonyms |
Cdp-840; Cdp 840; CHEMBL32442; 4-(2-(3-(Cyclopentyloxy)-4-methoxyphenyl)-2-phenylethyl)pyridine; (R)-4-(2-(3-(Cyclopentyloxy)-4-methoxyphenyl)-2-phenylethyl)pyridine; 4-[(2R)-2-(3-cyclopentyloxy-4-methoxyphenyl)-2-phenylethyl]pyridine; Pyridine, 4-(2-(3-(cyclopentyloxy)-4-methoxyphenyl)-2-phenylethyl)-, (R)-; 162542-90-7; AC1L2SW5; SCHEMBL84490; 4-[2-(3-Cyclopentyloxy-4-methoxy-phenyl)-2-phenyl-ethyl]-pyridine; GTPL9330; UTUUPXBCDMQYRR-HSZRJFAPSA-N; ZINC599006; PDSP2_001272; BDBM50064858; PDSP1_001288
Click to Show/Hide
|
|||
| External Link | ||||
| CE-1037 | Discontinued in Phase 2 | [119] | ||
| Synonyms |
SPWHS19WTL; UNII-SPWHS19WTL; CHEMBL56605; Mdl 201404YA; Mdl 201,404YA; CE 1037; 150493-09-7; ce1037; SCHEMBL8974527; BDBM50058491; Bis(4-(2'-(carboxy-2'-methylpropylsulfonyl)phenyl)2,2'-(1,4-phenylene))diisobutyrate; 3-{4-[2-(4-{1-[4-(2-Carboxy-2-methyl-propane-1-sulfonyl)-phenoxycarbonyl]-1-methyl-ethyl}-phenyl)-2-methyl-propionyloxy]-benzenesulfonyl}-2,2-dimethyl-propionic acid(CE-1037)
Click to Show/Hide
|
|||
| External Link | ||||
| MLN-977 | Discontinued in Phase 2 | [120] | ||
| Synonyms |
CHEMBL73148; CMI977; LDP-977; SCHEMBL16932371; LDP 977; CMI 977; HY-U00260; BDBM50144621; CS-7474; 175212-04-1; UNII-VNR0T3Q498 component YANONWCPCKIWEC-CABCVRRESA-N; N-(4-{(2S,5S)-5-[(4-fluorophenoxy)methyl]tetrahydrofuran-2-yl}but-3-ynyl)-N-hydroxyurea; Urea, N-(4-((2S,5S)-5-((4-fluorophenoxy)methyl)tetrahydro-2-furanyl)-3-butyn-1-yl)-N-hydroxy-
Click to Show/Hide
|
|||
| External Link | ||||
| GSK-159797 | Discontinued in Phase 2 | [33] | ||
| Synonyms |
Milveterol hydrochloride; UNII-1D1MD355SJ; 1D1MD355SJ; 804518-03-4; Milveterol hydrochloride [USAN]; Milveterol hydrochloride (USAN); GSK159797C; Milveterol HCl; SCHEMBL2014099; CHEMBL2107356; DTXSID50230335; QQPHRRSYJMOQOC-DKIIUIKKSA-N; D08945; N-{2-[4-((R)-2-hydroxy-2-phenylethylamino)phenyl]ethyl}-(R)-2-hydroxy-2-(3-formamido-4-hydroxyphenyl)ethylamine; 2-5A-anti-hTR
Click to Show/Hide
|
|||
| External Link | ||||
| Filaminast | Discontinued in Phase 2 | [121] | ||
| Synonyms |
Way-pda-641; UNII-CDD69JC61J; 141184-34-1; CDD69JC61J; (1E)-1-[3-(CYCLOPENTYLOXY)-4-METHOXYPHENYL]ETHANONE O-(AMINOCARBONYL)OXIME; FIL; Filaminast [USAN:INN]; Filaminast (USAN/INN); SCHEMBL73844; SCHEMBL73843; CHEMBL590754; BDBM14771; 1-(3-(Cyclopentyloxy)-4-methoxyphenyl)ethanone-(E)-O-(aminocarbonyl)oxime; DB02660; D04185; 3'-(Cyclopentyloxy)-4'-methoxyacetophenone (E)-O-carbamoyloxime; [1-(3-cyclopentyloxy-4-methoxy-phenyl)ethylideneamino] carbamate; (E)-{1-[3-(cyclopentyloxy)-4-methoxyphenyl]ethylidene}amino
Click to Show/Hide
|
|||
| External Link | ||||
| BRL-55834 | Discontinued in Phase 2 | [122] | ||
| Synonyms |
Brl 55834; 131899-25-7; brl55834; SCHEMBL6369898; AC1L2Z70; DTXSID80157243; 3,4-Dihydro-2,2-dimethyl-4-(oxopiperidin-1-yl)-6-pentafluoroethyl-2H-1-benzopyran-3-ol; 2-Piperidinone, 1-(3,4-dihydro-3-hydroxy-2,2-dimethyl-6-(pentafluoroethyl)-2H-1-benzopyran-4-yl)-, (3S-trans)-
Click to Show/Hide
|
|||
| External Link | ||||
| WC-3027 | Discontinued in Phase 2 | [123] | ||
| Synonyms |
SEGRA, Warner Chilcott; SEGRA, Warner Chilcott/Schering AG; Selective glucocorticoid receptor agonist (dermatitis), Warner Chilcott; Selective glucocorticoid receptor agonist (dermatitis), Warner Chilcott/Schering AG
Click to Show/Hide
|
|||
| External Link | ||||
| AZD1236 | Discontinued in Phase 2 | [124] | ||
| Synonyms |
(S)-5-(((4-((5-Chloropyridin-2-yl)oxy)piperidin-1-yl)sulfonyl)methyl)-5-methylimidazolidine-2,4-dione; 459814-90-5; AZD-1236; UNII-B4OQY51WZS; B4OQY51WZS; (S)-5-(((4-((5-Chloropyridin-2-yl)oxy)piperidin-1-yl)-sulfonyl)methyl)-5-methylimidazolidine-2,4-dione; SCHEMBL942315; GTPL7844; DTXSID30647184; SFJFBTPHDHUUPU-OAHLLOKOSA-N; AZD 1236; 6326AB; ZINC59688588; AKOS016011525; DB11961; AX8246058; KB-211575; AJ-113592; Piperidine, 4-((5-chloro-2-pyridinyl)oxy)-1-((((4S)-4-methyl-2,5-dioxo-4-imidazolidinyl)methyl)sulfonyl)
Click to Show/Hide
|
|||
| External Link | ||||
| PF-610355 | Discontinued in Phase 2 | [125] | ||
| Synonyms |
PF-00610355; Beta 2 adrenoceptor agonist (asthma), Pfizer
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-5423 | Discontinued in Phase 2 | [126] | ||
| Synonyms |
1034148-04-3; UNII-641H0Q518W; 641H0Q518W; 2,2,2-trifluoro-N-[(1R,2S)-1-[1-(4-fluorophenyl)indazol-5-yl]oxy-1-(3-methoxyphenyl)propan-2-yl]acetamide; 2,2,2-Tris(Fluoranyl)-~{n}-[(1~{r},2~{s})-1-[1-(4-Fluorophenyl)indazol-5-Yl]oxy-1-(3-Methoxyphenyl)propan-2-Yl]ethanamide; Acetamide, 2,2,2-trifluoro-N-[(1S,2R)-2-[[1-(4-fluorophenyl)-1H-indazol-5-yl]oxy]-2-(3-methoxyphenyl)-1-methylethyl]-;Acetamide, 2,2,2-trifluoro-N-[(1S,2R)-2-[[1-(4-fluorophenyl)-1H-indazol-5-yl]oxy]-2-(3-methoxyphenyl)-1-methylethyl]-
Click to Show/Hide
|
|||
| External Link | ||||
| EPI-12323 | Discontinued in Phase 2 | [127] | ||
| External Link | ||||
| Tolafentrine | Discontinued in Phase 2 | [128] | ||
| Synonyms |
BY-4070
Click to Show/Hide
|
|||
| External Link | ||||
| Milveterol+Fluticasone | Discontinued in Phase 2 | [129] | ||
| Synonyms |
S-(fluoromethyl) (6S,9R,10S,11S,13S,14S,16R,17R)-6,9-difluoro-11,17-dihydroxy-10,13,16-trimethyl-3-oxo-6,7,8,11,12,14,15,16-octahydrocyclopenta[a]phenanthrene-17-carbothioate; 90566-53-3; S-(fluoromethyl) (6a,8x,11b,16a,17a)-6,9-difluoro-11,17-dihydroxy-16-methyl-3-oxoandrosta-1,4-diene-17-carbothioate; AC1Q68ZV; AC1L1Y1V; AN-15808; s-(fluoromethyl)(6; A,8; I,11
Click to Show/Hide
|
|||
| External Link | ||||
| CS-003 | Discontinued in Phase 2 | [33] | ||
| External Link | ||||
| Darotropium | Discontinued in Phase 2 | [130] | ||
| External Link | ||||
| KCO-912 | Discontinued in Phase 2 | [131] | ||
| External Link | ||||
| AZD4818 | Discontinued in Phase 2 | [132] | ||
| Synonyms |
18-HYDROXYASCOMYCIN; AC1L9IBR; SCHEMBL14434002
Click to Show/Hide
|
|||
| External Link | ||||
| AZD5904 | Discontinued in Phase 1 | [133] | ||
| Synonyms |
UNII-62A9CG81VN; 62A9CG81VN; AZD-5904; 3-[[(2R)-oxolan-2-yl]methyl]-2-sulfanylidene-7H-purin-6-one; 3-(((2R)-Oxolan-2-yl)methyl)-2-sulfanylidene-7H-purin-6-one; 2-Thioxanthine, TX4; SCHEMBL2288062; GTPL7728; TX-4; BDBM92469; AZD 5904; 618913-30-7; HY-111341; CS-0035112; 6H-Purin-6-one, 1,2,3,9-tetrahydro-3-(((2R)-tetrahydro-2-furanyl)methyl)-2-thioxo-; (R)-3-((Tetrahydrofuran-2-yl)methyl)-2-thioxo-1,2,3,7-tetrahydro-6H-purin-6-one; 6H-Purin-6-one, 1,2,3,7-tetrahydro-3-(((2R)-tetrahydro-2-furanyl)methyl)-2-thioxo-
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-6553 | Discontinued in Phase 1 | [134] | ||
| Synonyms |
Neutrophil elastase inhibitor (oral, COPD), AstraZeneca/Quintiles
Click to Show/Hide
|
|||
| External Link | ||||
| ZD-0892 | Discontinued in Phase 1 | [135] | ||
| Synonyms |
CHEMBL55210; SCHEMBL7363455; CTK4D4091; BDBM50058391; HY-19254; 171964-73-1; CS-0014993; L-Prolinamide,N-(4-methoxybenzoyl)-L-valyl-N-[(1S)-3,3,3-trifluoro-1-(1-methylethyl)-2-oxopropyl]-; (S)-1-[(S)-2-(4-Methoxy-benzoylamino)-3-methyl-butyryl]-pyrrolidine-2-carboxylic acid
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-7140 | Discontinued in Phase 1 | [136] | ||
| External Link | ||||
| AZD-4407 | Discontinued in Phase 1 | [137] | ||
| Synonyms |
ZD-4407
Click to Show/Hide
|
|||
| External Link | ||||
| QAN-747 | Discontinued in Phase 1 | [138] | ||
| External Link | ||||
| Revatropate | Discontinued in Phase 1 | [33] | ||
| Synonyms |
UK-112166; UK-112,166; UK-112166-04; [(3R)-1-azabicyclo[2.2.2]octan-3-yl] (2R)-2-(hydroxymethyl)-4-methylsulfinyl-2-phenylbutanoate; [(3R)-1-azabicyclo[2.2.2]octan-3-yl] (2S)-2-(hydroxymethyl)-4-methylsulfinyl-2-phenylbutanoate; (3R-(3R*(S*(R*))))-1-Azabicyclo(2.2.2)oct-3-yl alpha-(hydroxymethyl)-alpha-(2-(methylsulfinyl)ethyl)benzenacetate
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-2315 | Discontinued in Phase 1 | [139] | ||
| Synonyms |
ZD-2315
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-8309 | Discontinued in Phase 1 | [140] | ||
| External Link | ||||
| AZD8075 | Discontinued in Phase 1 | [141] | ||
| External Link | ||||
| AZD8566 | Discontinued in Phase 1 | [142] | ||
| External Link | ||||
| AE-3763 | Discontinued in Phase 1 | [143] | ||
| External Link | ||||
| FR167653 | Discontinued in Phase 1 | [144] | ||
| Synonyms |
HMPQTEPEMQZWQH-ROUUACIJSA-N; 2-(5-AMINO-6-OXO-2-PHENYL-6H-PYRIMIDIN-1-YL)-N-[2-(5-TERT-BUTYL-1,3,4-OXADIAZOL-2-YL)-1-(METHYLETHYL)-2-HYDROXYETHYL]ACETAMIDE; AC1NR9VL; SCHEMBL15034365; 2-(5-amino-6-oxo-2-phenylpyrimidin-1-yl)-N-[(1S,2S)-1-(5-tert-butyl-1,3,4-oxadiazol-2-yl)-1-hydroxy-3-methylbutan-2-yl]acetamide; ONO-6818
Click to Show/Hide
|
|||
| External Link | ||||
| SB-332235 | Discontinued in Phase 1 | [145] | ||
| Synonyms |
1-(4-Chloro-2-hydroxy-3-sulfamoyl-phenyl)-3-(2,3-dichlorophenyl)urea; 276702-15-9; SB 332235; UNII-5HLP8UVL8M; 5HLP8UVL8M; CHEMBL3819292; WTLRWOHEKQGKDS-UHFFFAOYSA-N; SCHEMBL1535901; sb332235; MolPort-042-624-550; ZINC3990011; BDBM50182254; AKOS027470251; N-(4-Chloro-2hydroxy-3-aminosulfonylphenyl)-N'-(2,3-dichlorophenyl) urea; n-(4-chloro-2-hydroxy-3-aminosulfonylphenyl)-n'-(2,3-dichlorophenyl)urea; N-(4-Chloro-2-hydroxy-3-aminosulfonylphenyl)-N'-(2,3-dichlorophenyl) Urea; N-(3-(aminosulfonyl)-4-chloro-2-hydroxyphenyl)-N'-(2,3-dichlorophenyl) urea
Click to Show/Hide
|
|||
| External Link | ||||
| AZD5985 | Discontinued in Phase 1 | [146] | ||
| External Link | ||||
| Rilmakalim | Discontinued in Phase 1 | [147] | ||
| Synonyms |
Hoe-234
Click to Show/Hide
|
|||
| External Link | ||||
| SCH-351591 | Discontinued in Phase 1 | [148] | ||
| Synonyms |
SCH 351591; UNII-G6EF7V146S; CHEMBL250546; G6EF7V146S; CHEBI:77774; N-(3,5-Dichloro-1-oxido-4-pyridinyl)-8-methoxy-2-(trifluoromethyl)-5-quinoline carboxamide; 444659-43-2; N-(3,5-dichloro-1-oxidopyridin-4-yl)-8-methoxy-2-(trifluoromethyl)quinoline-5-carboxamide; SCH351591; SCHEMBL157018; DTXSID8044125; NOCAS_44125; BDBM50219270; D 4396; 8-methoxy-2-trifluoromethyl-quinoline-5-carboxylic acid (3,5-dichloro-1-oxy-pyridin-4-yl)-amide; 5-Quinolinecarboxamide, N-(3,5-dichloro-1-oxido-4-pyridinyl)-8-methoxy-2- (
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-3342 | Discontinued in Phase 1 | [149] | ||
| External Link | ||||
| RS-504393 | Preclinical | [150] | ||
| Synonyms |
300816-15-3; RS 504393; 6-Methyl-1'-(2-(5-methyl-2-phenyloxazol-4-yl)ethyl)spiro[benzo[d][1,3]oxazine-4,4'-piperidin]-2(1H)-one; RS504393; CHEMBL134074; 6-Methyl-1'-(2-(5-methyl-2-phenyloxazol-4-yl)ethyl)spiro-[benzo[d][1,3]oxazine-4,4'-piperidin]-2(1H)-one; 6-Methyl-1'-[2-(5-methyl-2-phenyl-4-oxazolyl)ethyl]spiro[4H-3,1-benzoxazine-4,4'-piperidin]-2(1H)-one; ACMC-20a25e; GTPL781; SCHEMBL9972645; CTK4G4374; CHEBI:93525; DTXSID20433290; MolPort-021-804-998; BCPP000086; HMS3269M19; BCP02713; ZINC13527116; ABP000463
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-7928 | Preclinical | [151] | ||
| External Link | ||||
| GW-3333 | Preclinical | [33] | ||
| Synonyms |
SMZPWUUYPYYHIV-HNJRGHQBSA-N; GW3333; CHEMBL514958; UNII-54J77T5T74; 54J77T5T74; 212609-68-2; AC1Q5NWC; AC1L50QR; BIDD:PXR0031; n2-[(2r,3s)-3-[formyl(hydroxy)amino]-4-methyl-2-(2-methylpropyl)pentanoyl]-n-pyridin-2-yl-l-isoleucinamide; SCHEMBL2875070; BDBM50247805; (2R,3S)-3-[formyl(hydroxy)amino]-4-methyl-N-[(2S,3S)-3-methyl-1-oxo-1-(pyridin-2-ylamino)pentan-2-yl]-2-(2-methylpropyl)pentanamide; (2R,3S)-3-(Formyl-hydroxyamino)-2-(2-methyl-1-propyl)-4-methylpentanoic acid, ((1S,2S)-2-methyl-1-(2-pyridylcarbamoyl)-1-butyl)amide; Gw 3333; 5-carboxymethylthio-3-(3'-chlorophenyl)-1,2,4-oxadiazol
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-0902 | Terminated | [152] | ||
| External Link | ||||
| AR-C-89855 | Terminated | [153] | ||
| Synonyms |
AR-C68164AA; AR-C68475AA; AR-C69457AA; AR-C89855AA
Click to Show/Hide
|
|||
| External Link | ||||
| ZARDAVERINE | Terminated | [154] | ||
| Synonyms |
101975-10-4; Zardaverina; Zardaverinum; Zardaverine [INN]; Zardaverinum [INN-Latin]; UNII-TQ358GWH6Y; Zardaverina [INN-Spanish]; 6-(4-DIFLUOROMETHOXY-3-METHOXY-PHENYL)-2H-PYRIDAZIN-3-ONE; C12H10F2N2O3; 6-(4-(Difluoromethoxy)-3-methoxyphenyl)-3(2H)-pyridazinone; TQ358GWH6Y; CHEMBL313842; CHEBI:46548; 6-[4-(difluoromethoxy)-3-methoxyphenyl]pyridazin-3(2H)-one; HJMQDJPMQIHLPB-UHFFFAOYSA-N; 6-(4-Difluoromethoxy-3-methoxyphenyl)-3(2H)-pyridazinone; NCGC00016106-04
Click to Show/Hide
|
|||
| External Link | ||||
| SSR-69071 | Terminated | [155] | ||
| Synonyms |
SSR 69071; 344930-95-6; SSR69071; DSSTox_RID_82301; DSSTox_CID_27368; DSSTox_GSID_47368; MLS006010264; SCHEMBL4295477; CHEMBL1354892; DTXSID1047368; CTK8E8740; CHEBI:93283; MolPort-023-276-460; HMS3269M07; ZINC1490807; Tox21_300247; AKOS024457139; NCGC00254231-01; NCGC00167751-02; NCGC00167751-01; NCGC00167751-03; SMR001822512; RT-015782; LS-191133; FT-0724044; CAS-344930-95-6; J-019626; BRD-K72895815-001-01-4
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-2914 | Terminated | [156] | ||
| External Link | ||||
| NIK-616 | Terminated | [157] | ||
| External Link | ||||
| AZD-0275 | Terminated | [158] | ||
| External Link | ||||
| AD-313 | Terminated | [159] | ||
| External Link | ||||
| ML-03 | Terminated | [160] | ||
| Synonyms |
Cystic fibrosis therapy, Milkhaus; HP-3; Respiratory therapeutics, Milkhaus
Click to Show/Hide
|
|||
| External Link | ||||
| PUP-1 | Investigative | [161] | ||
| Synonyms |
MMP-12 inhibitor (chronic obstryctive pulmonary disorder) Abiogen
Click to Show/Hide
|
|||
| External Link | ||||
| OT-010 | Investigative | [162] | ||
| Synonyms |
OT-010 (inhaled dry powder, asthma/COPD); OT-010 (inhaled dry powder, asthma/COPD), Oriel Therapeutics; OT-010 (inhaled dry powder, asthma/COPD), Sandoz
Click to Show/Hide
|
|||
| External Link | ||||
| TRN-101 | Investigative | [163] | ||
| Synonyms |
Dual ROCK1/ROCK2 inhibitor (inhalant, COPD), Theron Pharmaceuticals; Dual Rho kinase 1/ Rho kinase2 inhibitor (inhalant, COPD), Theron Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| PD-3766 | Investigative | [162] | ||
| Synonyms |
RAC GTPase inhibitor (COPD), P2D Biosciences; Ras related C3 botulinum substrate guanosine triphosphatase inhibitor (COPD), P2D Biosciences
Click to Show/Hide
|
|||
| External Link | ||||
| EP-102 | Investigative | [164] | ||
| External Link | ||||
| RBx-343E48F0 | Investigative | [165] | ||
| External Link | ||||
| ZP-003 | Investigative | [162] | ||
| External Link | ||||
| CHF-5480 | Investigative | [166] | ||
| Synonyms |
PDE 4 inhibitors (COPD/asthma); PDE 4 inhibitors (COPD/asthma), Chiesi
Click to Show/Hide
|
|||
| External Link | ||||
| ADC-7828 | Investigative | [110] | ||
| Synonyms |
Neutrophil elastase inhibitors (inhaled, CF/COPD); Neutrophil elastase inhibitor (inhaled, cystic fibrosis/chronic obstructive pulmonary disease), Argenta; Neutrophil elastase inhibitors (inhaled, CF/COPD), Argenta; Neutrophil elastase inhibitors (inhaled, CF/COPD), Pulmagen
Click to Show/Hide
|
|||
| External Link | ||||
| SOM-0525 | Investigative | [162] | ||
| External Link | ||||
| MBS-103 | Investigative | [162] | ||
| Synonyms |
Bismuth thiol based antibacterial agent (inhaled, pulmonary tuberculosis), Microbion; Bismuth thiol based agent (inhalant, pulmonary tuberculosis/cystic fibrosis/lung infection/COPD/pneumonia), Microbion
Click to Show/Hide
|
|||
| External Link | ||||
| ADS-0101 | Investigative | [167] | ||
| Synonyms |
ADS-0102; DOM-0101; DOM-0102; Domain antibody therapy (inhalant formulation, COPD/respiratory disease); Domain antibody therapy (inhalant formulation, COPD/respiratory disease), Domantis; DAbs (inhalant formulation, COPD/respiratory disorders), Domantis/Argenta
Click to Show/Hide
|
|||
| External Link | ||||
| DX-2300 | Investigative | [162] | ||
| Synonyms |
Kallikrein-1 inhibitors (asthma); Kallikrein-1 inhibitors (asthma), Dyax
Click to Show/Hide
|
|||
| External Link | ||||
| YPL-1101 | Investigative | [162] | ||
| External Link | ||||
| J-104135 | Investigative | [168] | ||
| Synonyms |
UNII-6137X5QNJF; 6137X5QNJF; J-104871; SCHEMBL15207668; SCHEMBL15207665; 1,3-Dioxolane-2,2,4-tricarboxylic acid, 5-((((1R,2R,4E)-2-(1,3-benzodioxol-5-yl)-5-(2-benzoxazolyl)-1-methyl-4-pentenyl)(2-naphthalenylmethyl)amino)carbonyl)-, (4R,5S)-; 1,3-Dioxolane-2,2,4-tricarboxylic acid, 5-((((1R,2R,4E)-2-(1,3-benzodioxol-5-yl)-5-(2-benzoxazolyl)-1-methyl-4-penten-1-yl)(2-naphthalenylmethyl)amino)carbonyl)-, (4R,5S)-; 191088-19-4; 1,3-Dioxolane-2,2,4-tricarboxylic acid, 5-(((2-(1,3-benzodioxol-5-yl)-5-(2-benzoxa
Click to Show/Hide
|
|||
| External Link | ||||
| SOM-1033 | Investigative | [162] | ||
| External Link | ||||
| PF-613322 | Investigative | [162] | ||
| Synonyms |
Beta 2 adrenoceptor agonists (COPD); UK-503590; Beta 2 adrenoceptor agonists (COPD),Pfizer
Click to Show/Hide
|
|||
| External Link | ||||
| MDT-011 | Investigative | [162] | ||
| External Link | ||||
| ZP-013 | Investigative | [162] | ||
| External Link | ||||
| PYM-60001 | Investigative | [162] | ||
| Synonyms |
TRPV1-modulators
Click to Show/Hide
|
|||
| External Link | ||||
| AMA-237 | Investigative | [163] | ||
| Synonyms |
AMA-63; ROCK inhibitors (COPD); ROCK inhibitors (COPD), Amakem
Click to Show/Hide
|
|||
| External Link | ||||
| CDP-146 | Investigative | [162] | ||
| Synonyms |
CT-8730; MAPK antagonists, Celltech; NG-1054; P38 MAP kinase inhibitor, Celltech; P38 kinase inhibitor, Celltech
Click to Show/Hide
|
|||
| External Link | ||||
| X-072-NAB | Investigative | [162] | ||
| Synonyms |
NATHMAB (COPD), XBiotech
Click to Show/Hide
|
|||
| External Link | ||||
| MMP-408 | Investigative | [161] | ||
| Synonyms |
MMP-118; MMP-145; MMP-12 inhibitors (COPD); MMP-12 inhibitors (COPD), Pfizer; MMP-12 inhibitors (COPD), Wyeth
Click to Show/Hide
|
|||
| External Link | ||||
| UR-5908 | Investigative | [162] | ||
| Synonyms |
COPD therapy, UBE Industries
Click to Show/Hide
|
|||
| External Link | ||||
References