m6A Target Gene Information
General Information of the m6A Target Gene (ID: M6ATAR00373)
Full List of m6A Methylation Regulator of This Target Gene and Corresponding Disease/Drug Response(s)
PPARGC1A
can be regulated by the following regulator(s), and cause disease/drug response(s). You can browse detail information of regulator(s) or disease/drug response(s).
Browse Regulator
Browse Disease
Fat mass and obesity-associated protein (FTO) [ERASER]
| Representative RNA-seq result indicating the expression of this target gene regulated by FTO | ||
| Cell Line | 253J cell line | Homo sapiens |
|
Treatment: siFTO 253J cells
Control: 253J cells
|
GSE150239 | |
| Regulation |
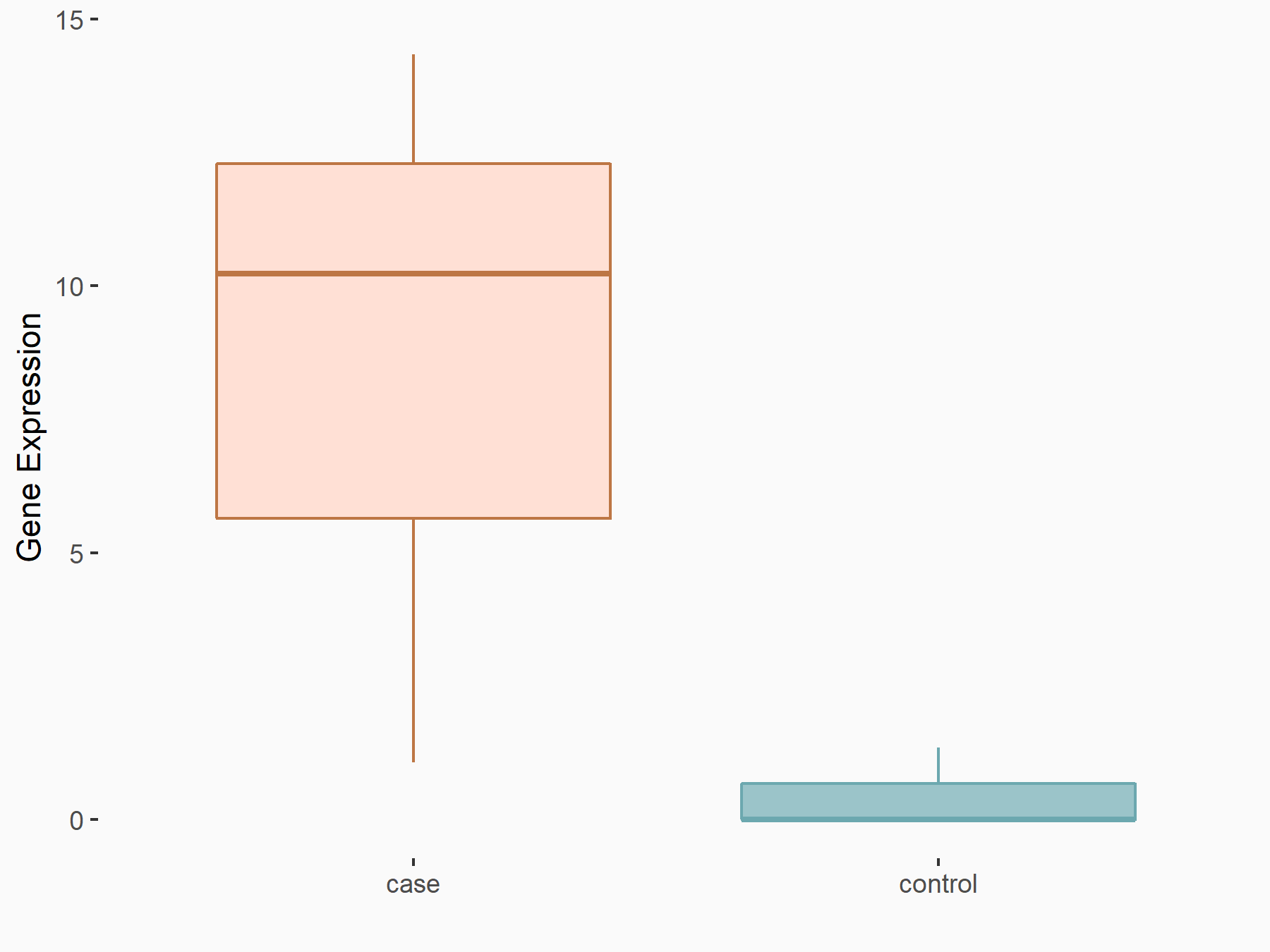  |
logFC: 4.58E+00 p-value: 7.10E-03 |
| More Results | Click to View More RNA-seq Results | |
| In total 2 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | FTO plays a critical anti-tumorigenic role in Clear Cell Renal Cell Carcinoma.Restored expression of FTO, through reducing m6A levels in mRNA transcripts of its critical target gene PPAR-gamma coactivator 1-alpha (PGC-1a/PPARGC1A), increases mitochondrial content, ROS production and oxidative damage, with the most important effect of repressed tumour growth. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | clear cell renal cell carcinoma | ICD-11: 2C90.0 | ||
| Cell Process | Oxidative stress | |||
| ROS production | ||||
| In-vitro Model | HEK293T | Normal | Homo sapiens | CVCL_0063 |
| 769-P | Renal cell carcinoma | Homo sapiens | CVCL_1050 | |
| 786-O | Renal cell carcinoma | Homo sapiens | CVCL_1051 | |
| In-vivo Model | Five- to 6-week-old male athymic nude mice purchased by Charles River were used for the xenograft model. 769-P cells stably expressing Ctrl, FTO and FTO-mut were trypsinized and washed twice to thrice with standardized PBS, and then, 5 × 106 cells in 100 uL of PBS was subcutaneously injected into the flanks of the mice (five mice per group). Mice were monitored twice every week for tumour growth, and tumour diameters were measured using a caliper. | |||
| Experiment 2 Reporting the m6A Methylation Regulator of This Target Gene | [2] | |||
| Response Summary | FTO downregulation suppressed mitochondria biogenesis and energy production, showing as the decreased mitochondria mass and mitochondrial DNA (mtDNA) content, the downregulated expression of mtDNA-encoding genes and PPAR-gamma coactivator 1-alpha (PGC-1a/PPARGC1A) gene, together with declined ATP level. These findings provide the first evidence for the contribution of FTO for skeletal muscle differentiation. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Muscular dystrophies | ICD-11: 8C70 | ||
| Cell Process | Myogenic differentiation | |||
| mTOR signaling pathway (hsa04150) | ||||
| In-vitro Model | MPM (Mouse primary myoblasts from about 10-day-old C57BL/6J were isolated) | |||
| C2C12 | Normal | Mus musculus | CVCL_0188 | |
| HEK293-FT | Normal | Homo sapiens | CVCL_6911 | |
| In-vivo Model | To generate doxycycline-inducible skeletal muscle-specific FTO deletion mice, FTOflox/flox mice were crossed with HSA-Cre mice to generate FTOflox/+ HSA-Cre mice, which were then crossed to FTOflox/flox mice to generate FTOflox/flox and FTOflox/flox HSA-Cre mice. | |||
Methyltransferase-like 3 (METTL3) [WRITER]
| Representative RNA-seq result indicating the expression of this target gene regulated by METTL3 | ||
| Cell Line | MOLM-13 cell line | Homo sapiens |
|
Treatment: shMETTL3 MOLM13 cells
Control: MOLM13 cells
|
GSE98623 | |
| Regulation |
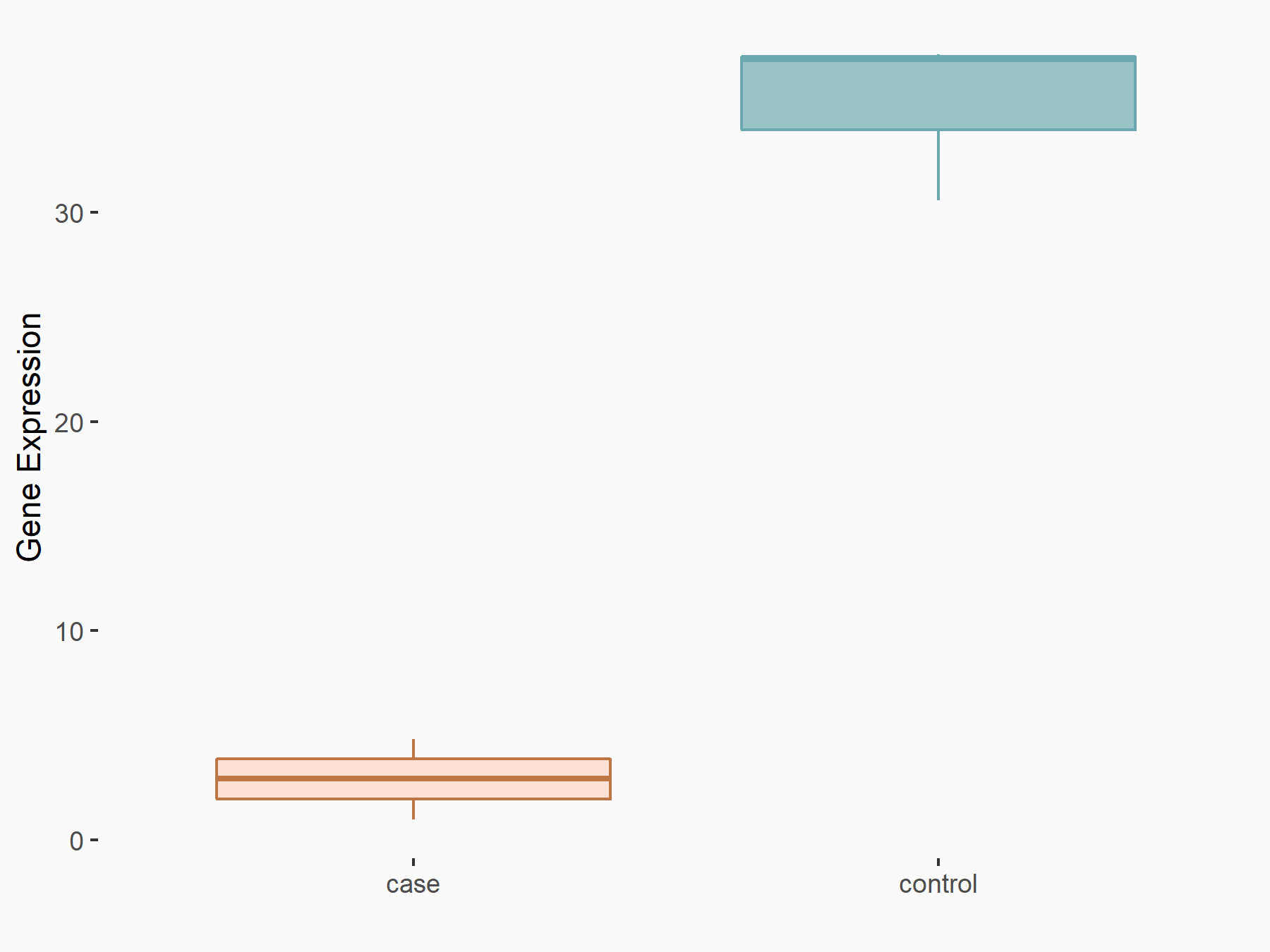  |
logFC: -3.59E+00 p-value: 8.55E-10 |
| More Results | Click to View More RNA-seq Results | |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [3] | |||
| Response Summary | Type 2 diabetes (T2D) is characterized by lack of insulin, insulin resistance and high blood sugar. METTL3 silence decreased the m6A methylated and total mRNA level of Fatty acid synthase (Fasn), subsequently inhibited fatty acid metabolism. The expression of Acc1, Acly, Dgat2, Ehhadh, Fasn, Foxo, PPAR-gamma coactivator 1-alpha (PGC-1a/PPARGC1A) and Sirt1, which are critical to the regulation of fatty acid synthesis and oxidation were dramatically decreased in livers of hepatocyte-specific METTL3 knockout mice. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Type 2 diabetes mellitus | ICD-11: 5A11 | ||
| Pathway Response | Insulin resistance | hsa04931 | ||
| Cell Process | Lipid metabolism | |||
| In-vitro Model | Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| In-vivo Model | Hepatocyte-specific METTL3 knockout mice (TBG-Cre, METTL3 fl/fl) were generated by crossing mice with TBG-Cre Tg mice. METTL3 flox (METTL3 fl/fl) and hepatocyte-specific METTL3 knockout mice (TBG-Cre, METTL3 fl/fl) were used for experiments. | |||
Renal cell carcinoma [ICD-11: 2C90]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [1] | |||
| Response Summary | FTO plays a critical anti-tumorigenic role in Clear Cell Renal Cell Carcinoma.Restored expression of FTO, through reducing m6A levels in mRNA transcripts of its critical target gene PPAR-gamma coactivator 1-alpha (PGC-1a/PPARGC1A), increases mitochondrial content, ROS production and oxidative damage, with the most important effect of repressed tumour growth. | |||
| Responsed Disease | clear cell renal cell carcinoma [ICD-11: 2C90.0] | |||
| Target Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | ||
| Target Regulation | Up regulation | |||
| Cell Process | Oxidative stress | |||
| ROS production | ||||
| In-vitro Model | HEK293T | Normal | Homo sapiens | CVCL_0063 |
| 769-P | Renal cell carcinoma | Homo sapiens | CVCL_1050 | |
| 786-O | Renal cell carcinoma | Homo sapiens | CVCL_1051 | |
| In-vivo Model | Five- to 6-week-old male athymic nude mice purchased by Charles River were used for the xenograft model. 769-P cells stably expressing Ctrl, FTO and FTO-mut were trypsinized and washed twice to thrice with standardized PBS, and then, 5 × 106 cells in 100 uL of PBS was subcutaneously injected into the flanks of the mice (five mice per group). Mice were monitored twice every week for tumour growth, and tumour diameters were measured using a caliper. | |||
Type 2 diabetes mellitus [ICD-11: 5A11]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [3] | |||
| Response Summary | Type 2 diabetes (T2D) is characterized by lack of insulin, insulin resistance and high blood sugar. METTL3 silence decreased the m6A methylated and total mRNA level of Fatty acid synthase (Fasn), subsequently inhibited fatty acid metabolism. The expression of Acc1, Acly, Dgat2, Ehhadh, Fasn, Foxo, PPAR-gamma coactivator 1-alpha (PGC-1a/PPARGC1A) and Sirt1, which are critical to the regulation of fatty acid synthesis and oxidation were dramatically decreased in livers of hepatocyte-specific METTL3 knockout mice. | |||
| Responsed Disease | Type 2 diabetes mellitus [ICD-11: 5A11] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Insulin resistance | hsa04931 | ||
| Cell Process | Lipid metabolism | |||
| In-vitro Model | Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| In-vivo Model | Hepatocyte-specific METTL3 knockout mice (TBG-Cre, METTL3 fl/fl) were generated by crossing mice with TBG-Cre Tg mice. METTL3 flox (METTL3 fl/fl) and hepatocyte-specific METTL3 knockout mice (TBG-Cre, METTL3 fl/fl) were used for experiments. | |||
Muscular dystrophies [ICD-11: 8C70]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [2] | |||
| Response Summary | FTO downregulation suppressed mitochondria biogenesis and energy production, showing as the decreased mitochondria mass and mitochondrial DNA (mtDNA) content, the downregulated expression of mtDNA-encoding genes and PPAR-gamma coactivator 1-alpha (PGC-1a/PPARGC1A) gene, together with declined ATP level. These findings provide the first evidence for the contribution of FTO for skeletal muscle differentiation. | |||
| Responsed Disease | Muscular dystrophies [ICD-11: 8C70] | |||
| Target Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | ||
| Target Regulation | Up regulation | |||
| Cell Process | Myogenic differentiation | |||
| mTOR signaling pathway (hsa04150) | ||||
| In-vitro Model | MPM (Mouse primary myoblasts from about 10-day-old C57BL/6J were isolated) | |||
| C2C12 | Normal | Mus musculus | CVCL_0188 | |
| HEK293-FT | Normal | Homo sapiens | CVCL_6911 | |
| In-vivo Model | To generate doxycycline-inducible skeletal muscle-specific FTO deletion mice, FTOflox/flox mice were crossed with HSA-Cre mice to generate FTOflox/+ HSA-Cre mice, which were then crossed to FTOflox/flox mice to generate FTOflox/flox and FTOflox/flox HSA-Cre mice. | |||
Full List of Crosstalk(s) between m6A Modification and Epigenetic Regulation Related to This Regulator
Non-coding RNA
m6A Regulator: Fat mass and obesity-associated protein (FTO)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT05346 | ||
| Epigenetic Regulator | MicroRNA 155 (MIR155) | |
| Regulated Target | FTO alpha-ketoglutarate dependent dioxygenase (FTO) | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | Clear cell renal cell carcinoma | |
RNA Modification Sequencing Data Associated with the Target (ID: M6ATAR00373)
| In total 1 m6A sequence/site(s) in this target gene | |||
| mod ID: A2ISITE000299 | Click to Show/Hide the Full List | ||
| mod site | chr4:23812014-23812015:- | [6] | |
| Sequence | TGAGGCAGGAGAATTGCTTGAACCAGGGAGGTGGAGGTTGC | ||
| Transcript ID List | ENST00000613098.4; ENST00000509702.5; ENST00000264867.7; ENST00000506055.5; rmsk_1263028 | ||
| External Link | RMBase: RNA-editing_site_103682 | ||
N6-methyladenosine (m6A)
| In total 84 m6A sequence/site(s) in this target gene | |||
| mod ID: M6ASITE065458 | Click to Show/Hide the Full List | ||
| mod site | chr4:23792203-23792204:- | [7] | |
| Sequence | AGTGTAATACTGTTGGTTGAACTATGCTGAAGAGGGAAAGT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633320 | ||
| mod ID: M6ASITE065459 | Click to Show/Hide the Full List | ||
| mod site | chr4:23792763-23792764:- | [7] | |
| Sequence | GGAGAGAATATTTCAAATGAACACGTGCACCCCATCATCAC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000264867.7; ENST00000509702.5 | ||
| External Link | RMBase: m6A_site_633321 | ||
| mod ID: M6ASITE065460 | Click to Show/Hide the Full List | ||
| mod site | chr4:23792846-23792847:- | [7] | |
| Sequence | AGCTTTTGTATCTTCTGCAGACACTGTGGGTAGCCCATCAA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633322 | ||
| mod ID: M6ASITE065461 | Click to Show/Hide the Full List | ||
| mod site | chr4:23792893-23792894:- | [8] | |
| Sequence | AAGATAGTTTTCCTCAATGGACTTCAAATTGCATCTAGAAT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633323 | ||
| mod ID: M6ASITE065462 | Click to Show/Hide the Full List | ||
| mod site | chr4:23793577-23793578:- | [7] | |
| Sequence | TGACATGACTTTTCTGGAAGACATGATACACCTACTACTCA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000264867.7; ENST00000509702.5 | ||
| External Link | RMBase: m6A_site_633324 | ||
| mod ID: M6ASITE065463 | Click to Show/Hide the Full List | ||
| mod site | chr4:23793957-23793958:- | [9] | |
| Sequence | TTCCTTTGTTTACTAAAGAGACATATTTATCAGTTGCAGAT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633325 | ||
| mod ID: M6ASITE065464 | Click to Show/Hide the Full List | ||
| mod site | chr4:23794103-23794104:- | [9] | |
| Sequence | ATAATTATAGTATGATTTTTACAATAATTAATGTGTGTCTG | ||
| Motif Score | 2.07285119 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000264867.7; ENST00000509702.5 | ||
| External Link | RMBase: m6A_site_633326 | ||
| mod ID: M6ASITE065465 | Click to Show/Hide the Full List | ||
| mod site | chr4:23795091-23795092:- | [8] | |
| Sequence | GAAACCACAGGTTCCATAGAACTAATATCCTGTCTCTCTCT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; A549 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633327 | ||
| mod ID: M6ASITE065466 | Click to Show/Hide the Full List | ||
| mod site | chr4:23795108-23795109:- | [7] | |
| Sequence | TATATGACAATCCTGAAGAAACCACAGGTTCCATAGAACTA | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; A549 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633328 | ||
| mod ID: M6ASITE065467 | Click to Show/Hide the Full List | ||
| mod site | chr4:23795238-23795239:- | [7] | |
| Sequence | AACCAACCAACCAACCACAAACCACCCTAAAATGACAGCCG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000264867.7; ENST00000509702.5 | ||
| External Link | RMBase: m6A_site_633329 | ||
| mod ID: M6ASITE065468 | Click to Show/Hide the Full List | ||
| mod site | chr4:23795272-23795273:- | [7] | |
| Sequence | TAAATTAAAAAGGAAAGAAAACTAACAACCAACCAACCAAC | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000264867.7; ENST00000509702.5 | ||
| External Link | RMBase: m6A_site_633330 | ||
| mod ID: M6ASITE065469 | Click to Show/Hide the Full List | ||
| mod site | chr4:23795362-23795363:- | [7] | |
| Sequence | AGACACGCTTAAAACCTAGAACTTCAAAATGTTCGTATTCT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; A549 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000613098.4; ENST00000264867.7; ENST00000509702.5 | ||
| External Link | RMBase: m6A_site_633331 | ||
| mod ID: M6ASITE065470 | Click to Show/Hide the Full List | ||
| mod site | chr4:23795369-23795370:- | [7] | |
| Sequence | TCCATGAAGACACGCTTAAAACCTAGAACTTCAAAATGTTC | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000613098.4; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633332 | ||
| mod ID: M6ASITE065471 | Click to Show/Hide the Full List | ||
| mod site | chr4:23795380-23795381:- | [7] | |
| Sequence | ATGGCAGCAGTTCCATGAAGACACGCTTAAAACCTAGAACT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000264867.7; ENST00000509702.5; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633333 | ||
| mod ID: M6ASITE065472 | Click to Show/Hide the Full List | ||
| mod site | chr4:23795410-23795411:- | [7] | |
| Sequence | ACCAGAATGCGCAAGGGCAAACCATTTCAAATGGCAGCAGT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000264867.7; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633334 | ||
| mod ID: M6ASITE065473 | Click to Show/Hide the Full List | ||
| mod site | chr4:23795444-23795445:- | [7] | |
| Sequence | ATGTGTGGGTACATGTGAGGACTGGGGGCACCTGACCAGAA | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000613098.4; ENST00000509702.5; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633335 | ||
| mod ID: M6ASITE065474 | Click to Show/Hide the Full List | ||
| mod site | chr4:23795609-23795610:- | [7] | |
| Sequence | AGCTGCTGAAGAGGCAAGAGACAGAATGATATCCAGTAAGC | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; HEK293T; iSLK | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000613098.4; ENST00000264867.7; ENST00000509702.5 | ||
| External Link | RMBase: m6A_site_633336 | ||
| mod ID: M6ASITE065475 | Click to Show/Hide the Full List | ||
| mod site | chr4:23795633-23795634:- | [7] | |
| Sequence | AACAACAATGGTTTACATGAACACAGCTGCTGAAGAGGCAA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HEK293T; A549; iSLK | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000264867.7; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633337 | ||
| mod ID: M6ASITE065476 | Click to Show/Hide the Full List | ||
| mod site | chr4:23795667-23795668:- | [7] | |
| Sequence | ACAACAACAATACAACAAGAACAACAACAACAATAACAACA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HEK293T; A549; iSLK | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000613098.4; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633338 | ||
| mod ID: M6ASITE065477 | Click to Show/Hide the Full List | ||
| mod site | chr4:23795687-23795688:- | [7] | |
| Sequence | CTGTACAAAAACAAAACAAAACAACAACAATACAACAAGAA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HEK293T; A549; iSLK | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000613098.4; ENST00000509702.5; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633339 | ||
| mod ID: M6ASITE065478 | Click to Show/Hide the Full List | ||
| mod site | chr4:23795692-23795693:- | [7] | |
| Sequence | ACTCTCTGTACAAAAACAAAACAAAACAACAACAATACAAC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HEK293T; A549; iSLK | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000264867.7; ENST00000613098.4; ENST00000509702.5 | ||
| External Link | RMBase: m6A_site_633340 | ||
| mod ID: M6ASITE065479 | Click to Show/Hide the Full List | ||
| mod site | chr4:23795697-23795698:- | [7] | |
| Sequence | TTTACACTCTCTGTACAAAAACAAAACAAAACAACAACAAT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HEK293T; A549; iSLK | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000506055.5; ENST00000613098.4; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633341 | ||
| mod ID: M6ASITE065480 | Click to Show/Hide the Full List | ||
| mod site | chr4:23795751-23795752:- | [7] | |
| Sequence | CAGCGCGTCCTTCCCTAAAGACTATTGCAAGTCATACTTAG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; HEK293T; HepG2; A549; iSLK | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000506055.5; ENST00000509702.5; ENST00000264867.7; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633342 | ||
| mod ID: M6ASITE065481 | Click to Show/Hide the Full List | ||
| mod site | chr4:23795772-23795773:- | [7] | |
| Sequence | GATGGCGAATACCTCATGGGACAGCGCGTCCTTCCCTAAAG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HEK293T; HepG2; A549; HEK293A-TOA; iSLK | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000264867.7; ENST00000509702.5; ENST00000613098.4; ENST00000506055.5 | ||
| External Link | RMBase: m6A_site_633343 | ||
| mod ID: M6ASITE065482 | Click to Show/Hide the Full List | ||
| mod site | chr4:23795918-23795919:- | [7] | |
| Sequence | GTGTTTTTTTTCAGATTCAAACTCAGATGACTTTGACCCTG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; A549 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000613098.4; ENST00000264867.7; ENST00000506055.5; ENST00000509702.5 | ||
| External Link | RMBase: m6A_site_633344 | ||
| mod ID: M6ASITE065483 | Click to Show/Hide the Full List | ||
| mod site | chr4:23801734-23801735:- | [7] | |
| Sequence | TTTCAAGTCTAACTATGCAGACCTAGGTATGGATTTTATTG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; A549 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000613098.4; ENST00000509702.5; ENST00000506055.5; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633345 | ||
| mod ID: M6ASITE065484 | Click to Show/Hide the Full List | ||
| mod site | chr4:23801878-23801879:- | [7] | |
| Sequence | TTTCTTTGTCCGTTGCAGAGACAGCTATGGTTTCATTACCT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000613098.4; ENST00000509702.5; ENST00000506055.5; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633346 | ||
| mod ID: M6ASITE065485 | Click to Show/Hide the Full List | ||
| mod site | chr4:23802280-23802281:- | [7] | |
| Sequence | AACACGGACAGAACTGAGGGACCGTTTTGAAGTTTTTGGTG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000264867.7; ENST00000506055.5; ENST00000509702.5; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633347 | ||
| mod ID: M6ASITE065486 | Click to Show/Hide the Full List | ||
| mod site | chr4:23802288-23802289:- | [7] | |
| Sequence | CCTGACACAACACGGACAGAACTGAGGGACCGTTTTGAAGT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000506055.5; ENST00000613098.4; ENST00000264867.7; ENST00000509702.5 | ||
| External Link | RMBase: m6A_site_633348 | ||
| mod ID: M6ASITE065487 | Click to Show/Hide the Full List | ||
| mod site | chr4:23802293-23802294:- | [7] | |
| Sequence | TCAGACCTGACACAACACGGACAGAACTGAGGGACCGTTTT | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000506055.5; ENST00000509702.5; ENST00000264867.7; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633349 | ||
| mod ID: M6ASITE065488 | Click to Show/Hide the Full List | ||
| mod site | chr4:23802309-23802310:- | [7] | |
| Sequence | ATTTATGTCGGTAAAATCAGACCTGACACAACACGGACAGA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000506055.5; ENST00000264867.7; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633350 | ||
| mod ID: M6ASITE065489 | Click to Show/Hide the Full List | ||
| mod site | chr4:23813035-23813036:- | [10] | |
| Sequence | ATCACGTTCAAGATCGCCCTACAGCCGTCGGCCCAGGTAAT | ||
| Motif Score | 2.078666667 | ||
| Cell/Tissue List | kidney | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000264867.7; ENST00000613098.4; ENST00000509702.5; ENST00000506055.5 | ||
| External Link | RMBase: m6A_site_633351 | ||
| mod ID: M6ASITE065490 | Click to Show/Hide the Full List | ||
| mod site | chr4:23813091-23813092:- | [7] | |
| Sequence | TATGAGTCAAGCCACTACAGACACCGCACGCACCGAAATTC | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000506055.5; ENST00000264867.7; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633352 | ||
| mod ID: M6ASITE065491 | Click to Show/Hide the Full List | ||
| mod site | chr4:23813799-23813800:- | [7] | |
| Sequence | AAATCCTTATTTTCTCAAAGACCCCAAAGGATGCGCTCTCG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; iSLK | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000264867.7; ENST00000613098.4; ENST00000506055.5 | ||
| External Link | RMBase: m6A_site_633353 | ||
| mod ID: M6ASITE065492 | Click to Show/Hide the Full List | ||
| mod site | chr4:23813922-23813923:- | [7] | |
| Sequence | AGCGAAGATGAAAGTGATAAACTGAGCTACCCTTGGGATGG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; GSC-11; iSLK; MSC; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000506055.5; ENST00000509702.5; ENST00000264867.7; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633354 | ||
| mod ID: M6ASITE065493 | Click to Show/Hide the Full List | ||
| mod site | chr4:23813970-23813971:- | [7] | |
| Sequence | CCTATGTTTATAAATTCAGGACTAGCCATGGATGGCCTGTT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TREX; GSCs | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000264867.7; ENST00000509702.5; ENST00000506055.5; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633355 | ||
| mod ID: M6ASITE065494 | Click to Show/Hide the Full List | ||
| mod site | chr4:23813994-23813995:- | [7] | |
| Sequence | AGTAATGAACAATTCTCCAAACTACCTATGTTTATAAATTC | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TREX; endometrial; GSCs | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000264867.7; ENST00000506055.5; ENST00000613098.4; ENST00000509702.5 | ||
| External Link | RMBase: m6A_site_633356 | ||
| mod ID: M6ASITE065495 | Click to Show/Hide the Full List | ||
| mod site | chr4:23814006-23814007:- | [7] | |
| Sequence | GACAGTGATTTCAGTAATGAACAATTCTCCAAACTACCTAT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; kidney; A549; hESC-HEK293T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TREX; endometrial; GSCs | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-REF-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000613098.4; ENST00000506055.5; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633357 | ||
| mod ID: M6ASITE065496 | Click to Show/Hide the Full List | ||
| mod site | chr4:23814025-23814026:- | [7] | |
| Sequence | CAAGACCGGTGAACTGAGGGACAGTGATTTCAGTAATGAAC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; hESC-HEK293T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TREX; endometrial; GSCs | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000264867.7; ENST00000506055.5; ENST00000509702.5; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633358 | ||
| mod ID: M6ASITE065497 | Click to Show/Hide the Full List | ||
| mod site | chr4:23814033-23814034:- | [7] | |
| Sequence | GAAGCAGACAAGACCGGTGAACTGAGGGACAGTGATTTCAG | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TREX; endometrial; GSCs | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000506055.5; ENST00000264867.7; ENST00000509702.5; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633359 | ||
| mod ID: M6ASITE065498 | Click to Show/Hide the Full List | ||
| mod site | chr4:23814041-23814042:- | [7] | |
| Sequence | TTGACGACGAAGCAGACAAGACCGGTGAACTGAGGGACAGT | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TREX; endometrial; GSCs | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000264867.7; ENST00000509702.5; ENST00000506055.5; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633360 | ||
| mod ID: M6ASITE065499 | Click to Show/Hide the Full List | ||
| mod site | chr4:23814046-23814047:- | [7] | |
| Sequence | TGTTTTTGACGACGAAGCAGACAAGACCGGTGAACTGAGGG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; hESC-HEK293T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; TREX; endometrial; GSCs | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000509702.5; ENST00000613098.4; ENST00000264867.7; ENST00000506055.5 | ||
| External Link | RMBase: m6A_site_633361 | ||
| mod ID: M6ASITE065500 | Click to Show/Hide the Full List | ||
| mod site | chr4:23814055-23814056:- | [11] | |
| Sequence | CAGTCAAGCTGTTTTTGACGACGAAGCAGACAAGACCGGTG | ||
| Motif Score | 2.839505952 | ||
| Cell/Tissue List | A549 | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000509702.5; ENST00000613098.4; ENST00000506055.5; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633362 | ||
| mod ID: M6ASITE065501 | Click to Show/Hide the Full List | ||
| mod site | chr4:23814058-23814059:- | [11] | |
| Sequence | TCCCAGTCAAGCTGTTTTTGACGACGAAGCAGACAAGACCG | ||
| Motif Score | 2.833690476 | ||
| Cell/Tissue List | A549 | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000264867.7; ENST00000506055.5; ENST00000509702.5; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633363 | ||
| mod ID: M6ASITE065502 | Click to Show/Hide the Full List | ||
| mod site | chr4:23814094-23814095:- | [7] | |
| Sequence | GGAAATCCGAGCCGAGCTGAACAAGCACTTCGGTCATCCCA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; endometrial; GSCs | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000264867.7; ENST00000509702.5; ENST00000506055.5; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633364 | ||
| mod ID: M6ASITE065503 | Click to Show/Hide the Full List | ||
| mod site | chr4:23814118-23814119:- | [7] | |
| Sequence | CACAAGAAAACAGCTCCAAGACCAGGAAATCCGAGCCGAGC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; endometrial; GSCs | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000506055.5; ENST00000613098.4; ENST00000509702.5; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633365 | ||
| mod ID: M6ASITE065504 | Click to Show/Hide the Full List | ||
| mod site | chr4:23814129-23814130:- | [7] | |
| Sequence | TCTCCTTGCAGCACAAGAAAACAGCTCCAAGACCAGGAAAT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; endometrial; GSCs | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000264867.7; ENST00000509702.5; ENST00000506055.5; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633366 | ||
| mod ID: M6ASITE065505 | Click to Show/Hide the Full List | ||
| mod site | chr4:23814173-23814174:- | [7] | |
| Sequence | ACCAGTGCTACCTGAGAGAGACTTTGGAGGCAAGCAAGCAG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; peripheral-blood; GSC-11; HEK293A-TOA | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000613098.4; ENST00000509702.5; ENST00000506055.5; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633367 | ||
| mod ID: M6ASITE065506 | Click to Show/Hide the Full List | ||
| mod site | chr4:23814193-23814194:- | [7] | |
| Sequence | TTGTTCTTCCACAGATTCAGACCAGTGCTACCTGAGAGAGA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; GSC-11 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000506055.5; ENST00000264867.7; ENST00000509702.5; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633368 | ||
| mod ID: M6ASITE065507 | Click to Show/Hide the Full List | ||
| mod site | chr4:23814258-23814259:- | [7] | |
| Sequence | CAGGAGCTCCAAGACTCTAGACAACTAGAAAATAAAGATGT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; HEK293T; A549 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000613098.4; ENST00000506055.5; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633369 | ||
| mod ID: M6ASITE065508 | Click to Show/Hide the Full List | ||
| mod site | chr4:23814265-23814266:- | [7] | |
| Sequence | TATATCACAGGAGCTCCAAGACTCTAGACAACTAGAAAATA | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; HEK293T; A549 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000264867.7; ENST00000506055.5; ENST00000509702.5; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633370 | ||
| mod ID: M6ASITE065509 | Click to Show/Hide the Full List | ||
| mod site | chr4:23814302-23814303:- | [7] | |
| Sequence | GCCAGTCAATTAATTCCAAAACAGAAATACTCATTAATATA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HEK293T; A549 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000613098.4; ENST00000264867.7; ENST00000506055.5 | ||
| External Link | RMBase: m6A_site_633371 | ||
| mod ID: M6ASITE065510 | Click to Show/Hide the Full List | ||
| mod site | chr4:23814365-23814366:- | [7] | |
| Sequence | GTGGACACGAGGAAAGGAAGACCAAGCGGCCCAGTCTGCGG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HEK293T | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000513205.5; ENST00000509702.5; ENST00000506055.5; ENST00000613098.4; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633372 | ||
| mod ID: M6ASITE065511 | Click to Show/Hide the Full List | ||
| mod site | chr4:23814381-23814382:- | [7] | |
| Sequence | TCCTCAGTCCTCACTGGTGGACACGAGGAAAGGAAGACCAA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000513205.5; ENST00000613098.4; ENST00000264867.7; ENST00000506055.5 | ||
| External Link | RMBase: m6A_site_633373 | ||
| mod ID: M6ASITE065512 | Click to Show/Hide the Full List | ||
| mod site | chr4:23814518-23814519:- | [7] | |
| Sequence | AGCTGAAGTCCTCTTGCAAGACTGTGGTGCCACCACCATCA | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000264867.7; ENST00000613098.4; ENST00000513205.5; ENST00000509702.5; ENST00000509642.5; ENST00000506055.5 | ||
| External Link | RMBase: m6A_site_633374 | ||
| mod ID: M6ASITE065513 | Click to Show/Hide the Full List | ||
| mod site | chr4:23820449-23820450:- | [7] | |
| Sequence | GGTGACGGTTTAAACCACAGACACGAAGGAATTTGGGTAGG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000613098.4; ENST00000509702.5; ENST00000264867.7; ENST00000509642.5; ENST00000506055.5; ENST00000513205.5 | ||
| External Link | RMBase: m6A_site_633375 | ||
| mod ID: M6ASITE065514 | Click to Show/Hide the Full List | ||
| mod site | chr4:23820456-23820457:- | [7] | |
| Sequence | TCAACAAGGTGACGGTTTAAACCACAGACACGAAGGAATTT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000506055.5; ENST00000513205.5; ENST00000509702.5; ENST00000613098.4; ENST00000509642.5; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633376 | ||
| mod ID: M6ASITE065515 | Click to Show/Hide the Full List | ||
| mod site | chr4:23820629-23820630:- | [7] | |
| Sequence | TCTCTTTTACAGGTGTAAAAACTAATTTGATTTCAAAATAG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000513205.5; ENST00000617484.4; ENST00000509642.5; ENST00000264867.7; ENST00000613098.4; ENST00000509702.5; ENST00000506055.5 | ||
| External Link | RMBase: m6A_site_633377 | ||
| mod ID: M6ASITE065516 | Click to Show/Hide the Full List | ||
| mod site | chr4:23824285-23824286:- | [7] | |
| Sequence | TAAGTGTGGAACTCTCTGGAACTGCAGGTAAGCCTTATATT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000506055.5; ENST00000513205.5; ENST00000509702.5; ENST00000264867.7; ENST00000613098.4; ENST00000617484.4; ENST00000509642.5 | ||
| External Link | RMBase: m6A_site_633378 | ||
| mod ID: M6ASITE065517 | Click to Show/Hide the Full List | ||
| mod site | chr4:23824295-23824296:- | [7] | |
| Sequence | GAACGCACCTTAAGTGTGGAACTCTCTGGAACTGCAGGTAA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000264867.7; ENST00000509642.5; ENST00000613098.4; ENST00000509702.5; ENST00000617484.4; ENST00000513205.5; ENST00000506055.5 | ||
| External Link | RMBase: m6A_site_633379 | ||
| mod ID: M6ASITE065518 | Click to Show/Hide the Full List | ||
| mod site | chr4:23824321-23824322:- | [7] | |
| Sequence | GTTCCCCATTTGAGAACAAGACTATTGAACGCACCTTAAGT | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000506055.5; ENST00000617484.4; ENST00000613098.4; ENST00000509642.5; ENST00000513205.5; ENST00000509702.5; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633380 | ||
| mod ID: M6ASITE065519 | Click to Show/Hide the Full List | ||
| mod site | chr4:23824326-23824327:- | [7] | |
| Sequence | CAAGGGTTCCCCATTTGAGAACAAGACTATTGAACGCACCT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000613098.4; ENST00000513205.5; ENST00000506055.5; ENST00000509642.5; ENST00000617484.4; ENST00000264867.7; ENST00000509702.5 | ||
| External Link | RMBase: m6A_site_633381 | ||
| mod ID: M6ASITE065520 | Click to Show/Hide the Full List | ||
| mod site | chr4:23824503-23824504:- | [7] | |
| Sequence | TTTTTTTTTTCTTTAGCCAAACCAACAACTTTATCTCTTCC | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000613098.4; ENST00000509642.5; ENST00000509702.5; ENST00000506055.5; ENST00000513205.5; ENST00000264867.7; ENST00000612355.1; ENST00000617484.4 | ||
| External Link | RMBase: m6A_site_633382 | ||
| mod ID: M6ASITE065521 | Click to Show/Hide the Full List | ||
| mod site | chr4:23828455-23828456:- | [7] | |
| Sequence | GAACAGAAACAGCAGCAGAGACAAATGCACCTCCAAAAAGA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000613098.4; ENST00000509702.5; ENST00000617484.4; ENST00000264867.7; ENST00000506055.5; ENST00000509642.5; ENST00000513205.5; ENST00000612355.1 | ||
| External Link | RMBase: m6A_site_633383 | ||
| mod ID: M6ASITE065522 | Click to Show/Hide the Full List | ||
| mod site | chr4:23828467-23828468:- | [7] | |
| Sequence | CAAACCCACAGAGAACAGAAACAGCAGCAGAGACAAATGCA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000612355.1; ENST00000617484.4; ENST00000509642.5; ENST00000509702.5; ENST00000264867.7; ENST00000506055.5; ENST00000513205.5; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633384 | ||
| mod ID: M6ASITE065523 | Click to Show/Hide the Full List | ||
| mod site | chr4:23828473-23828474:- | [7] | |
| Sequence | TCACACCAAACCCACAGAGAACAGAAACAGCAGCAGAGACA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000613098.4; ENST00000509642.5; ENST00000612355.1; ENST00000264867.7; ENST00000509702.5; ENST00000617484.4; ENST00000506055.5; ENST00000513205.5 | ||
| External Link | RMBase: m6A_site_633385 | ||
| mod ID: M6ASITE065524 | Click to Show/Hide the Full List | ||
| mod site | chr4:23828484-23828485:- | [7] | |
| Sequence | GATGACCCTCCTCACACCAAACCCACAGAGAACAGAAACAG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000509642.5; ENST00000513205.5; ENST00000612355.1; ENST00000506055.5; ENST00000613098.4; ENST00000509702.5; ENST00000264867.7; ENST00000617484.4 | ||
| External Link | RMBase: m6A_site_633386 | ||
| mod ID: M6ASITE065525 | Click to Show/Hide the Full List | ||
| mod site | chr4:23828562-23828563:- | [10] | |
| Sequence | AAAGCGAAGAGTATTTGTCAACAGCAAAAGCCACAAAGACG | ||
| Motif Score | 2.173910714 | ||
| Cell/Tissue List | kidney | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000506055.5; ENST00000508380.1; ENST00000514479.1; ENST00000509642.5; ENST00000513205.5; ENST00000264867.7; ENST00000612355.1; ENST00000617484.4; ENST00000509702.5; ENST00000613098.4 | ||
| External Link | RMBase: m6A_site_633387 | ||
| mod ID: M6ASITE065526 | Click to Show/Hide the Full List | ||
| mod site | chr4:23829482-23829483:- | [7] | |
| Sequence | ATCACAATCACAGGATCAGAACAAACCCTGCAATTGTTAAG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000617484.4; ENST00000514479.1; ENST00000508380.1; ENST00000506055.5; ENST00000264867.7; ENST00000613098.4; ENST00000509702.5; ENST00000612355.1; ENST00000509642.5; ENST00000513205.5 | ||
| External Link | RMBase: m6A_site_633388 | ||
| mod ID: M6ASITE065527 | Click to Show/Hide the Full List | ||
| mod site | chr4:23829511-23829512:- | [7] | |
| Sequence | CAGTGGTCTCAGTACCCAGAACCATGCAAATCACAATCACA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000514479.1; ENST00000264867.7; ENST00000508380.1; ENST00000612355.1; ENST00000509702.5; ENST00000513205.5; ENST00000613098.4; ENST00000509642.5; ENST00000506055.5; ENST00000617484.4 | ||
| External Link | RMBase: m6A_site_633389 | ||
| mod ID: M6ASITE065528 | Click to Show/Hide the Full List | ||
| mod site | chr4:23831549-23831550:- | [7] | |
| Sequence | AAGAGCCGTCTCTAGTAAGAACCCTTCCTACTGTTTAGCAG | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000509642.5; ENST00000509702.5; ENST00000512169.1; ENST00000513205.5; ENST00000613098.4; ENST00000508380.1; ENST00000612355.1; ENST00000617484.4; ENST00000264867.7; ENST00000506055.5 | ||
| External Link | RMBase: m6A_site_633390 | ||
| mod ID: M6ASITE065529 | Click to Show/Hide the Full List | ||
| mod site | chr4:23831695-23831696:- | [7] | |
| Sequence | AGTCCTCACAGAGACACTAGACAGTCTCCCTGTGGATGAAG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000513205.5; ENST00000264867.7; ENST00000617484.4; ENST00000506055.5; ENST00000612355.1; ENST00000613098.4; ENST00000512169.1; ENST00000509642.5; ENST00000508380.1; ENST00000509702.5 | ||
| External Link | RMBase: m6A_site_633391 | ||
| mod ID: M6ASITE065530 | Click to Show/Hide the Full List | ||
| mod site | chr4:23831702-23831703:- | [7] | |
| Sequence | TGCTAGCAGTCCTCACAGAGACACTAGACAGTCTCCCTGTG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000512169.1; ENST00000509702.5; ENST00000617484.4; ENST00000613098.4; ENST00000612355.1; ENST00000513205.5; ENST00000509642.5; ENST00000264867.7; ENST00000506055.5; ENST00000508380.1 | ||
| External Link | RMBase: m6A_site_633392 | ||
| mod ID: M6ASITE065531 | Click to Show/Hide the Full List | ||
| mod site | chr4:23831725-23831726:- | [7] | |
| Sequence | AGATGAAGAGAATGAGGCAAACTTGCTAGCAGTCCTCACAG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000508380.1; ENST00000613098.4; ENST00000513205.5; ENST00000509702.5; ENST00000612355.1; ENST00000264867.7; ENST00000512169.1; ENST00000506055.5; ENST00000507342.5; ENST00000617484.4; ENST00000509642.5 | ||
| External Link | RMBase: m6A_site_633393 | ||
| mod ID: M6ASITE065532 | Click to Show/Hide the Full List | ||
| mod site | chr4:23866132-23866133:- | [7] | |
| Sequence | AAAGAAAATGCAGTTTTAAAACACTAGGCTGGCTATTCCTG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000509702.5; ENST00000264867.7; ENST00000508380.1; ENST00000515534.5; ENST00000617484.4; ENST00000509642.5; ENST00000513205.5; ENST00000506055.5; ENST00000507342.5; ENST00000512169.1; ENST00000613098.4; ENST00000612355.1 | ||
| External Link | RMBase: m6A_site_633394 | ||
| mod ID: M6ASITE065533 | Click to Show/Hide the Full List | ||
| mod site | chr4:23884823-23884824:- | [7] | |
| Sequence | ACAGACAGCTTTCTGGGTGGACTCAAGTGGTGCAGTGACCA | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000264867.7; ENST00000515534.5; ENST00000612355.1; ENST00000507380.1; ENST00000506055.5; ENST00000513205.5; ENST00000514494.1; ENST00000507342.5; ENST00000617484.4; ENST00000503714.1 | ||
| External Link | RMBase: m6A_site_633395 | ||
| mod ID: M6ASITE065534 | Click to Show/Hide the Full List | ||
| mod site | chr4:23884839-23884840:- | [7] | |
| Sequence | TGTGAACGACTTGGATACAGACAGCTTTCTGGGTGGACTCA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000513205.5; ENST00000264867.7; ENST00000514494.1; ENST00000612355.1; ENST00000515534.5; ENST00000507342.5; ENST00000617484.4; ENST00000503714.1; ENST00000507380.1; ENST00000506055.5 | ||
| External Link | RMBase: m6A_site_633396 | ||
| mod ID: M6ASITE065535 | Click to Show/Hide the Full List | ||
| mod site | chr4:23884865-23884866:- | [7] | |
| Sequence | CCTGAACTTGATCTTTCTGAACTAGATGTGAACGACTTGGA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000507342.5; ENST00000264867.7; ENST00000514494.1; ENST00000617484.4; ENST00000506055.5; ENST00000503714.1; ENST00000515534.5; ENST00000612355.1; ENST00000507380.1; ENST00000513205.5 | ||
| External Link | RMBase: m6A_site_633397 | ||
| mod ID: M6ASITE065536 | Click to Show/Hide the Full List | ||
| mod site | chr4:23884880-23884881:- | [7] | |
| Sequence | CTTTGCCCAGATCTTCCTGAACTTGATCTTTCTGAACTAGA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000507342.5; ENST00000513205.5; ENST00000514494.1; ENST00000503714.1; ENST00000507380.1; ENST00000612355.1; ENST00000506055.5; ENST00000264867.7; ENST00000515534.5; ENST00000617484.4 | ||
| External Link | RMBase: m6A_site_633398 | ||
| mod ID: M6ASITE065537 | Click to Show/Hide the Full List | ||
| mod site | chr4:23884908-23884909:- | [7] | |
| Sequence | TGCTGCTCTGGTTGGTGAAGACCAGCCTCTTTGCCCAGATC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000503714.1; ENST00000507342.5; ENST00000612355.1; ENST00000513205.5; ENST00000506055.5; ENST00000514494.1; ENST00000264867.7; ENST00000515534.5; ENST00000507380.1; ENST00000617484.4 | ||
| External Link | RMBase: m6A_site_633399 | ||
| mod ID: M6ASITE065538 | Click to Show/Hide the Full List | ||
| mod site | chr4:23889910-23889911:- | [10] | |
| Sequence | CTCTGAGTCTGTATGGAGTGACATCGAGGTGAGCTGGGGCA | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | kidney | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000515534.5; ENST00000617484.4; ENST00000507342.5; ENST00000514494.1; ENST00000506055.5; ENST00000507380.1; ENST00000264867.7; ENST00000513205.5; ENST00000612355.1 | ||
| External Link | RMBase: m6A_site_633400 | ||
| mod ID: M6ASITE065539 | Click to Show/Hide the Full List | ||
| mod site | chr4:23889931-23889932:- | [7] | |
| Sequence | GTGGGACATGTGCAACCAGGACTCTGAGTCTGTATGGAGTG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000513205.5; ENST00000507380.1; ENST00000617484.4; ENST00000612355.1; ENST00000514494.1; ENST00000506055.5; ENST00000515534.5; ENST00000507342.5; ENST00000264867.7 | ||
| External Link | RMBase: m6A_site_633401 | ||
| mod ID: M6ASITE065540 | Click to Show/Hide the Full List | ||
| mod site | chr4:23889946-23889947:- | [7] | |
| Sequence | CAGGAGCTGGATGGCGTGGGACATGTGCAACCAGGACTCTG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000513205.5; ENST00000507342.5; ENST00000617484.4; ENST00000514494.1; ENST00000264867.7; ENST00000506055.5; ENST00000507380.1; ENST00000515534.5; ENST00000612355.1 | ||
| External Link | RMBase: m6A_site_633402 | ||
| mod ID: M6ASITE065541 | Click to Show/Hide the Full List | ||
| mod site | chr4:23890069-23890070:- | [7] | |
| Sequence | TGACTGGGGACTGTAGTAAGACAGGTGCCTTCAGTTCACTC | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000514494.1; ENST00000507342.5; ENST00000617484.4; ENST00000612355.1 | ||
| External Link | RMBase: m6A_site_633403 | ||
References