m6A Target Gene Information
General Information of the m6A Target Gene (ID: M6ATAR00217)
Full List of m6A Methylation Regulator of This Target Gene and Corresponding Disease/Drug Response(s)
CYP1B1
can be regulated by the following regulator(s), and cause disease/drug response(s). You can browse detail information of regulator(s) or disease/drug response(s).
Browse Regulator
Browse Disease
Methyltransferase-like 3 (METTL3) [WRITER]
| Representative RNA-seq result indicating the expression of this target gene regulated by METTL3 | ||
| Cell Line | Caco-2 cell line | Homo sapiens |
|
Treatment: shMETTL3 Caco-2 cells
Control: shNTC Caco-2 cells
|
GSE167075 | |
| Regulation |
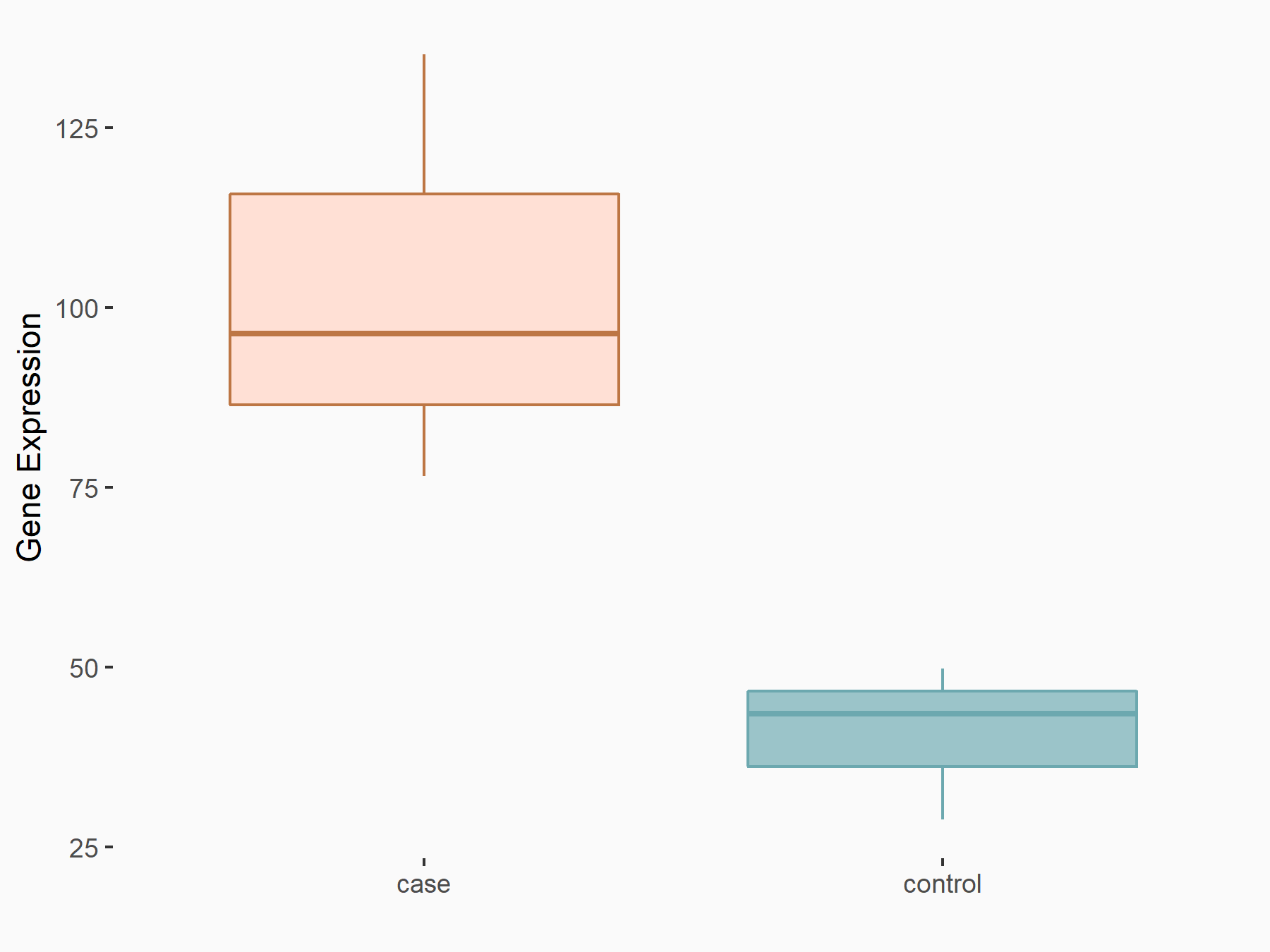  |
logFC: 1.33E+00 p-value: 1.93E-05 |
| More Results | Click to View More RNA-seq Results | |
| Representative RIP-seq result supporting the interaction between CYP1B1 and the regulator | ||
| Cell Line | MDA-MB-231 | Homo sapiens |
| Regulation | logFC: 1.72E+00 | GSE60213 |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | METTL3 contributes to renal ischemia-reperfusion injury by regulating Foxd1 methylation. When METTL3 was inhibited, m6A levels were accordingly decreased and cell apoptosis was suppressed in the H/R in vitro model. Based on MeRIP sequencing, transcription factor activating enhancer binding protein 2-alpha (tfap2a), Cytochrome P450 1B1 (CYP1B1), and forkhead box D1 (foxd1) were significantly differentially expressed, as was m6A, which is involved in the negative regulation of cell proliferation and kidney development. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Injury of kidney | ICD-11: NB92.0 | ||
| Cell Process | Cell proliferation | |||
| Cell apoptosis | ||||
| In-vitro Model | NRK-52E | Normal | Rattus norvegicus | CVCL_0468 |
| In-vivo Model | Rats were anesthetized and incised through the midline of the abdomen, and the left renal vertebral arch and arteries were blocked for 45 min, thereby resulting in left kidney ischemia. At the same time, the right kidney was removed, further aggravating the degree of left kidney injury. | |||
Methyltransferase-like 14 (METTL14) [WRITER]
| Representative RNA-seq result indicating the expression of this target gene regulated by METTL14 | ||
| Cell Line | MDA-MB-231 | Homo sapiens |
|
Treatment: siMETTL14 MDA-MB-231 cells
Control: MDA-MB-231 cells
|
GSE81164 | |
| Regulation |
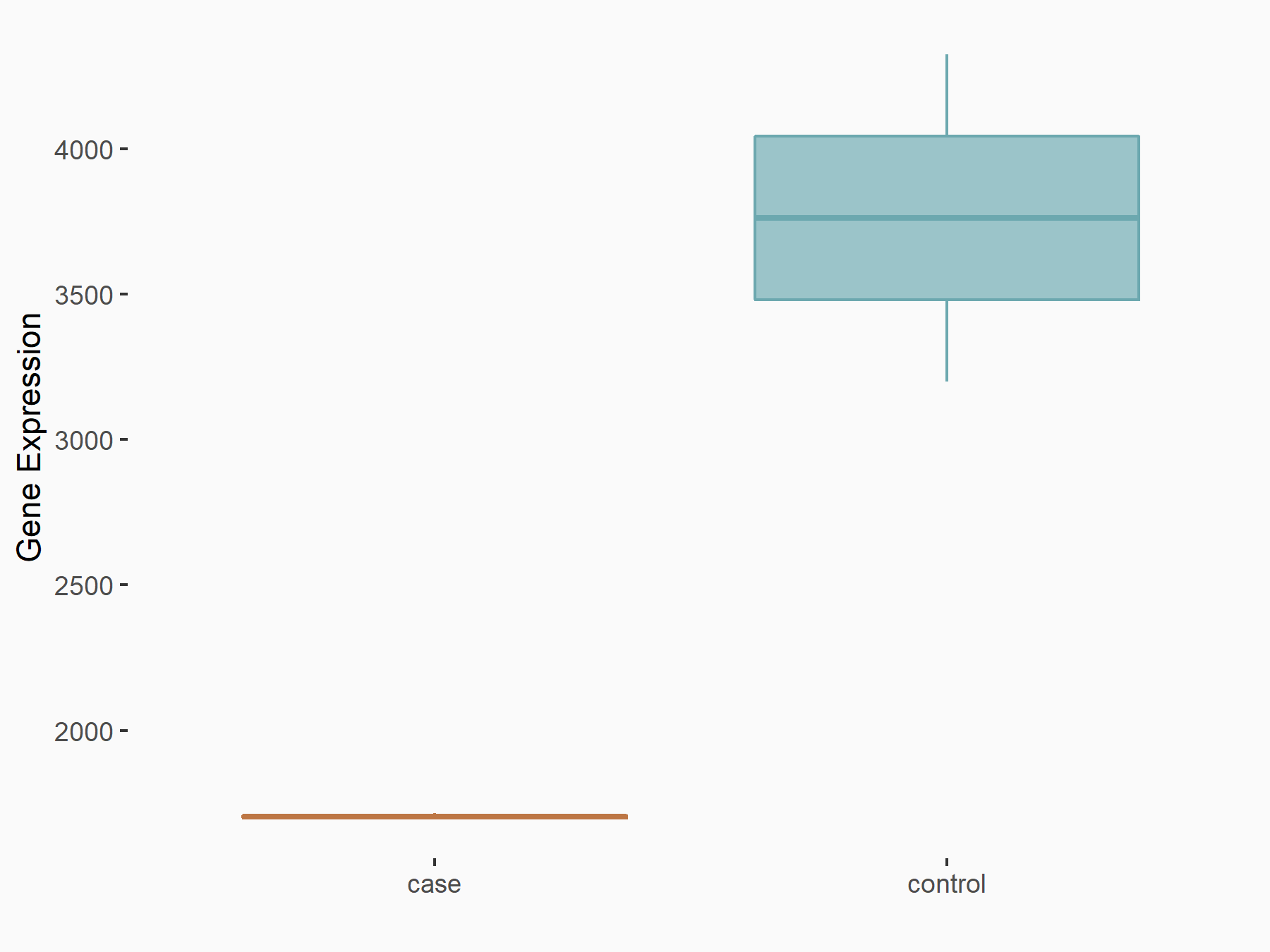  |
logFC: -1.14E+00 p-value: 9.74E-11 |
| More Results | Click to View More RNA-seq Results | |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [2] | |||
| Response Summary | LNC942-METTL14-CXCR4/Cytochrome P450 1B1 (CYP1B1) signaling axis, which provides new targets and crosstalk m6A epigenetic modification mechanism for breast cancer prevention and treatment. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | ||
| Cell Process | Cell apoptosis | |||
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [2] | |||
| Response Summary | LNC942-METTL14-CXCR4/Cytochrome P450 1B1 (CYP1B1) signaling axis, which provides new targets and crosstalk m6A epigenetic modification mechanism for breast cancer prevention and treatment. | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
| Target Regulator | Methyltransferase-like 14 (METTL14) | WRITER | ||
| Target Regulation | Up regulation | |||
| Cell Process | Cell apoptosis | |||
Urinary/pelvic organs injury [ICD-11: NB92]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [1] | |||
| Response Summary | METTL3 contributes to renal ischemia-reperfusion injury by regulating Foxd1 methylation. When METTL3 was inhibited, m6A levels were accordingly decreased and cell apoptosis was suppressed in the H/R in vitro model. Based on MeRIP sequencing, transcription factor activating enhancer binding protein 2-alpha (tfap2a), Cytochrome P450 1B1 (CYP1B1), and forkhead box D1 (foxd1) were significantly differentially expressed, as was m6A, which is involved in the negative regulation of cell proliferation and kidney development. | |||
| Responsed Disease | Injury of kidney [ICD-11: NB92.0] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Cell Process | Cell proliferation | |||
| Cell apoptosis | ||||
| In-vitro Model | NRK-52E | Normal | Rattus norvegicus | CVCL_0468 |
| In-vivo Model | Rats were anesthetized and incised through the midline of the abdomen, and the left renal vertebral arch and arteries were blocked for 45 min, thereby resulting in left kidney ischemia. At the same time, the right kidney was removed, further aggravating the degree of left kidney injury. | |||
Full List of Crosstalk(s) between m6A Modification and Epigenetic Regulation Related to This Regulator
DNA modification
m6A Regulator: Methyltransferase-like 14 (METTL14)
| In total 3 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT02205 | ||
| Epigenetic Regulator | DNA (cytosine-5)-methyltransferase 3B (DNMT3B) | |
| Regulated Target | Methyltransferase-like protein 14 (METTL14) | |
| Crosstalk relationship | DNA modification → m6A | |
| Disease | Breast cancer | |
| Crosstalk ID: M6ACROT02229 | ||
| Epigenetic Regulator | Cysteine methyltransferase DNMT3A (DNMT3A) | |
| Regulated Target | Methyltransferase-like protein 14 (METTL14) | |
| Crosstalk relationship | DNA modification → m6A | |
| Disease | Breast cancer | |
| Crosstalk ID: M6ACROT02253 | ||
| Epigenetic Regulator | DNA (cytosine-5)-methyltransferase 1 (DNMT1) | |
| Regulated Target | Methyltransferase-like protein 14 (METTL14) | |
| Crosstalk relationship | DNA modification → m6A | |
| Disease | Breast cancer | |
Non-coding RNA
m6A Regulator: Methyltransferase-like 14 (METTL14)
| In total 2 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT05193 | ||
| Epigenetic Regulator | piR-14633 | |
| Regulated Target | Methyltransferase-like protein 14 (METTL14) | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | Cervical cancer | |
| Crosstalk ID: M6ACROT05353 | ||
| Epigenetic Regulator | Long intergenic non-protein coding RNA 942 (LINC00942) | |
| Regulated Target | Methyltransferase-like protein 14 (METTL14) | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | Breast cancer | |
RNA Modification Sequencing Data Associated with the Target (ID: M6ATAR00217)
| In total 1 m6A sequence/site(s) in this target gene | |||
| mod ID: M5CSITE002290 | Click to Show/Hide the Full List | ||
| mod site | chr2:38075379-38075380:- | [5] | |
| Sequence | TCTGTCCCCAGCATGGGCACCAGCCTCAGCCCGAACGACCC | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000613082.1; ENST00000494864.1; ENST00000610745.5; ENST00000490576.1; ENST00000614273.1 | ||
| External Link | RMBase: m5C_site_26349 | ||
N6-methyladenosine (m6A)
| In total 70 m6A sequence/site(s) in this target gene | |||
| mod ID: M6ASITE044018 | Click to Show/Hide the Full List | ||
| mod site | chr2:38067642-38067643:- | [6] | |
| Sequence | ATCTCATTACTTATACTGGGACACCATTACCAAAATAATAA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468851 | ||
| mod ID: M6ASITE044019 | Click to Show/Hide the Full List | ||
| mod site | chr2:38067717-38067718:- | [6] | |
| Sequence | ACAAAGTACATTCTGGGGAAACAACATTTATATGTAGCCTT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468852 | ||
| mod ID: M6ASITE044020 | Click to Show/Hide the Full List | ||
| mod site | chr2:38067737-38067738:- | [6] | |
| Sequence | GCTACAGTGCATAGTTGTAGACAAAGTACATTCTGGGGAAA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468853 | ||
| mod ID: M6ASITE044021 | Click to Show/Hide the Full List | ||
| mod site | chr2:38067842-38067843:- | [6] | |
| Sequence | TAAAAGGTATACTTTAGTAGACATTTATAACTCAAGGATAC | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; kidney | ||
| Seq Type List | m6A-seq; m6A-REF-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468854 | ||
| mod ID: M6ASITE044022 | Click to Show/Hide the Full List | ||
| mod site | chr2:38067877-38067878:- | [7] | |
| Sequence | TTTGTGTGTTTTTAGCTGTGACACAACTGTGTGATTAAAAG | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468855 | ||
| mod ID: M6ASITE044023 | Click to Show/Hide the Full List | ||
| mod site | chr2:38067990-38067991:- | [6] | |
| Sequence | AAAAGCTTTCATGTCCCAGAACTTAGCCTTTACCTGTGAAG | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468856 | ||
| mod ID: M6ASITE044024 | Click to Show/Hide the Full List | ||
| mod site | chr2:38068047-38068048:- | [6] | |
| Sequence | GCAAATTGCTTTTTTCCAAAACAAAAAGATGTCTCAGGTTT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T | ||
| Seq Type List | m6A-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468857 | ||
| mod ID: M6ASITE044025 | Click to Show/Hide the Full List | ||
| mod site | chr2:38068164-38068165:- | [7] | |
| Sequence | GAGAGAGAATGTATTTGCTGACAACCATTAAAGTCAGAAGT | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468858 | ||
| mod ID: M6ASITE044026 | Click to Show/Hide the Full List | ||
| mod site | chr2:38068205-38068206:- | [6] | |
| Sequence | GATTACTGCTTTAATCAGAAACCCTCATTGTGTTTCTACCG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468859 | ||
| mod ID: M6ASITE044027 | Click to Show/Hide the Full List | ||
| mod site | chr2:38068271-38068272:- | [6] | |
| Sequence | GTTAAAGAAAGGAAAAGAAAACAAAAAACGAATGAAAATAA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T | ||
| Seq Type List | m6A-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468860 | ||
| mod ID: M6ASITE044028 | Click to Show/Hide the Full List | ||
| mod site | chr2:38068463-38068464:- | [6] | |
| Sequence | AGCTGCAAAAATAATCATGAACCAATCTGGATGCCTCATTA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468861 | ||
| mod ID: M6ASITE044029 | Click to Show/Hide the Full List | ||
| mod site | chr2:38068533-38068534:- | [6] | |
| Sequence | TATGCCCCACCAAGGCTGAGACAGTGAATTTGGGCTGCTGT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468862 | ||
| mod ID: M6ASITE044030 | Click to Show/Hide the Full List | ||
| mod site | chr2:38068612-38068613:- | [7] | |
| Sequence | TAAAGGCATTAGAGTCAACTACACAAAGCAGGCTTGCCCAG | ||
| Motif Score | 2.078666667 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468863 | ||
| mod ID: M6ASITE044031 | Click to Show/Hide the Full List | ||
| mod site | chr2:38068702-38068703:- | [6] | |
| Sequence | GTGGTTTGGAATATTAAAAAACTTCATGTAATTTTATTTTA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468864 | ||
| mod ID: M6ASITE044032 | Click to Show/Hide the Full List | ||
| mod site | chr2:38068740-38068741:- | [7] | |
| Sequence | GGCTATTAAAAAAGTCTACAACATAGCAGATCTGTTTTGTG | ||
| Motif Score | 2.173910714 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468865 | ||
| mod ID: M6ASITE044033 | Click to Show/Hide the Full List | ||
| mod site | chr2:38068743-38068744:- | [7] | |
| Sequence | ATAGGCTATTAAAAAAGTCTACAACATAGCAGATCTGTTTT | ||
| Motif Score | 2.078666667 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468866 | ||
| mod ID: M6ASITE044034 | Click to Show/Hide the Full List | ||
| mod site | chr2:38068807-38068808:- | [6] | |
| Sequence | GCACAAAATTCAAAGCATGGACATTTAGAAGAAAGATGTTT | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T | ||
| Seq Type List | m6A-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468867 | ||
| mod ID: M6ASITE044035 | Click to Show/Hide the Full List | ||
| mod site | chr2:38068863-38068864:- | [6] | |
| Sequence | AAAGAAGGAAAGATGGTTAAACATTTTCCCACTCATTCTGA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468868 | ||
| mod ID: M6ASITE044036 | Click to Show/Hide the Full List | ||
| mod site | chr2:38068890-38068891:- | [6] | |
| Sequence | TGGTAAGAGAAAAGAGAGAAACACTGAAAAGAAGGAAAGAT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468869 | ||
| mod ID: M6ASITE044037 | Click to Show/Hide the Full List | ||
| mod site | chr2:38069100-38069101:- | [7] | |
| Sequence | CAAATGAAATGTATGCATTCACATTTAGAAAAGTGAATTGA | ||
| Motif Score | 2.047297619 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468870 | ||
| mod ID: M6ASITE044038 | Click to Show/Hide the Full List | ||
| mod site | chr2:38069190-38069191:- | [6] | |
| Sequence | TTGGAAGCTGTTTGGAAAAGACAGTGGAGATGAGGTCAGTT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468871 | ||
| mod ID: M6ASITE044039 | Click to Show/Hide the Full List | ||
| mod site | chr2:38069254-38069255:- | [7] | |
| Sequence | AGATGATTTTTTGAAAGTTAACATTAATGCCTGCTTTTTGG | ||
| Motif Score | 2.168095238 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468872 | ||
| mod ID: M6ASITE044040 | Click to Show/Hide the Full List | ||
| mod site | chr2:38069297-38069298:- | [7] | |
| Sequence | TTGGGGACAGAACTCCCATTACAACTGACCAAGTTTCTCTT | ||
| Motif Score | 2.07285119 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468873 | ||
| mod ID: M6ASITE044041 | Click to Show/Hide the Full List | ||
| mod site | chr2:38069306-38069307:- | [6] | |
| Sequence | TGACAAGAGTTGGGGACAGAACTCCCATTACAACTGACCAA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; peripheral-blood | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468874 | ||
| mod ID: M6ASITE044042 | Click to Show/Hide the Full List | ||
| mod site | chr2:38069311-38069312:- | [6] | |
| Sequence | CGAGGTGACAAGAGTTGGGGACAGAACTCCCATTACAACTG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; peripheral-blood | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468875 | ||
| mod ID: M6ASITE044043 | Click to Show/Hide the Full List | ||
| mod site | chr2:38069420-38069421:- | [6] | |
| Sequence | TGAAGTTAGTGGAAATCTGAACATTCTCCTGTGGAAGGCAG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468876 | ||
| mod ID: M6ASITE044044 | Click to Show/Hide the Full List | ||
| mod site | chr2:38069577-38069578:- | [7] | |
| Sequence | GATGTGTTCTTATTTTTATAACATCTTTATTGAAATTCTAT | ||
| Motif Score | 2.168095238 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468877 | ||
| mod ID: M6ASITE044045 | Click to Show/Hide the Full List | ||
| mod site | chr2:38069664-38069665:- | [7] | |
| Sequence | AAATCAACCACCTATTTTTGACATGGAAATGAAGCAGGGTT | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468878 | ||
| mod ID: M6ASITE044046 | Click to Show/Hide the Full List | ||
| mod site | chr2:38069724-38069725:- | [7] | |
| Sequence | AATATTGAAAATTGAAAAGTACAACTAACGCAACCAAGTGT | ||
| Motif Score | 2.856142857 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468879 | ||
| mod ID: M6ASITE044047 | Click to Show/Hide the Full List | ||
| mod site | chr2:38069986-38069987:- | [6] | |
| Sequence | ATGCATTATTAAATTGTAAAACTCCAAGGTGATGTTGTACC | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468880 | ||
| mod ID: M6ASITE044048 | Click to Show/Hide the Full List | ||
| mod site | chr2:38070158-38070159:- | [6] | |
| Sequence | TTTTCAGGAAAATAACTTAGACTCTAGTATTTATGGGTGGA | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000491456.1; ENST00000494864.1 | ||
| External Link | RMBase: m6A_site_468881 | ||
| mod ID: M6ASITE044049 | Click to Show/Hide the Full List | ||
| mod site | chr2:38070199-38070200:- | [6] | |
| Sequence | TGATAAGGGAAAAGATAAAGACCAGAAATTCCCTTTTCACC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000494864.1; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468882 | ||
| mod ID: M6ASITE044050 | Click to Show/Hide the Full List | ||
| mod site | chr2:38070254-38070255:- | [6] | |
| Sequence | ATATTGTTGAAGAGACAGAGACAAGTAATTTCAGTGTAAAG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000491456.1; ENST00000494864.1 | ||
| External Link | RMBase: m6A_site_468883 | ||
| mod ID: M6ASITE044051 | Click to Show/Hide the Full List | ||
| mod site | chr2:38070260-38070261:- | [6] | |
| Sequence | CAGAGTATATTGTTGAAGAGACAGAGACAAGTAATTTCAGT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000494864.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468884 | ||
| mod ID: M6ASITE044052 | Click to Show/Hide the Full List | ||
| mod site | chr2:38070289-38070290:- | [6] | |
| Sequence | ATAGACAAAAAGTATATTAAACAAAGTTTCAGAGTATATTG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000494864.1; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468885 | ||
| mod ID: M6ASITE044053 | Click to Show/Hide the Full List | ||
| mod site | chr2:38070305-38070306:- | [6] | |
| Sequence | TCTCCCATATGCAGAAATAGACAAAAAGTATATTAAACAAA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000610745.5; ENST00000494864.1 | ||
| External Link | RMBase: m6A_site_468886 | ||
| mod ID: M6ASITE044054 | Click to Show/Hide the Full List | ||
| mod site | chr2:38070385-38070386:- | [6] | |
| Sequence | CACCCAAACACTTACACCAAACTACTGAATGAAGCAGTATT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; A549; GM12878 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000494864.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468887 | ||
| mod ID: M6ASITE044055 | Click to Show/Hide the Full List | ||
| mod site | chr2:38070398-38070399:- | [6] | |
| Sequence | ATATAGACACATACACCCAAACACTTACACCAAACTACTGA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; A549; GM12878 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000494864.1; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468888 | ||
| mod ID: M6ASITE044056 | Click to Show/Hide the Full List | ||
| mod site | chr2:38070412-38070413:- | [6] | |
| Sequence | GAGTTAAAATGCACATATAGACACATACACCCAAACACTTA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; A549; GM12878 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000494864.1; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468889 | ||
| mod ID: M6ASITE044057 | Click to Show/Hide the Full List | ||
| mod site | chr2:38070420-38070421:- | [7] | |
| Sequence | ATTCTTTGGAGTTAAAATGCACATATAGACACATACACCCA | ||
| Motif Score | 2.830589286 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000491456.1; ENST00000610745.5; ENST00000494864.1 | ||
| External Link | RMBase: m6A_site_468890 | ||
| mod ID: M6ASITE044058 | Click to Show/Hide the Full List | ||
| mod site | chr2:38070473-38070474:- | [7] | |
| Sequence | GGCCCAATGAATTATTATATACATACTGCATCTTGGTTATT | ||
| Motif Score | 2.110482143 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000494864.1; ENST00000610745.5; ENST00000491456.1 | ||
| External Link | RMBase: m6A_site_468891 | ||
| mod ID: M6ASITE044059 | Click to Show/Hide the Full List | ||
| mod site | chr2:38070501-38070502:- | [6] | |
| Sequence | GAGATTTTTTTGAGTCAAAGACTTAAAGGGCCCAATGAATT | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; A549; GM12878 | ||
| Seq Type List | m6A-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000614273.1; ENST00000494864.1; ENST00000491456.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468892 | ||
| mod ID: M6ASITE044060 | Click to Show/Hide the Full List | ||
| mod site | chr2:38070732-38070733:- | [6] | |
| Sequence | AAAATTTACAAGCCAAGGAAACTTGCCAATAAGAAGCAAGA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; A549; GM12878; HEK293A-TOA; iSLK; MSC | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000614273.1; ENST00000494864.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468893 | ||
| mod ID: M6ASITE044061 | Click to Show/Hide the Full List | ||
| mod site | chr2:38070745-38070746:- | [7] | |
| Sequence | GATAGTGCTGTCCAAAATTTACAAGCCAAGGAAACTTGCCA | ||
| Motif Score | 2.07285119 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000494864.1; ENST00000610745.5; ENST00000614273.1 | ||
| External Link | RMBase: m6A_site_468894 | ||
| mod ID: M6ASITE044062 | Click to Show/Hide the Full List | ||
| mod site | chr2:38070817-38070818:- | [6] | |
| Sequence | AGTTATGGTCTAACCATTAAACCCAAGTCATTTAAAGTCAA | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; HEK293T; A549; HepG2; H1A; H1B; fibroblasts; GM12878; LCLs; MM6; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000494864.1; ENST00000614273.1 | ||
| External Link | RMBase: m6A_site_468895 | ||
| mod ID: M6ASITE044063 | Click to Show/Hide the Full List | ||
| mod site | chr2:38070931-38070932:- | [6] | |
| Sequence | AGGCGGTGCATTGGCGAAGAACTTTCTAAGATGCAGCTTTT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; HEK293T; A549; HepG2; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000494864.1; ENST00000610745.5; ENST00000614273.1 | ||
| External Link | RMBase: m6A_site_468896 | ||
| mod ID: M6ASITE044064 | Click to Show/Hide the Full List | ||
| mod site | chr2:38070989-38070990:- | [6] | |
| Sequence | GGATGGCCTCATCAACAAGGACCTGACCAGCAGAGTGATGA | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HEK293T; A549; HepG2; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000492443.1; ENST00000494864.1; ENST00000610745.5; ENST00000614273.1 | ||
| External Link | RMBase: m6A_site_468897 | ||
| mod ID: M6ASITE044065 | Click to Show/Hide the Full List | ||
| mod site | chr2:38070995-38070996:- | [7] | |
| Sequence | GGACAAGGATGGCCTCATCAACAAGGACCTGACCAGCAGAG | ||
| Motif Score | 2.173910714 | ||
| Cell/Tissue List | hESC-HEK293T; CD8T | ||
| Seq Type List | MAZTER-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000494864.1; ENST00000614273.1; ENST00000610745.5; ENST00000492443.1 | ||
| External Link | RMBase: m6A_site_468898 | ||
| mod ID: M6ASITE044066 | Click to Show/Hide the Full List | ||
| mod site | chr2:38071013-38071014:- | [6] | |
| Sequence | TGATCCAGCTCGATTCTTGGACAAGGATGGCCTCATCAACA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HEK293T; A549; hESC-HEK293T; HepG2; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; CD8T; H1299; MM6; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000614273.1; ENST00000492443.1; ENST00000610745.5; ENST00000494864.1 | ||
| External Link | RMBase: m6A_site_468899 | ||
| mod ID: M6ASITE044067 | Click to Show/Hide the Full List | ||
| mod site | chr2:38071037-38071038:- | [6] | |
| Sequence | GAAGTGGCCTAACCCGGAGAACTTTGATCCAGCTCGATTCT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; HEK293T; A549; HepG2; H1A; H1B; hNPCs; fibroblasts; GM12878; LCLs; H1299; MM6; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000494864.1; ENST00000492443.1; ENST00000614273.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468900 | ||
| mod ID: M6ASITE044068 | Click to Show/Hide the Full List | ||
| mod site | chr2:38071103-38071104:- | [6] | |
| Sequence | GGGCTACCACATTCCCAAGGACACTGTGGTTTTTGTCAACC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HEK293T; A549; hESC-HEK293T; HepG2; H1A; H1B; hNPCs; fibroblasts; GM12878; LCLs; MM6; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000614273.1; ENST00000610745.5; ENST00000494864.1; ENST00000492443.1 | ||
| External Link | RMBase: m6A_site_468901 | ||
| mod ID: M6ASITE044069 | Click to Show/Hide the Full List | ||
| mod site | chr2:38071253-38071254:- | [6] | |
| Sequence | GGATCAGGTCGTGGGGAGGGACCGTCTGCCTTGTATGGGTG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HEK293T; A549; HepG2; fibroblasts; GM12878; MT4; MM6; CD4T; peripheral-blood; HEK293A-TOA; iSLK; MSC; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000614273.1; ENST00000494864.1; ENST00000492443.1; ENST00000613082.1 | ||
| External Link | RMBase: m6A_site_468902 | ||
| mod ID: M6ASITE044070 | Click to Show/Hide the Full List | ||
| mod site | chr2:38071293-38071294:- | [6] | |
| Sequence | ACAGGTATCCTGATGTGCAGACTCGAGTGCAGGCAGAATTG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; A549; HEK293T; HepG2; H1A; H1B; fibroblasts; GM12878; CD4T; peripheral-blood; HEK293A-TOA; iSLK; MSC; endometrial; NB4; MM6 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000494864.1; ENST00000614273.1; ENST00000492443.1; ENST00000613082.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468903 | ||
| mod ID: M6ASITE044071 | Click to Show/Hide the Full List | ||
| mod site | chr2:38073598-38073599:- | [6] | |
| Sequence | GCGCTTGGCTCAGTTAGCGGACCCCCTGATGCCCACGTTGG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; A549; CD4T; peripheral-blood; HEK293A-TOA; iSLK; MSC | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000613082.1; ENST00000494864.1; ENST00000614273.1; ENST00000492443.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468904 | ||
| mod ID: M6ASITE044072 | Click to Show/Hide the Full List | ||
| mod site | chr2:38073708-38073709:- | [6] | |
| Sequence | GAGAAATGCCGGGGTAGAAGACAAGAAGCAGTCACTTTTAC | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; peripheral-blood | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000492443.1; ENST00000614273.1; ENST00000494864.1; ENST00000610745.5; ENST00000613082.1 | ||
| External Link | RMBase: m6A_site_468905 | ||
| mod ID: M6ASITE044073 | Click to Show/Hide the Full List | ||
| mod site | chr2:38073747-38073748:- | [6] | |
| Sequence | GGCAGCGCTAGGTTTATAAAACCTCCGCGCTAGGAGTTTGA | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; peripheral-blood | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000613082.1; ENST00000494864.1; ENST00000492443.1; ENST00000614273.1 | ||
| External Link | RMBase: m6A_site_468906 | ||
| mod ID: M6ASITE044074 | Click to Show/Hide the Full List | ||
| mod site | chr2:38073794-38073795:- | [6] | |
| Sequence | GACAGGTAACCCGCACAGAAACTTTCAGAAGGCGGCCACAA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; peripheral-blood | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000614273.1; ENST00000494864.1; ENST00000492443.1; ENST00000613082.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468907 | ||
| mod ID: M6ASITE044075 | Click to Show/Hide the Full List | ||
| mod site | chr2:38074390-38074391:- | [6] | |
| Sequence | CATCTTCGGCGCCAGCCAGGACACCCTGTCCACCGCGCTGC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; A549; HEK293T; HepG2; H1B; H1A; fibroblasts; GM12878; MM6; CD4T; peripheral-blood; HEK293A-TOA; iSLK; MSC; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000494864.1; ENST00000613082.1; ENST00000614273.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468910 | ||
| mod ID: M6ASITE044076 | Click to Show/Hide the Full List | ||
| mod site | chr2:38074468-38074469:- | [6] | |
| Sequence | GGAAAAGAAGGCGGCCGGGGACTCGCACGGTGGTGGCGCGC | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HEK293T; A549; HepG2; H1B; H1A; fibroblasts; GM12878; LCLs; MM6; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000613082.1; ENST00000610745.5; ENST00000614273.1; ENST00000494864.1 | ||
| External Link | RMBase: m6A_site_468911 | ||
| mod ID: M6ASITE044077 | Click to Show/Hide the Full List | ||
| mod site | chr2:38074507-38074508:- | [8] | |
| Sequence | CGCCCCCCGCGACATGATGGACGCCTTTATCCTCTCTGCGG | ||
| Motif Score | 3.616982143 | ||
| Cell/Tissue List | A549 | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000610745.5; ENST00000613082.1; ENST00000494864.1; ENST00000614273.1 | ||
| External Link | RMBase: m6A_site_468912 | ||
| mod ID: M6ASITE044078 | Click to Show/Hide the Full List | ||
| mod site | chr2:38074516-38074517:- | [9] | |
| Sequence | GCCCGGGGCCGCCCCCCGCGACATGATGGACGCCTTTATCC | ||
| Motif Score | 2.865571429 | ||
| Cell/Tissue List | kidney | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000613082.1; ENST00000494864.1; ENST00000614273.1 | ||
| External Link | RMBase: m6A_site_468913 | ||
| mod ID: M6ASITE044079 | Click to Show/Hide the Full List | ||
| mod site | chr2:38074567-38074568:- | [6] | |
| Sequence | CTTCAGCAACTTCATCCTGGACAAGTTCTTGAGGCACTGCG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HEK293T; A549; HepG2; H1A; H1B; fibroblasts; GM12878; LCLs; MM6; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000610745.5; ENST00000614273.1; ENST00000494864.1; ENST00000613082.1 | ||
| External Link | RMBase: m6A_site_468914 | ||
| mod ID: M6ASITE044080 | Click to Show/Hide the Full List | ||
| mod site | chr2:38075327-38075328:- | [6] | |
| Sequence | ACCCGCTGTCCATCCAGCAGACCACGCTCCTGCTACTCCTG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; peripheral-blood; endometrial; NB4; MM6 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000494864.1; ENST00000610745.5; ENST00000613082.1; ENST00000614273.1; ENST00000490576.1 | ||
| External Link | RMBase: m6A_site_468917 | ||
| mod ID: M6ASITE044081 | Click to Show/Hide the Full List | ||
| mod site | chr2:38075347-38075348:- | [6] | |
| Sequence | GAACGACCCTTGGCCGCTAAACCCGCTGTCCATCCAGCAGA | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; peripheral-blood; endometrial; NB4; MM6 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000494864.1; ENST00000490576.1; ENST00000613082.1; ENST00000614273.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468918 | ||
| mod ID: M6ASITE044082 | Click to Show/Hide the Full List | ||
| mod site | chr2:38075795-38075796:- | [6] | |
| Sequence | GCTGTCCCGCGCCACTGGAAACCGCACCTCCCCGCAGGTCA | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; peripheral-blood; endometrial; NB4; MM6 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000613082.1; ENST00000494864.1; ENST00000614273.1; ENST00000610745.5; ENST00000490576.1 | ||
| External Link | RMBase: m6A_site_468921 | ||
| mod ID: M6ASITE044083 | Click to Show/Hide the Full List | ||
| mod site | chr2:38075927-38075928:- | [6] | |
| Sequence | GGCACTGACGACGCCAAGAGACTCGAGTGGGAGTTAAAGCT | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; H1A; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; endometrial; NB4; MM6 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000494864.1; ENST00000614273.1; ENST00000613082.1; ENST00000490576.1; ENST00000610745.5 | ||
| External Link | RMBase: m6A_site_468927 | ||
| mod ID: M6ASITE044084 | Click to Show/Hide the Full List | ||
| mod site | chr2:38075940-38075941:- | [8] | |
| Sequence | TCCAAGCGCTCCTGGCACTGACGACGCCAAGAGACTCGAGT | ||
| Motif Score | 2.833690476 | ||
| Cell/Tissue List | A549 | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000614273.1; ENST00000494864.1; ENST00000613082.1; ENST00000610745.5; ENST00000490576.1 | ||
| External Link | RMBase: m6A_site_468928 | ||
| mod ID: M6ASITE044085 | Click to Show/Hide the Full List | ||
| mod site | chr2:38076033-38076034:- | [6] | |
| Sequence | AATCTCAACGCTGTGAGGAAACCTCGACTTTGCCAGGTCCC | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; A549; H1B; H1A; MM6; CD4T; peripheral-blood; HEK293T; HEK293A-TOA; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000613082.1; ENST00000494864.1; ENST00000490576.1; ENST00000610745.5; ENST00000614273.1 | ||
| External Link | RMBase: m6A_site_468930 | ||
| mod ID: M6ASITE044086 | Click to Show/Hide the Full List | ||
| mod site | chr2:38109712-38109713:- | [6] | |
| Sequence | TTATGTCAGAAGAAGTCCAGACCTCTCACAGTGACGAGGGG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; iSLK | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000494864.1 | ||
| External Link | RMBase: m6A_site_468935 | ||
| mod ID: M6ASITE044087 | Click to Show/Hide the Full List | ||
| mod site | chr2:38109794-38109795:- | [6] | |
| Sequence | TAATTCACTGATCTGCTTAAACTGCCTTTTAATGCTTAGAA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000494864.1 | ||
| External Link | RMBase: m6A_site_468936 | ||
References