m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT02021
|
[1] | |||
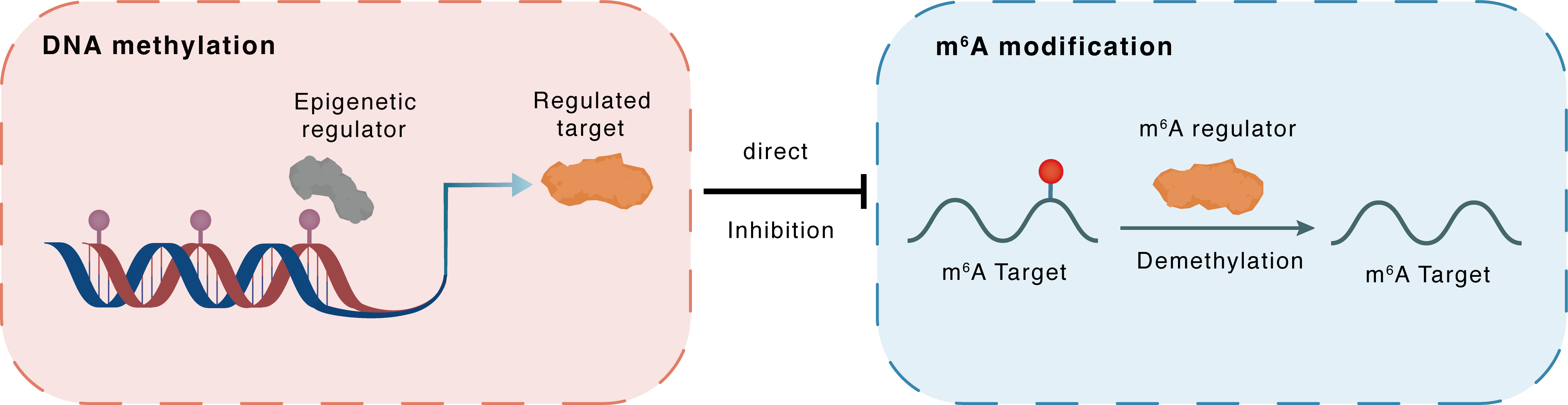 DNA methylation
DNMT3B
FTO
Direct
Inhibition
m6A modification
PPARA
PPARA
FTO
Demethylation
DNA methylation
DNMT3B
FTO
Direct
Inhibition
m6A modification
PPARA
PPARA
FTO
Demethylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | |||
| m6A Target | Peroxisome proliferator-activated receptor alpha (PPARalpha/PPARA) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | DNA methylation (DNAMeth) | ||||
| Epigenetic Regulator | DNA (cytosine-5)-methyltransferase 3B (DNMT3B) | WRITER | View Details | ||
| Regulated Target | FTO alpha-ketoglutarate dependent dioxygenase (FTO) | View Details | |||
| Crosstalk Relationship | DNA methylation → m6A | Inhibition | |||
| Crosstalk Mechanism | DNA methylation directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | Inhibition of DNA methyltransferases (DNMT1, DNMT3A, and DNMT3B) by 5-azacytidine (5-aza) reversed alcohol-induced kidney injury and decreased the mRNA and protein levels of FTO. Importantly, we found that FTO, the m6A demethylase, epigenetically modified Peroxisome proliferator-activated receptor alpha (PPARalpha/PPARA) in a YTH domain family 2 (YTHDF2)-dependent manner, which resulted in inflammation in alcoholic kidney injury models. In conclusion, our findings indicate that alcohol increases the methylation of PPAR-alpha m6A by FTO-mediated YTHDF2 epigenetic modification, which ultimately leads to the activation of NLRP3 inflammasomes and NF-kappaB-driven renal inflammation in the kidney. | ||||
| Responsed Disease | Renal ischemia-reperfusion injury | ICD-11: NB92.0Y | |||
| Responsed Drug | 5-azacytidine | ||||
| Pathway Response | RNA degradation | hsa03018 | |||
| Cell Process | mRNA stability | ||||
In-vitro Model |
HK2 | Normal | Acipenser baerii | CVCL_YE28 | |
| In-vivo Model | Male C57BL/6 mice (aged 6-8 weeks) were acclimated with the Lieber-DeCarli liquid diet control ad libitum for 1 week. Afterward, mice received either Lieber-DeCarli diet ethanol (6% vol/vol) or Lieber-DeCarli diet control (isocaloric maltose dextrin) for 4 and 8 weeks. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| DNA (cytosine-5)-methyltransferase 3B (DNMT3B) | 22 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Curcumin | Phase 3 | [2] | ||
| Synonyms |
458-37-7; Diferuloylmethane; Natural yellow 3; Turmeric yellow; Turmeric; Curcuma; Kacha haldi; Gelbwurz; Indian saffron; Curcumin I; Souchet; Halud; Halad; Haidr; Haldar; Merita earth; Yellow Ginger; Terra Merita; Yellow Root; Safran d'Inde; Yo-Kin; Golden seal; Curcuma oil; Orange Root; Oils, curcuma; CI Natural Yellow 3; Curcumine; Hydrastis; Indian turmeric; Yellow puccoon; Turmeric extract; Diferaloylmethane; Kurkumin [Czech]; (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione; Tumeric yellow; Turmeric oil
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27376512-Compound-MTC-424 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1940 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-427 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 295 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-422 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1430 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-423 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 363 nM | |||
| External Link | ||||
| NSC-622444 | Investigative | [2] | ||
| Synonyms |
NSC622444; CHEMBL116347; AC1Q3LXD; AC1L7GK5; SCHEMBL9755151; dichlorinated diacylmethane fragment; ZINC1616868; BDBM50048522; 5,3'-dicarboxy-4,4'-dihydrodiphenylmethane; 5,5''-methylenebis(3-chloro-2-hydroxybenzoic acid); 5,5'-Methylenebis(3-chloro-2-hydroxybenzoic acid); 3,3'-methanediylbis(5-chloro-6-hydroxybenzoic acid); 5-(3-carboxy-5-chloro-4-hydroxybenzyl)-3-chloro-2-hydroxybenzoic acid; 3',3-Dichloro-4',4-dimethoxy-5',5-bis(methoxycarbonyl)-1,1-diphenylmethane
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-138419 | Investigative | [2] | ||
| Synonyms |
NSC138419; n-[4-(methylamino)benzoyl]glutamic acid; AC1Q5SG8; AC1L5YW4; SCHEMBL5925511; CHEMBL591443; CTK1H0013; 2-[(4-methylaminobenzoyl)amino]pentanedioic acid; A816490; 2-[[4-(methylamino)benzoyl]amino]pentanedioic acid; 2-[[4-(methylamino)phenyl]carbonylamino]pentanedioic acid; 2-[[[4-(methylamino)phenyl]-oxomethyl]amino]pentanedioic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-319745 | Investigative | [2] | ||
| Synonyms |
61629-60-5; HB 093; BRN 2168571; 4-(2-((5-Chloro-2-methoxybenzoyl)amino)ethyl)hydrocinnamic acid; 3-[4-[2-[(5-CHLORO-2-METHOXY-BENZOYL)AMINO]ETHYL]PHENYL]PROPANOIC ACID; 3-(4-(2-(5-Chlor-2-methoxy-benzamido)-aethyl)phenyl)-propionsaeure [German]; 3-[4-[2-[(5-chloro-2-methoxybenzoyl)amino]ethyl]phenyl]propanoic acid; HYDROCINNAMIC ACID, 4-(2-((5-CHLORO-2-METHOXYBENZOYL)AMINO)ETHYL)-; AC1L2AFL; CHEMBL597112; SCHEMBL11481071; CTK5B3505; DTXSID00210642; AIEFQKOARQRACO-UHFFFAOYSA-N; ZINC1572211; HB-093; NSC319745
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-348926 | Investigative | [2] | ||
| Synonyms |
NSC348926; 2-phthalimidoadipic acid; AC1L7IP1; SCHEMBL9741723; CHEMBL599367; 2-(1,3-dioxoisoindol-2-yl)hexanedioic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-401077 | Investigative | [2] | ||
| Synonyms |
NSC401077; MLS000757170; DNA Methyltransferase Inhibitor; CHEMBL383475; 32675-71-1; 2-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-3-(1H-indol-3-yl)-propionic acid; 2-(1,3-dioxo-2,3-dihydro-1H-isoindol-2-yl)-3-(1H-indol-3-yl)propanoic acid; 2-(1,3-Dioxo-1,3-dihydro-2H-isoindol-2-yl)-3-(1H-indol-3-yl)propanoic acid; 2-(1,3-Dioxo-1,3-dihydro-2H-isoindol-2-yl)-3-(1H-indol-3-yl)propionic acid; SMR000413613; AC1Q71QA; Oprea1_475901; Oprea1_410805; MLS000777218; MLS006011919; SCHEMBL562060
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| S-tubercidinylhomocysteine | Investigative | [4] | ||
| Synonyms |
CHEMBL552309; 57344-98-6; AC1L3YAS; AC1Q5QMO; (S)-7-(5-S-(3-amino-3-carboxypropyl)-5-thio-beta-D-ribofuranosyl)-7H-pyrrolo(2,3-d)pyrimidin-4-amine; (2s)-2-amino-4-({[(2s,3s,4r,5r)-5-(4-amino-7h-pyrrolo[2,3-d]pyrimidin-7-yl)-3,4-dihydroxytetrahydrofuran-2-yl]methyl}sulfanyl)butanoic acid(non-preferred name); BDBM50294482; (2S)-2-amino-4-[[(2S,3S,4R,5R)-5-(4-aminopyrrolo[2,3-d]pyrimidin-7-yl)-3,4-dihydroxyoxolan-2-yl]methylsulfanyl]butanoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 300 nM | |||
| External Link | ||||
| NSC-106084 | Investigative | [2] | ||
| Synonyms |
CHEMBL597113; NSC106084; AC1L6H8Q; CTK7J5419; ZINC1868549; BDBM50308983; {4-[5-bromo-2-(carboxymethoxy)benzoyl]phenoxy}acetic acid; 2-(4-bromo-2-(4-(carboxymethoxy)benzoyl)phenoxy)acetic acid; 2-[4-[5-bromo-2-(carboxymethyloxy)benzoyl]phenoxy]acetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-154957 | Investigative | [2] | ||
| Synonyms |
NSC154957; AC1L6EF2; CHEMBL586418; 3-benzhydrylsulfanyl-2-formamidopropanoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-54162 | Investigative | [2] | ||
| Synonyms |
NSC54162; AC1Q5WTA; AC1L6CM2; CHEMBL611994; 2,2'-{[(2-hydroxyphenyl)methanediyl]disulfanediyl}diacetic acid; ZINC1685025; Acetic acid, (salicylidenedithio)di-; 4265-51-4; Acetic acid, [(o-hydroxybenzylidene)dithio]di-; Acetic acid,2'-[[(2-hydroxyphenyl)methylene]bis(thio)]bis-; 2-[carboxymethylsulfanyl-(2-hydroxyphenyl)methyl]sulfanylacetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-57893 | Investigative | [2] | ||
| Synonyms |
MLS002667915; 7399-94-2; 4-[(1h-benzimidazol-2-ylmethyl)(formyl)amino]benzoic acid; NSC57893; AC1L6GFK; AC1Q5TWY; NCIOpen2_002368; CHEMBL599366; 4-[1H-benzimidazol-2-ylmethyl(formyl)amino]benzoic acid; CTK5D9099; DTXSID30288854; HMS3089M13; ZINC1688755; AKOS030547711
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-56071 | Investigative | [2] | ||
| Synonyms |
32230-52-7; NSC56071; AC1L6EJV; AC1Q7ES0; NCIOpen2_007380; CHEMBL596910; CTK4G8394; DTXSID80288485; ZINC1686711; 2,2'-[piperazine-1,4-diylbis(carbonothioylsulfanediyl)]diacetic acid; AKOS030574801; Acetic acid,2,2'-[1,4-piperazinediylbis(carbonothioylthio)]bis- (9CI); 2-[4-(carboxymethylsulfanylcarbothioyl)piperazine-1-carbothioyl]sulfanylacetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-137546 | Investigative | [2] | ||
| Synonyms |
NSC137546; CHEMBL591202; AC1L5Y49; AKOS008984447; 2-[(2,6-dichlorobenzoyl)amino]pentanedioic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-623548 | Investigative | [2] | ||
| Synonyms |
2581-36-4; NSC 408488; o-Cresotic acid, 5,5'-methylenedi-; 2,3-CRESOTIC ACID, 5,5'-METHYLENEDI-; UNII-S3D8KC88KC; 5,5'-Methylenedi-2,3-cresotic acid; NSC 623548; BRN 3433298; S3D8KC88KC; CHEMBL113835; 5,5'-Methylenedi-o-cresotic acid; NSC623548; NSC408488; 5,5'-Methylenebis(2-hydroxy-3-methylbenzoic acid); 2, 5,5'-methylenedi-; AC1L29YK; Oprea1_231968; 2-10-00-00398 (Beilstein Handbook Reference); SCHEMBL9755153; CTK4F6504; DTXSID90180466; o-Cresotic acid,5'-methylenedi-; MolPort-000-698-522; ZINC4028795; STL511095
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-345763 | Investigative | [2] | ||
| Synonyms |
7-(8-hydroxyquinolin-5-yl)-4,7-dioxoheptanoic acid; NSC345763; AC1L7HSU; CHEMBL597114
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-158324 | Investigative | [2] | ||
| Synonyms |
Acediasulfone; UNII-30YP2YHH8W; 30YP2YHH8W; CHEMBL48396; N-[4-[(4-AMINOPHENYL)SULPHONYL]PHENYL]GLYCINE; 2-[4-(4-aminophenyl)sulfonylanilino]acetic acid; Acediasulfonum; N-(4-((4-Aminophenyl)sulphonyl)phenyl)glycine; EINECS 201-243-7; AC1L25EF; ZINC862; SCHEMBL143660; CTK5E7379; DTXSID00229991; CHEBI:135300; BDBM50099670; AKOS027327086; DB08926; Glycine,N-[4-[(4-aminophenyl)sulfonyl]phenyl]-; {4-[(4-aminophenyl)sulfonyl]anilino}acetic acid; 2-(4-(4-aminophenylsulfonyl)phenylamino)acetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (L-)-S-adenosyl-L-homocysteine | Investigative | [5] | ||
| Synonyms |
S-Adenosyl-L-homocysteine; S-adenosylhomocysteine; 979-92-0; AdoHcy; S-(5'-adenosyl)-L-homocysteine; adenosylhomocysteine; Formycinylhomocysteine; Adenosyl-L-homocysteine; S-(5'-deoxyadenosin-5'-yl)-L-homocysteine; 2-S-adenosyl-L-homocysteine; 5'-Deoxy-S-adenosyl-L-homocysteine; S-adenosyl-homocysteine; S-Adenosyl Homocysteine; L-S-Adenosylhomocysteine; L-Homocysteine, S-(5'-deoxyadenosin-5'-yl)-; adenosylhomo-cys; adenosyl-homo-cys; UNII-8K31Q2S66S; (S)-5'-(S)-(3-Amino-3-carboxypropyl)-5'-thioadenosine; BRN 5166233; SAH
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 200 nM | |||
| External Link | ||||
| NSC-622445 | Investigative | [2] | ||
| Synonyms |
5,5'-Methylenedisalicylic acid; 122-25-8; 5,5'-Methylenebis(2-hydroxybenzoic acid); Methylenebis(salicylic acid); 5,5-Methylenebis(salicylic acid); UNII-2KF4FVV76N; 5,5-Methylenedisalicylic acid; 5-(3-Carboxy-4-hydroxybenzyl)salicylic acid; 4,4'-Dihydroxy-3,3'-dicarboxydiphenylmethane; 3,3'-Dicarboxy-4,4'-dihydroxydiphenylmethane; NSC 14778; 2KF4FVV76N; 4,4'-Dihydroxydiphenylmethane-3,3'-dicarboxylic acid; 3,3'-Methylenebis(6-hydroxybenzoic acid); CHEMBL115145; Benzoic acid, 3,3'-methylenebis[6-hydroxy-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 17000 nM | |||
| External Link | ||||
| Peroxisome proliferator-activated receptor alpha (PPARalpha/PPARA) | 76 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Bezafibrate | Approved | [6] | ||
| Synonyms |
Azufibrat; Befibrat; Befizal; BezaLande; BezaPuren; Bezabeta; Bezacur; Bezafibrat; Bezafibrato; Bezafibratum; Bezafisal;Bezalip; Bezamerck; Bezatol; Cedur; Difaterol; Durabezur; Eulitop; Lipox; Reducterol; Sklerofibrat; Solibay; Azupharma Brand of Bezafibrate; Bayer Brand of Bezafibrate; Berlin Chemie Brand of Bezafibrate; Betapharm Brand of Bezafibrate; Beza Lande; Beza Puren; Bezafibrat PB; Bezafibrate Azupharma Brand; Bezafibrate Bayer Brand; Bezafibrate Betapharm Brand; Bezafibrate Cryopharma Brand; Bezafibrate Elfar Brand; Bezafibrate Hennig Brand; Bezafibrate Hexal Brand; Bezafibrate Isis Brand; Bezafibrate Lakeside Brand; Bezafibrate Merckle Brand; Bezafibrate Roche Brand; Bezafibrate Synthelabo Brand; Bezafibrate TAD Brand; Bezafibrate Teva Brand; Bezafibrato [Spanish]; Bezalip Retard; Bezatol SR; Boehringer Mannheim Brand of Bezafibrate; Cryopharma Brand of Bezafibrate; Elfar Brand of Bezafibrate;Hennig Brand of Bezafibrate; Hexal Brand of Bezafibrate; Isis Brand of Bezafibrate; Lakeside Brand of Bezafibrate; Merckle Brand of Bezafibrate; Regadrin B; Roche Brand of Bezafibrate; Synthelabo Brand of Bezafibrate; TAD Brand of Bezafibrate; Teva Brand of Bezafibrate; BM 15075; LO 44; BF-759; BM 15.075; BM-15075; BM15.075; Berlin-Chemie Brand of Bezafibrate; Beza-Lande; Beza-Puren; Bezafibrate Berlin-Chemie Brand; Bezafibrato [INN-Spanish]; Bezafibratum [INN-Latin]; Bezalip (TN); Bezatol SR (TN); PB, Bezafibrat; BM-15.075; Bezafibrate (JP15/USAN/INN); Bezafibrate [USAN:BAN:INN:JAN]; A-[4-(4-chlorobenzoylaminoethyl)phenoxy]isobutyric acid; 2-(4-{2-[(4-chlorobenzoyl)amino]ethyl}phenoxy)-2-methylpropanoic acid; 2-(p-(2-(p-Chlorobenzamido)ethyl)phenoxy)-2-methylpropionic acid; 2-[4-(2-[4-Chlorobenzamido]ethyl)-phenoxy]-2-methylpropanoic acid; 2-[4-[2-(4-Chlorobenzamido)ethyl]phenoxy]-2-methylpropanoic acid; 2-[4-[2-(4-Chlorobenzamido)ethyl]phenoxy]isobutyric Acid; 2-[4-[2-[(4-chlorobenzoyl)amino]ethyl]phenoxy]-2-methylpropanoic acid
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 7100 nM | |||
| External Link | ||||
| Pemafibrate | Approved | [7] | ||
| Synonyms |
K-877
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 1 nM | |||
| External Link | ||||
| Fenofibrate | Approved | [8] | ||
| Synonyms |
Ankebin; Antara; Controlip; Durafenat; Elasterate; Elasterin; FNF; Fenobeta; Fenobrate; Fenofanton; Fenofibrato; Fenofibratum; Fenogal; Fenoglide; Fenomax; Fenotard; Finofibrate; Fulcro; Lipanthyl; Lipantil; Liparison; Lipidex; Lipidil; Lipifen; Lipirex; Lipoclar; Lipofen; Lipofene; Liposit; Lipsin; Lofibra; Luxacor; Nolipax; Pharmavit; Phenofibrate; Procetofen; Procetofene; Proctofene; Protolipan; Secalip; Sedufen; Supralip; Tricor; Triglide; AbZ Brand of Procetofen; Abbott Brand of Procetofen; Aliud Brand of Procetofen; Antara Micronized Procetofen; Anto Brand of Procetofen; Apo Feno Micro; Apo Fenofibrate; Apotex Brand of Procetofen; Azupharma Brand of Procetofen; Betapharm Brand of Procetofen; Bouchara Brand of Procetofen; Ct Arzneimittel Brand of Procetofen; Fenofibrat AL; Fenofibrat AZU; Fenofibrat AbZ; Fenofibrat FPh; Fenofibrat Heumann; Fenofibrat Hexal; Fenofibrat Stada; Fenofibrat ratiopharm; Fenofibrat von ct; Fenofibrate Debat; Fenofibrate MSD; Fournier Brand of Procetofen; GNR Pharma Brand of Procetofen; Gate Brand of Procetofen; Gen Fenofibrate; Genpharm Brand of Procetofen; Heumann Brand of Procetofen; Hexal Brand of Procetofen; Knoll Brand of Procetofen; Lichtenstein Brand of Procetofen; Lipidil Micro; Lipidil Supra; Lipidil Ter; MTW Brand of Procetofen; MTW Fenofibrat; Merck dura Brand of Procetofen; Novartis Brand of Procetofen; Novo Fenofibrate; Novopharm Brand of Procetofen; Nu Fenofibrate; Nu Pharm Brand of Procetofen; PMS Fenofibrate Micro;Pharmascience Brand of Procetofen; Procetofen Reliant Brand; Q Pharm Brand of Procetofen; Ratiopharm Brand of Procetofen; Reliant Brand of Procetofen; Schering Plough Brand of Procetofen; Stadapharm Brand of Procetofen; United Drug Brand of Procetofen; F 6020; LF 178; LF178; AZU, Fenofibrat; Antara (TN); Antara (micronized); Apo-Fenofibrate; CIP-Fenofibrate; Ct-Arzneimittel Brand of Procetofen; Debat, Fenofibrate; FENOFIBRATE (MICRONIZED); Fenofibrat-ratiopharm; Fenofibrate IDD-P; Fenofibrate [INN:BAN]; Fenofibrato [INN-Spanish]; Fenofibratum [INN-Latin]; Fenogal (TN); GNR-Pharma Brand of Procetofen; GRS-027; Gen-Fenofibrate; Heumann, Fenofibrat; Hexal, Fenofibrat; LCP-Feno; LCP-FenoChol; LF-178; Lipanthyl (TN); Lipantil (TN); Lipidil (TN); Lipidil-Ter; Lipofen (TN); Lofibra (TN); MTW-Fenofibrat; Micronized Procetofen, Antara; Novo-Fenofibrate; Nu-Fenofibrate; Nu-Pharm Brand of Procetofen; PMS-Fenofibrate Micro; Procetofen, Antara Micronized; Q-Pharm Brand of Procetofen; Schering-Plough Brand of Procetofen; Stada, Fenofibrat; TRICOR (MICRONIZED); Tricor (TN); Triglide (TN); Trilipix (TN); Apo-Feno-Micro; Fenocor-67 (TN); Fenofibrate (JAN/INN); Isopropyl 2-[4-(4-chlorobenzoyl)phenoxy]-2-methylpropanoate; Isopropyl 2-(4-(4-chlorobenzoyl)phenoxy)-2-methylpropionate; Isopropyl 2-(p-(p-chlorobenzoyl)phenoxy)-2-methylpropionate
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | IC50 = 1000 nM | |||
| External Link | ||||
| Ciprofibrate | Approved | [8] | ||
| Synonyms |
Ciprofibrato; Ciprofibratum; Ciprol; Hiperlipen; Hyperlipen; Lipanor; Modalim; Oroxadin; Sanofi Synthelabo brand of ciprofibrate; Sanofi Winthrop brand of ciprofibrate; C 0330; WIN 35833; Ciprofibrato [INN-Spanish]; Ciprofibratum [INN-Latin]; Win 35,833; Win-35833; Ciprofibrate (USAN/INN); Ciprofibrate [USAN:BAN:INN]; 2-(4-(2,2-Dichlorocyclopropyl)phenoxy)2-methylpropanoic acid; 2-(p-(2,2-Dichlorocyclopropyl)phenoxy)-2-methylpropionic acid; 2-[4-(2,2-Dichlorocyclopropyl)phenoxy]-2-methylpropanoic acid; 2-[p-(2,2-Dichlorocyclopropyl)phenoxy]-2-methylpropanoic acid; 2-{4-[2,2-dichlorocyclopropyl]phenoxy}-2-methylpropanoic acid; 2-{[4-(2,2-dichlorocyclopropyl)phenyl]oxy}-2-methylpropanoic acid
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 900 nM | |||
| External Link | ||||
| Lobeglitazone | Approved | [9] | ||
| Synonyms |
Lobeglitazone sulfate; CKD-501; Dual PPARalpha/delta (type 2 diabetes), Chong Kun Dang
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Ragaglitazar | Phase 3 | [10] | ||
| Synonyms |
DRF-2725; (2S)-2-ETHOXY-3-{4-[2-(10H-PHENOXAZIN-10-YL)ETHOXY]PHENYL}PROPANOIC ACID; CHEMBL24038; (2S)-2-ethoxy-3-[4-(2-phenoxazin-10-ylethoxy)phenyl]propanoic acid; DRF; 1nyx; DRF2725; AC1L9KVW; 2-Ethoxy-3-[4-(2-phenoxazin-10-yl-ethoxy)-phenyl]-propionic acid; GTPL2664; SCHEMBL4822459; WMUIIGVAWPWQAW-DEOSSOPVSA-N; NN-622; BDBM50109551; DB07675; NNC-61-0029; (-)-DRF-2725; (S)-2-Ethoxy-3-[4-(2-phenoxazin-10-yl-ethoxy)-phenyl]-propionic acid; (2S)-2-ethoxy-3-{4-[2-(phenoxazin-10-yl)ethoxy]phenyl}propanoic acid
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | Ki = 980 nM | |||
| External Link | ||||
| CS-038 | Phase 3 | [11] | ||
| Synonyms |
Chiglitazar; CS-00098; PPAR alpha/gamma agonist (diabetes), Chipscreen Biosciences
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| TESAGLITAZAR | Phase 3 | [12] | ||
| Synonyms |
251565-85-2; Galida; (S)-2-Ethoxy-3-(4-(4-((methylsulfonyl)oxy)phenethoxy)phenyl)propanoic acid; AZ 242; AZ-242; AR-H039242XX; (S)-2-Ethoxy-3-{4-[2-(4-methanesulfonyloxyphenyl)ethoxy]phenyl}propionic acid; UNII-6734037O3L; (S)-2-Ethoxy-3-{4-[2-(4-methanesulfonyloxy-phenyl)-ethoxy]-phenyl}-propionic acid; (2S)-2-ethoxy-3-[4-[2-(4-methylsulfonyloxyphenyl)ethoxy]phenyl]propanoic acid; BR-44608; 6734037O3L; (S)-2-Ethoxy-3-[4-[2-(4-methanesulfonyloxyphenyl)ethoxy]phenyl]propanoic acid
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | EC50 = 37 nM | |||
| External Link | ||||
| GFT-505 | Phase 3 | [13] | ||
| Synonyms |
GFT-1007; PPAR activator (dyslipidemia/type 2 diabetes), Genfit
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | EC50 = 10 nM | |||
| External Link | ||||
| MURAGLITAZAR | Phase 3 | [14] | ||
| Synonyms |
331741-94-7; Pargluva; BMS-298585; UNII-W1MKM70WQI; BMS 298585; W1MKM70WQI; CHEMBL186179; N-[(4-Methoxyphenoxy)carbonyl]-N-[[4-[2-(5-methyl-2-phenyl-4-oxazolyl)ethoxy]phenyl]methyl]glycine; N-((4-methoxyphenoxy)carbonyl)-N-((4-(2-(5-methyl-2-phenyl-4-oxazolyl)ethoxy)phenyl)methyl)glycine; BMS298585; Muraglitazar [USAN:INN]; CCRIS 9258; AC1L4FVP; Muraglitazar (USAN/INN); DSSTox_CID_31508; DSSTox_RID_97393; DSSTox_GSID_57719; SCHEMBL676469; DTXSID9057719; CTK8E8901; MolPort-006-395-259; IRLWJILLXJGJTD-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | EC50 = 40 nM | |||
| External Link | ||||
| ZYH-1 | Phase 3 | [15] | ||
| Synonyms |
Metabolism disorder treatment, Zydus-Cadila; PPAR alpha/gamma modulator (dyslipdemia), Zydus-Cadila
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Imiglitazar | Phase 3 | [16] | ||
| Synonyms |
TAK-559
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | EC50 = 5 nM | |||
| External Link | ||||
| LY-518674 | Phase 2 | [17] | ||
| Synonyms |
LY-674; 2-Methyl-2-[4-[3-[1-(4-methylbenzyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-3-yl]propyl]phenoxy]propionic acid
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 42 nM | |||
| External Link | ||||
| GFT14 | Phase 2 | [18] | ||
| MOA | Agonist | |||
| External Link | ||||
| ZYH7 | Phase 2 | [19] | ||
| MOA | Agonist | |||
| External Link | ||||
| Naveglitazar | Phase 2 | [20] | ||
| Synonyms |
LY 519818; LY-818; PPAR alpha/gamma co-agonists, Lilly/Ligand
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | EC50 = 2800 nM | |||
| External Link | ||||
| ONO-5129 | Phase 2 | [21] | ||
| Synonyms |
Dual PPAR alpha/gamma agonist (metabolic disorder), Ono
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| CDT-fenofibrate | Phase 1 | [22] | ||
| MOA | Agonist | |||
| External Link | ||||
| AVE0897 | Phase 1 | [23] | ||
| MOA | Agonist | |||
| External Link | ||||
| TPST-1120 | Phase 1 | [24] | ||
| MOA | Antagonist | |||
| External Link | ||||
| Oxeglitazar | Phase 1 | [25] | ||
| Synonyms |
EMD-336340; EML-16156; EML-4156; LM-4156
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| GW-409544 | Phase 1 | [26] | ||
| Synonyms |
GW-409544X; GW-544; GW-6471; Lipid regulators, Ligand/Glaxo
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | Ki = 2 nM | |||
| External Link | ||||
| PMID25416646-Compound-Figure5-H | Patented | [27] | ||
| MOA | Agonist | |||
| External Link | ||||
| Flavonoid derivative 8 | Patented | [27] | ||
| Synonyms |
PMID25416646-Compound-Figure5-B
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| PMID25416646-Compound-Figure5-A | Patented | [27] | ||
| MOA | Agonist | |||
| External Link | ||||
| Aleglitazar | Discontinued in Phase 3 | [28] | ||
| Synonyms |
RO7; Aleglitazar (USAN); 2-methoxy-3-[4-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy]benzothiophen-7-yl]propanoic Acid
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 50 nM | |||
| External Link | ||||
| NS-220 | Discontinued in Phase 2 | [29] | ||
| Synonyms |
R-1593; Cis-2-Methyl-5-[4-[5-methyl-2-(4-methylphenyl)oxazol-4-yl]butyl]-1,3-dioxane-2-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 10 nM | |||
| External Link | ||||
| KRP-101 | Discontinued in Phase 2 | [30] | ||
| Synonyms |
PPAR alpha agonist (hyperlipidemia), Kyorin
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 0.615 nM | |||
| External Link | ||||
| BM-17.0744 | Discontinued in Phase 2 | [31] | ||
| Synonyms |
K-111; 12-(4-Chlorophenyl)-2,2-dichlorododecanoic acid
Click to Show/Hide
|
|||
| MOA | Activator | |||
| External Link | ||||
| AVE-8134 | Discontinued in Phase 2 | [32] | ||
| Synonyms |
PPAR alpha agonist, Genfit
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| KRP-297 | Discontinued in Phase 2 | [33] | ||
| Synonyms |
MK 767; MK-767; KRP297; KRP 297; 213252-19-8; L410198; L 410198; 5-((2,4-Dioxo-5-thiazolidinyl)methyl)-2-methoxy-N-((4-(trifluoromethyl)phenyl)methyl)benzamide; Benzamide, 5-((2,4-dioxo-5-thiazolidinyl)methyl)-2-methoxy-N-((4-(trifluoromethyl)phenyl)methyl)-; 5-[(2,4-dioxo-1,3-thiazolidin-5-yl)methyl]-2-methoxy-N-[[4-(trifluoromethyl)phenyl]methyl]benzamide; NFFXEUUOMTXWCX-UHFFFAOYSA-N; SCHEMBL3922; AC1L45TL; MLS006010319; GTPL2677; CTK4E6495; MolPort-018-657-358; AKOS005067111; NCGC00263123-01; SMR004701384
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Reglixane | Discontinued in Phase 2 | [21] | ||
| MOA | Modulator | |||
| External Link | ||||
| AVE-0847 | Discontinued in Phase 2 | [34] | ||
| Synonyms |
PPAR alpha/gamma agonists (diabetes/dyslipidemia), Genfit; PPAR alpha/gamma agonists (diabetes/dyslipidemia), aventis; PPAR alpha/gamma agonists (diabetes/dyslipidemia), sanofi-aventis
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Sodelglitazar | Discontinued in Phase 2 | [35] | ||
| Synonyms |
447406-78-2; UNII-6G973E04VI; 6G973E04VI; GW677954; Sodelglitazar [USAN:INN]; 2-[4-[[[2-[2-Fluoro-4-(trifluoromethyl)phenyl]-4-methyl-1,3-thiazol-5-yl]methyl]sulfanyl]-2-methylphenoxy]-2-methylpropanoic acid; 2-(4-(((2-(2-Fluoro-4-(trifluoromethyl)phenyl)-4-methyl-1,3-thiazol-5-yl)methyl)sulfanyl)-2-methylphenoxy)-2-methylpropanoic acid; Sodelglitazar (USAN); SCHEMBL4822839; CHEMBL2104984; DTXSID90196289; ZUGQWAYOWCBWGM-UHFFFAOYSA-N; ZINC1553281; AN-28238; ACM447406782; FT-0743554; D06647; 406S782; Propanoic ac
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Indeglitazar | Discontinued in Phase 2 | [36] | ||
| Synonyms |
PLX-204
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| GSK-677954 | Discontinued in Phase 2 | [37] | ||
| Synonyms |
SCHEMBL2065429
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| AR-H049020 | Discontinued in Phase 1 | [38] | ||
| MOA | Agonist | |||
| External Link | ||||
| LG-101280 | Discontinued in Phase 1 | [39] | ||
| Synonyms |
LSN-862; LY-WWW; LY-YYY; PPAR modulators, Ligand/Lilly
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| DRF 10945 | Discontinued in Phase 1 | [40] | ||
| MOA | Agonist | |||
| External Link | ||||
| MP-136 | Discontinued in Phase 1 | [41] | ||
| MOA | Agonist | |||
| External Link | ||||
| E-3030 | Discontinued in Phase 1 | [42] | ||
| Synonyms |
Dual PPAR alpha/gamma agonists, Eisai
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| LY-929 | Discontinued in Phase 1 | [43] | ||
| Synonyms |
LY-510929; Dual PPAR-alpha/PPAR-gamma agonists
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 8.913 nM | |||
| External Link | ||||
| MC-3001 | Preclinical | [44] | ||
| Synonyms |
MC-3004; PPAR-alpha agonists, MaxoCore
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| PIRINIXIC ACID | Preclinical | [45] | ||
| Synonyms |
50892-23-4; WY-14643; [4-Chloro-6-(2,3-xylidino)-2-pyrimidinylthio]acetic acid; WY-14,643; WY14643; Wyeth 14,643; Wy 14643; Pirinixic acid [INN]; WY-14643 (Pirinixic Acid); (4-Chloro-6-(2,3-xylidino)-2-pyrimidinylthio)acetic acid; UNII-86C4MRT55A; C14H14ClN3O2S; CCRIS 133; Acidum pirinixicum [INN-Latin]; Acide pirinixique [INN-French]; Acido pirinixico [INN-Spanish]; NSC310038; NSC-310038; NSC 310038; BRN 0759945; ((4-Chloro-6-((2,3-dimethylphenyl)amino)-2-pyrimidinyl)thio)acetic acid; Acetic acid, ((4-chloro-6-(
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 130 nM | |||
| External Link | ||||
| Romazarit | Preclinical | [46] | ||
| Synonyms |
ORE-5007; Metabolic modulator (obesity/hyperlipidemia), Ore Pharmaceuticals
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| MC-3002 | Preclinical | [21] | ||
| Synonyms |
PPAR dual agonists, MaxoCore
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| CS-207 | Terminated | [47] | ||
| Synonyms |
CS-307; PPAR alpha agonists (cardiovascular disease associated with dyslipidemia); PPAR alpha agonists (cardiovascular disease associated with dyslipidemia), Chipscreen Biosciences
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| KRP-105 | Terminated | [48] | ||
| MOA | Agonist | |||
| External Link | ||||
| BVT-142 | Terminated | [49] | ||
| Synonyms |
BVT-13; BVT-142 analogs, Biovitrum; Dual PPAR alpha/gamma agonists (type II diabetes); Dual PPAR alpha/gamma agonists (type II diabetes), Biovitrum
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Sipoglitazar | Terminated | [15] | ||
| Synonyms |
TAK-654
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| CS-204 | Terminated | [50] | ||
| Synonyms |
CS-00088; PPAR alpha/gamma/delta agonist (type 2 diabetes), Chipscreen Bioscience
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| GW7647 | Investigative | [51] | ||
| Synonyms |
GW-7647; GW 7647
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | Ki = 0.0541 nM | |||
| External Link | ||||
| CP-775146 | Investigative | [52] | ||
| Synonyms |
CP775146
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| GSK-9578 | Investigative | [8] | ||
| Synonyms |
KYQNYMXQHLMADB-UHFFFAOYSA-N; GW 9578; GW9578; 247923-29-1; GW-9578; UNII-H32ABL87X4; H32ABL87X4; CHEMBL278590; SCHEMBL68327; GTPL2673; BDBM28799; CTK8E7867; GSK9578; DTXSID10179493; MolPort-009-019-367; ZINC14115100; Propanoic acid, 2-((4-(2-((((2,4-difluorophenyl)amino)carbonyl)heptylamino)ethyl)phenyl)thio)-2-methyl-; RT-013144; J-015674; 289722-11-8; 2-{[4-(2-{[(2,4-difluorophenyl)carbamoyl](heptyl)amino}ethyl)phenyl]sulfanyl}-2-methylpropanoic acid; 2-[4-[2-[(2,4-difluorophenyl)carbamoyl-heptylamino]ethyl]phenyl]sulfanyl-2-methylpropanoic
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 6 nM | |||
| External Link | ||||
| Fibrates | Investigative | [53] | ||
| Synonyms |
clofibric acid; 882-09-7; 2-(4-Chlorophenoxy)-2-methylpropanoic acid; Chlorofibrinic acid; Clofibrinic acid; Chlorfibrinic acid; Clofibrin; 2-(4-CHLOROPHENOXY)-2-METHYLPROPIONIC ACID; Chlorophibrinic acid; PCIB; Clofibrate free acid; Clofibrinsaeure; Regulipid; Regadrin; PCPIB; 2-(p-Chlorophenoxy)-2-methylpropionic acid; 2-(4-Chlorophenoxy)isobutyric Acid; 4-CPIB; 2-(p-Chlorophenoxy)isobutyric acid; Propanoic acid, 2-(4-chlorophenoxy)-2-methyl-; alpha-(p-Chlorophenoxy)isobutyric acid; Acido clofibrico; Acide clofibrique; Acidum c
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 39600 nM | |||
| External Link | ||||
| BMS-687453 | Investigative | [54] | ||
| Synonyms |
1000998-59-3; UNII-39TL5L7XDX; BMS 687453; 39TL5L7XDX; 2-{[(3-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-yl]methoxy}phenyl)methyl](methoxycarbonyl)amino}acetic acid; SCHEMBL2742714; CHEMBL1089501; BDBM28800; EX-A592; BCP14808; ZINC44460341; AKOS030526188; CS-5523; DA-48472; HY-10678; FT-0749275; J-690001; 2-[[3-[[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-yl]methoxy]phenyl]methyl-methoxycarbonylamino]acetic acid; 7HA; N-(3-{[2-(4-chlorophenyl)-5-methyl-1,3-oxazol-4-yl]methoxy}benzyl)-N-(methoxycarbonyl)glycine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 9.6 nM | |||
| External Link | ||||
| pristanic acid | Investigative | [55] | ||
| Synonyms |
UNII-6P06977L1A; 6P06977L1A; (2S,6R,10R)-2,6,10,14-tetramethylpentadecanoic acid; L,d,D-pristanic acid; GTPL2676; Pristanic acid, (2S,6R,10R)-; UNII-5FMQ2908AP component PAHGJZDQXIOYTH-KURKYZTESA-N; Pentadecanoic acid, 2,6,10,14-tetramethyl-, (2S,6R,10R)-
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| eicosatetranoic acid | Investigative | [56] | ||
| Synonyms |
ETYA; 5,8,11,14-eicosatetraynoic acid; 1191-85-1; icosa-5,8,11,14-tetraynoic acid; Octadehydroarachidonic acid; MLS000069514; SMR000058640; MLS-0002886.0001; Ro 31428; Ro 3-1428; Opera_ID_402; AC1Q5VYB; Spectrum5_001952; cid_1780; CBiol_001864; SCHEMBL68751; KBioSS_000169; BSPBio_001449; KBioGR_000169; GTPL2669; CHEMBL458328; BML2-F04; AC1L1C80; CTK0H5766; CHEBI:94483; KBio3_000338; KBio2_002737; KBio3_000337; KBio2_005305; KBio2_000169; BDBM31752; DTXSID20152318; MGLDCXPLYOWQRP-UHFFFAOYSA-N; HMS3402I11; Bio1_001128
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| 8S-HETE | Investigative | [57] | ||
| Synonyms |
8-hydroxyeicosatetraenoic acid
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| LL-6531 | Investigative | [41] | ||
| Synonyms |
PPAR modulators (diabetes), Lupin
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Deoxy-Bigchap | Investigative | [58] | ||
| Synonyms |
Big CHAP, Deoxy; N,N-bis-(3-D-Gluconamidopropyl)deoxycholamide; AC1MTX5H; DTXSID70394140; AN-35629; FT-0629429; N,N -Bis(3-D -gluconamidopropyl)- deoxy-cholamide; N-[3-[4-(3,12-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoyl-[3-(2,3,4,5,6-pentahydroxyhexanoylamino)propyl]amino]propyl]-2,3,4,5,6-pentahydroxyhexanamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (E)-4-(3,5-dimethoxystyryl)phenol | Investigative | [41] | ||
| Synonyms |
Pterostilbene; 537-42-8; 4-(3,5-Dimethoxystyryl)phenol; 3',5'-Dimethoxy-4-stilbenol; trans-pterostilbene; 4-[(E)-2-(3,5-dimethoxyphenyl)ethenyl]phenol; pterostilben; 4-[(E)-2-(3,5-dimethoxyphenyl)vinyl]phenol; UNII-26R60S6A5I; 3,5-Dimethoxy-4'-hydroxy-trans-stilbene; 3,5-Dimethoxy-4'-hydroxystilbene; 18259-15-9; trans-3,5-dimethoxy-4'-hydroxystilbene; CHEMBL83527; CHEBI:8630; VLEUZFDZJKSGMX-ONEGZZNKSA-N; 4-((E)-2-(3,5-dimethoxyphenyl)ethenyl)phenol; 26R60S6A5I; Pterostilbene, Pterocarpus
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| AD-5061 | Investigative | [16] | ||
| Synonyms |
AD-7057; AD7057; AD 7057; AD5061; AD 5061
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| GW-2331 | Investigative | [59] | ||
| Synonyms |
190844-95-2; PPAR ligand, Glaxo Wellcome
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | EC50 < 20 nM | |||
| External Link | ||||
| TZD18 | Investigative | [60] | ||
| Synonyms |
TZD 18; TZD-18
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| (9Z,12E)-12-nitrooctadeca-9,12-dienoic acid | Investigative | [61] | ||
| Synonyms |
12-Nitrolinoleic acid; 12-Nitro-9Z,12Z-octadecadienoic acid; CHEMBL554608; CHEBI:34150; AC1NQZV1; C13958; SCHEMBL2371323; BDBM50295045; LMFA01120002; 12-Nitro-9-cis,12-cis-octadecadienoic acid; (9Z,12Z)-12-Nitrooctadeca-9,12-dienoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 9600 nM | |||
| External Link | ||||
| reglitazar | Investigative | [62] | ||
| Synonyms |
JTT-501
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| DRF 2519 | Investigative | [63] | ||
| Synonyms |
5-[[4-[2-(4-Oxo-2H-1,3-benzoxazin3(4H)-yl)ethoxy]phenyl]methyl2,4-thiazolidinedione; SCHEMBL6953746; GTPL2671; CHEMBL1491825; DTXSID5040754; NOCAS_40754; CTK8F9377; API0008459; NCGC00165785-01; NCGC00164420-01; SR-05000000444; SR-05000000444-2
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| (E)-12-Nitrooctadec-12-enoic Acid | Investigative | [61] | ||
| Synonyms |
CHEMBL549351; BDBM50295043; (E)-12-Nitro-12-octadecenoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1600 nM | |||
| External Link | ||||
| (E)-13-Nitrooctadec-12-enoic Acid | Investigative | [61] | ||
| Synonyms |
CHEMBL540732
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1100 nM | |||
| External Link | ||||
| ZY H2 | Investigative | [64] | ||
| Synonyms |
ZYH2
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| LY-465608 | Investigative | [65] | ||
| Synonyms |
LY465608; LY 465608
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 149.5 nM | |||
| External Link | ||||
| DB-900 | Investigative | [66] | ||
| MOA | Agonist | |||
| External Link | ||||
| L-796449 | Investigative | [67] | ||
| Synonyms |
UNII-O937X0Z5EM; CHEMBL278994; O937X0Z5EM; 194608-80-5; KAPDPGZDHUCILF-UHFFFAOYSA-N; GTPL2689; SCHEMBL4296303; BDBM50085040; Benzeneacetic acid, 3-chloro-4-((3-((3-phenyl-7-propyl-6-benzofuranyl)oxy)propyl)thio)-; L796449; L 796449; L-796,449; 3-chloro-4-(3-(3-phenyl-7-propylbenzofuran-6-yloxy)propylthio)-phenylacetic acid; {3-Chloro-4-[3-(3-phenyl-7-propyl-benzofuran-6-yloxy)-propylsulfanyl]-phenyl}-acetic acid; 2-[3-chloro-4-[3-[(3-phenyl-7-propyl-1-benzofuran-6-yl)oxy]propylsulfanyl]phenyl]acetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 4.1 nM | |||
| External Link | ||||
| L-165461 | Investigative | [67] | ||
| Synonyms |
CHEMBL279053; SCHEMBL6753428; GTPL2690; BDBM50126016; AKOS027321335; L165461; L 165461; 3-Chloro-4-[3-(3-ethyl-7-propyl-1,2-benzisoxazole-6-yloxy)propylthio]benzeneacetic acid; 2-(3-chloro-4-(3-(3-ethyl-7-propylbenzo[d]isoxazol-6-yloxy)propylthio)phenyl)acetic acid; {3-Chloro-4-[3-(3-ethyl-7-propyl-benzo[d]isoxazol-6-yloxy)-propylsulfanyl]-phenyl}-acetic acid; 2-[3-chloro-4-[3-[(3-ethyl-7-propyl-1,2-benzoxazol-6-yl)oxy]propylsulfanyl]phenyl]acetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 247 nM | |||
| External Link | ||||
| N-oleoylethanolamide | Investigative | [68] | ||
| Synonyms |
n-Oleoylethanolamine; oleoylethanolamide; N-(2-Hydroxyethyl)oleamide; 111-58-0; Oleylethanolamide; N-oleoyl ethanolamine; Oleamide MEA; Oleoyl monoethanolamide; Oleoyl Ethanolamide; N-(2-Hydroxyethyl)-9-octadecenamide; N-(Hydroxyethyl)oleamide; UNII-1HI5J9N8E6; (Z)-N-(2-hydroxyethyl)octadec-9-enamide; Oleic acid ethanolamide; N-(9Z-octadecenoyl)-ethanolamine; EINECS 203-884-8; OEA; MLS002153155; CHEMBL280065; 1HI5J9N8E6; (9Z)-N-(2-hydroxyethyl)octadec-9-enamide; Monoethanolamine oleic acid amide; CHEBI:71466; NOE
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 110 nM | |||
| External Link | ||||
References