m6A Target Gene Information
General Information of the m6A Target Gene (ID: M6ATAR00162)
Full List of m6A Methylation Regulator of This Target Gene and Corresponding Disease/Drug Response(s)
SLC1A5
can be regulated by the following regulator(s), and cause disease/drug response(s). You can browse detail information of regulator(s) or disease/drug response(s).
Browse Regulator
Browse Disease
Browse Drug
Fat mass and obesity-associated protein (FTO) [ERASER]
| Representative RNA-seq result indicating the expression of this target gene regulated by FTO | ||
| Cell Line | 253J cell line | Homo sapiens |
|
Treatment: siFTO 253J cells
Control: 253J cells
|
GSE150239 | |
| Regulation |
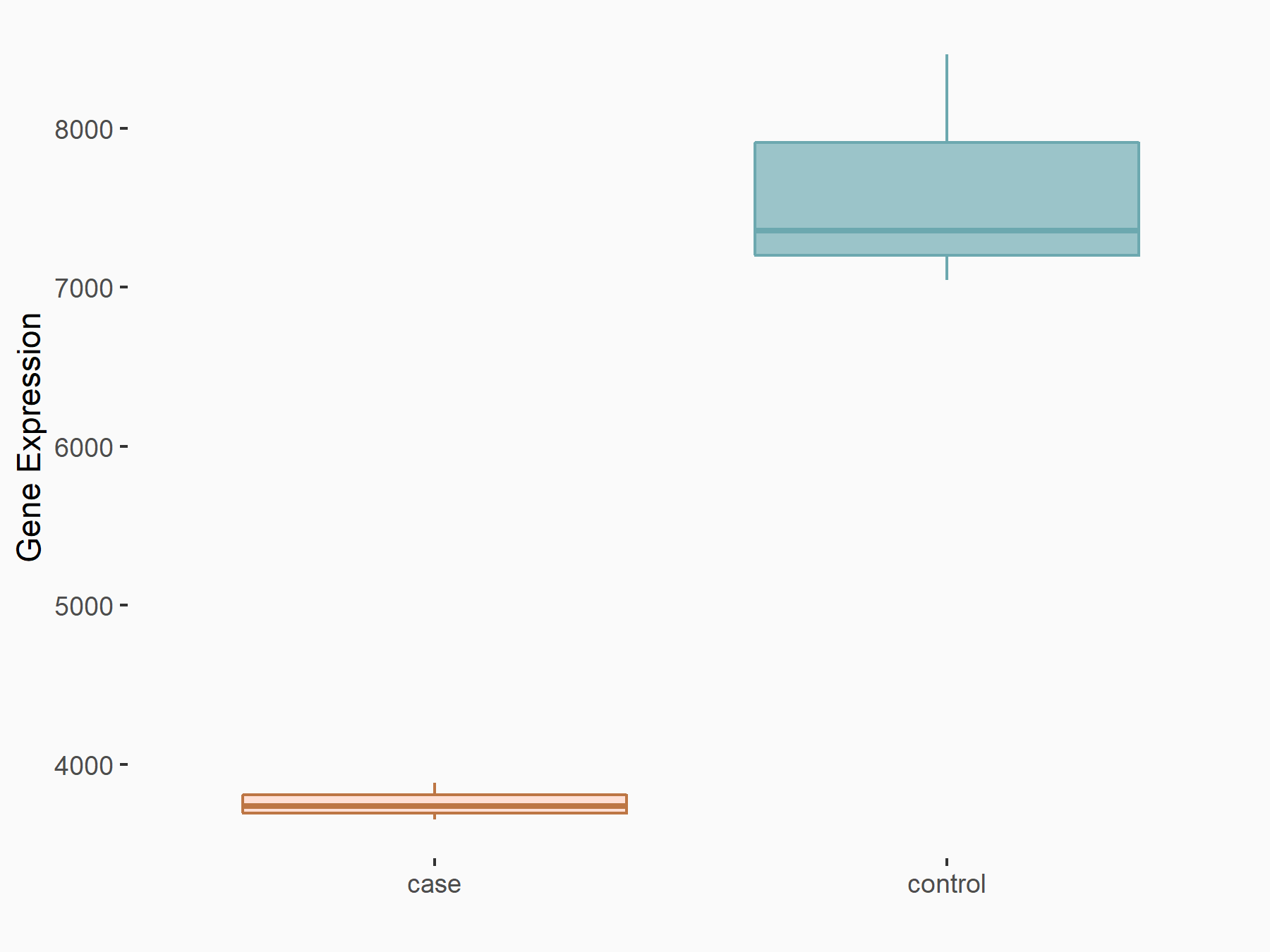 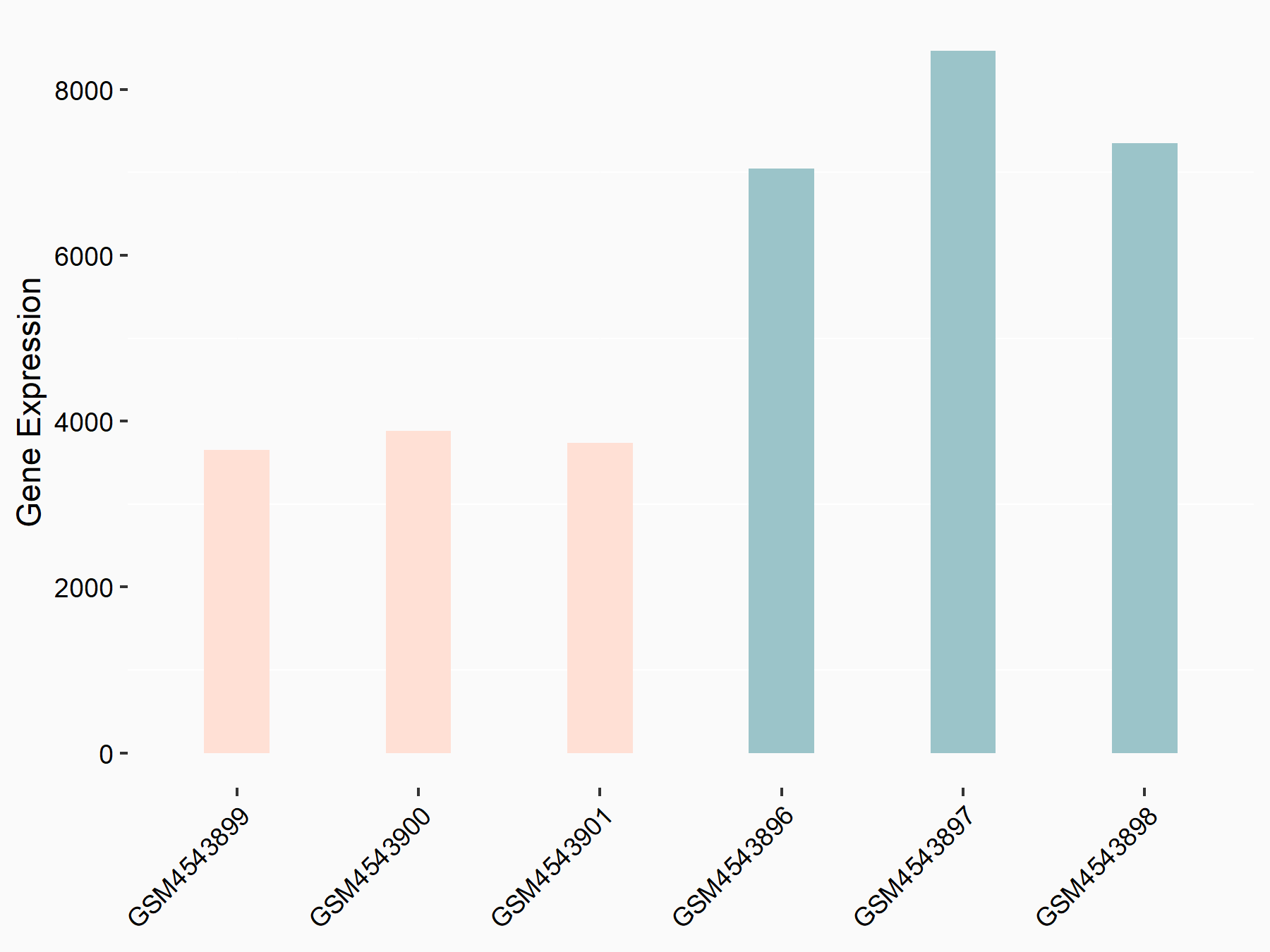 |
logFC: -1.02E+00 p-value: 5.80E-30 |
| More Results | Click to View More RNA-seq Results | |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | Genetic inactivation of FTO using multiple orthogonal approaches revealed that FTO inhibition selectively reduces the growth and survival of VHL-deficient cells in vitro and in vivo. Integrated analysis of transcriptome-wide m6A-seq and mRNA-seq analysis identified the glutamine transporter Neutral amino acid transporter B(0) (SLC1A5) as an FTO target that promotes metabolic reprogramming and survival of VHL-deficient ccRCC cells. GLS1 inhibitors that target mitochondrial glutaminase and the conversion of glutamine to glutamate are currently being evaluated in early-phase clinical trials in ccRCC. These findings identify FTO as a potential HIF-independent therapeutic target for the treatment of VHL-deficient renal cell carcinoma. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Renal cell carcinoma | ICD-11: 2C90 | ||
| Responsed Drug | GLS-IN-968 | Investigative | ||
| Pathway Response | Central carbon metabolism in cancer | hsa05230 | ||
| HIF-1 signaling pathway | hsa04066 | |||
| Central carbon metabolism in cancer | hsa05230 | |||
| Metabolic pathways | hsa01100 | |||
| VEGF signaling pathway | hsa04370 | |||
| In-vitro Model | UMRC2-vec (CCRCC isogenic cell lines that are VHL-deficient) | |||
Renal cell carcinoma [ICD-11: 2C90]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [1] | |||
| Response Summary | Genetic inactivation of FTO using multiple orthogonal approaches revealed that FTO inhibition selectively reduces the growth and survival of VHL-deficient cells in vitro and in vivo. Integrated analysis of transcriptome-wide m6A-seq and mRNA-seq analysis identified the glutamine transporter Neutral amino acid transporter B(0) (SLC1A5) as an FTO target that promotes metabolic reprogramming and survival of VHL-deficient ccRCC cells. GLS1 inhibitors that target mitochondrial glutaminase and the conversion of glutamine to glutamate are currently being evaluated in early-phase clinical trials in ccRCC. These findings identify FTO as a potential HIF-independent therapeutic target for the treatment of VHL-deficient renal cell carcinoma. | |||
| Responsed Disease | Renal cell carcinoma [ICD-11: 2C90] | |||
| Target Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | ||
| Target Regulation | Up regulation | |||
| Responsed Drug | GLS-IN-968 | Investigative | ||
| Pathway Response | Central carbon metabolism in cancer | hsa05230 | ||
| HIF-1 signaling pathway | hsa04066 | |||
| Central carbon metabolism in cancer | hsa05230 | |||
| Metabolic pathways | hsa01100 | |||
| VEGF signaling pathway | hsa04370 | |||
| In-vitro Model | UMRC2-vec (CCRCC isogenic cell lines that are VHL-deficient) | |||
GLS-IN-968
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response | [1] | |||
| Response Summary | Genetic inactivation of FTO using multiple orthogonal approaches revealed that FTO inhibition selectively reduces the growth and survival of VHL-deficient cells in vitro and in vivo. Integrated analysis of transcriptome-wide m6A-seq and mRNA-seq analysis identified the glutamine transporter Neutral amino acid transporter B(0) (SLC1A5) as an FTO target that promotes metabolic reprogramming and survival of VHL-deficient ccRCC cells. GLS1 inhibitors that target mitochondrial glutaminase and the conversion of glutamine to glutamate are currently being evaluated in early-phase clinical trials in ccRCC. These findings identify FTO as a potential HIF-independent therapeutic target for the treatment of VHL-deficient renal cell carcinoma. | |||
| Target Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | ||
| Target Regulation | Up regulation | |||
| Responsed Disease | Renal cell carcinoma | ICD-11: 2C90 | ||
| Pathway Response | Central carbon metabolism in cancer | hsa05230 | ||
| HIF-1 signaling pathway | hsa04066 | |||
| Central carbon metabolism in cancer | hsa05230 | |||
| Metabolic pathways | hsa01100 | |||
| VEGF signaling pathway | hsa04370 | |||
| In-vitro Model | UMRC2-vec (CCRCC isogenic cell lines that are VHL-deficient) | |||
RNA Modification Sequencing Data Associated with the Target (ID: M6ATAR00162)
| In total 17 m6A sequence/site(s) in this target gene | |||
| mod ID: A2ISITE009243 | Click to Show/Hide the Full List | ||
| mod site | chr19:46776375-46776376:- | [2] | |
| Sequence | ATCTCCACTAAAAATACAAAAAAATTAGCCGGGTATGGTGG | ||
| Transcript ID List | ENST00000594991.5; ENST00000434726.6; ENST00000542575.6; rmsk_5014510; ENST00000412532.6 | ||
| External Link | RMBase: RNA-editing_site_71360 | ||
| mod ID: A2ISITE009244 | Click to Show/Hide the Full List | ||
| mod site | chr19:46779984-46779985:- | [3] | |
| Sequence | TAGCACGCGCCTATAGTCCCAGCTACTTGGGAGGCTGAGGC | ||
| Transcript ID List | ENST00000542575.6; ENST00000594991.5; ENST00000412532.6; ENST00000593713.1; ENST00000434726.6; rmsk_5014518 | ||
| External Link | RMBase: RNA-editing_site_71361 | ||
| mod ID: A2ISITE009245 | Click to Show/Hide the Full List | ||
| mod site | chr19:46779992-46779993:- | [3] | |
| Sequence | GGGCGTGGTAGCACGCGCCTATAGTCCCAGCTACTTGGGAG | ||
| Transcript ID List | ENST00000434726.6; ENST00000593713.1; ENST00000412532.6; ENST00000542575.6; ENST00000594991.5; rmsk_5014518 | ||
| External Link | RMBase: RNA-editing_site_71362 | ||
| mod ID: A2ISITE009246 | Click to Show/Hide the Full List | ||
| mod site | chr19:46780000-46780001:- | [3] | |
| Sequence | AATTAGCTGGGCGTGGTAGCACGCGCCTATAGTCCCAGCTA | ||
| Transcript ID List | ENST00000542575.6; ENST00000412532.6; ENST00000434726.6; rmsk_5014518; ENST00000593713.1; ENST00000594991.5 | ||
| External Link | RMBase: RNA-editing_site_71363 | ||
| mod ID: A2ISITE009247 | Click to Show/Hide the Full List | ||
| mod site | chr19:46780003-46780004:- | [3] | |
| Sequence | AAAAATTAGCTGGGCGTGGTAGCACGCGCCTATAGTCCCAG | ||
| Transcript ID List | ENST00000594991.5; ENST00000593713.1; ENST00000542575.6; rmsk_5014518; ENST00000412532.6; ENST00000434726.6 | ||
| External Link | RMBase: RNA-editing_site_71364 | ||
| mod ID: A2ISITE009248 | Click to Show/Hide the Full List | ||
| mod site | chr19:46780016-46780017:- | [3] | |
| Sequence | TACTAAAAATACAAAAAATTAGCTGGGCGTGGTAGCACGCG | ||
| Transcript ID List | ENST00000412532.6; ENST00000593713.1; ENST00000542575.6; ENST00000594991.5; ENST00000434726.6; rmsk_5014518 | ||
| External Link | RMBase: RNA-editing_site_71365 | ||
| mod ID: A2ISITE009249 | Click to Show/Hide the Full List | ||
| mod site | chr19:46780048-46780049:- | [3] | |
| Sequence | CCATCCTGGCTAACACAGAAAAACCCTGTCTCTACTAAAAA | ||
| Transcript ID List | ENST00000593713.1; ENST00000542575.6; rmsk_5014518; ENST00000434726.6; ENST00000594991.5; ENST00000412532.6 | ||
| External Link | RMBase: RNA-editing_site_71366 | ||
| mod ID: A2ISITE009250 | Click to Show/Hide the Full List | ||
| mod site | chr19:46780049-46780050:- | [3] | |
| Sequence | ACCATCCTGGCTAACACAGAAAAACCCTGTCTCTACTAAAA | ||
| Transcript ID List | ENST00000542575.6; ENST00000434726.6; ENST00000412532.6; rmsk_5014518; ENST00000594991.5; ENST00000593713.1 | ||
| External Link | RMBase: RNA-editing_site_71367 | ||
| mod ID: A2ISITE009251 | Click to Show/Hide the Full List | ||
| mod site | chr19:46780052-46780053:- | [3] | |
| Sequence | GAGACCATCCTGGCTAACACAGAAAAACCCTGTCTCTACTA | ||
| Transcript ID List | ENST00000542575.6; ENST00000594991.5; ENST00000412532.6; ENST00000593713.1; ENST00000434726.6; rmsk_5014518 | ||
| External Link | RMBase: RNA-editing_site_71368 | ||
| mod ID: A2ISITE009252 | Click to Show/Hide the Full List | ||
| mod site | chr19:46780056-46780057:- | [3] | |
| Sequence | GATCGAGACCATCCTGGCTAACACAGAAAAACCCTGTCTCT | ||
| Transcript ID List | ENST00000593713.1; ENST00000594991.5; ENST00000434726.6; rmsk_5014518; ENST00000542575.6; ENST00000412532.6 | ||
| External Link | RMBase: RNA-editing_site_71369 | ||
| mod ID: A2ISITE009253 | Click to Show/Hide the Full List | ||
| mod site | chr19:46780080-46780081:- | [3] | |
| Sequence | GGCAGGCAGATCACGAGGTCAGGAGATCGAGACCATCCTGG | ||
| Transcript ID List | ENST00000593713.1; rmsk_5014518; ENST00000434726.6; ENST00000412532.6; ENST00000594991.5; ENST00000542575.6 | ||
| External Link | RMBase: RNA-editing_site_71370 | ||
| mod ID: A2ISITE009254 | Click to Show/Hide the Full List | ||
| mod site | chr19:46780093-46780094:- | [3] | |
| Sequence | TTTGGGAGGCTGAGGCAGGCAGATCACGAGGTCAGGAGATC | ||
| Transcript ID List | rmsk_5014518; ENST00000434726.6; ENST00000412532.6; ENST00000542575.6; ENST00000593713.1; ENST00000594991.5 | ||
| External Link | RMBase: RNA-editing_site_71371 | ||
| mod ID: A2ISITE009255 | Click to Show/Hide the Full List | ||
| mod site | chr19:46780097-46780098:- | [3] | |
| Sequence | GCACTTTGGGAGGCTGAGGCAGGCAGATCACGAGGTCAGGA | ||
| Transcript ID List | ENST00000594991.5; ENST00000412532.6; ENST00000542575.6; rmsk_5014518; ENST00000593713.1; ENST00000434726.6 | ||
| External Link | RMBase: RNA-editing_site_71372 | ||
| mod ID: A2ISITE009256 | Click to Show/Hide the Full List | ||
| mod site | chr19:46780101-46780102:- | [3] | |
| Sequence | CCCAGCACTTTGGGAGGCTGAGGCAGGCAGATCACGAGGTC | ||
| Transcript ID List | ENST00000542575.6; ENST00000412532.6; ENST00000434726.6; rmsk_5014518; ENST00000593713.1; ENST00000594991.5 | ||
| External Link | RMBase: RNA-editing_site_71373 | ||
| mod ID: A2ISITE009257 | Click to Show/Hide the Full List | ||
| mod site | chr19:46780126-46780127:- | [3] | |
| Sequence | CAGTACGGAGGCTCATGCCTATAATCCCAGCACTTTGGGAG | ||
| Transcript ID List | ENST00000594991.5; ENST00000593713.1; ENST00000412532.6; rmsk_5014518; ENST00000542575.6; ENST00000434726.6 | ||
| External Link | RMBase: RNA-editing_site_71374 | ||
| mod ID: A2ISITE009258 | Click to Show/Hide the Full List | ||
| mod site | chr19:46780145-46780146:- | [3] | |
| Sequence | TTTAAGAAACTGGGTGGCCCAGTACGGAGGCTCATGCCTAT | ||
| Transcript ID List | rmsk_5014518; ENST00000594991.5; ENST00000542575.6; ENST00000434726.6; ENST00000412532.6; ENST00000593713.1 | ||
| External Link | RMBase: RNA-editing_site_71375 | ||
| mod ID: A2ISITE009259 | Click to Show/Hide the Full List | ||
| mod site | chr19:46780577-46780578:- | [2] | |
| Sequence | ATTGCTTGAGGCCAGGAGTTAGAGACTAGCCTGGTCAACAT | ||
| Transcript ID List | ENST00000542575.6; ENST00000412532.6; ENST00000594991.5; rmsk_5014520; ENST00000593713.1; ENST00000434726.6 | ||
| External Link | RMBase: RNA-editing_site_71376 | ||
5-methylcytidine (m5C)
| In total 8 m6A sequence/site(s) in this target gene | |||
| mod ID: M5CSITE002063 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775577-46775578:- | [4] | |
| Sequence | CCACTGAGGAAGGAAACCCCCTCCTCAAACACTATCGGGGG | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000594991.5; ENST00000542575.6; ENST00000434726.6; ENST00000412532.6 | ||
| External Link | RMBase: m5C_site_25031 | ||
| mod ID: M5CSITE002064 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775578-46775579:- | [4] | |
| Sequence | CCCACTGAGGAAGGAAACCCCCTCCTCAAACACTATCGGGG | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000434726.6; ENST00000594991.5; ENST00000412532.6; ENST00000542575.6 | ||
| External Link | RMBase: m5C_site_25032 | ||
| mod ID: M5CSITE002065 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775579-46775580:- | [4] | |
| Sequence | CCCCACTGAGGAAGGAAACCCCCTCCTCAAACACTATCGGG | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000434726.6; ENST00000542575.6; ENST00000412532.6; ENST00000594991.5 | ||
| External Link | RMBase: m5C_site_25033 | ||
| mod ID: M5CSITE002066 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775580-46775581:- | [4] | |
| Sequence | TCCCCACTGAGGAAGGAAACCCCCTCCTCAAACACTATCGG | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000412532.6; ENST00000542575.6; ENST00000434726.6; ENST00000594991.5 | ||
| External Link | RMBase: m5C_site_25034 | ||
| mod ID: M5CSITE002067 | Click to Show/Hide the Full List | ||
| mod site | chr19:46778680-46778681:- | [4] | |
| Sequence | GGCCACTGCCTTTGGGACCTCTTCCAGGTACGGCCTTGGGG | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000412532.6; ENST00000434726.6; ENST00000593713.1; ENST00000594991.5; ENST00000542575.6 | ||
| External Link | RMBase: m5C_site_25035 | ||
| mod ID: M5CSITE002068 | Click to Show/Hide the Full List | ||
| mod site | chr19:46780620-46780621:- | [4] | |
| Sequence | TCACACCTGTAATCCCAGCACTTTGGGAGGCTGAGGGGGGA | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000412532.6; rmsk_5014520; ENST00000434726.6; ENST00000594991.5; ENST00000593713.1; ENST00000542575.6 | ||
| External Link | RMBase: m5C_site_25036 | ||
| mod ID: M5CSITE002069 | Click to Show/Hide the Full List | ||
| mod site | chr19:46780625-46780626:- | [4] | |
| Sequence | GTACCTCACACCTGTAATCCCAGCACTTTGGGAGGCTGAGG | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000542575.6; rmsk_5014520; ENST00000434726.6; ENST00000593713.1; ENST00000594991.5; ENST00000412532.6 | ||
| External Link | RMBase: m5C_site_25037 | ||
| mod ID: M5CSITE002070 | Click to Show/Hide the Full List | ||
| mod site | chr19:46780626-46780627:- | [4] | |
| Sequence | GGTACCTCACACCTGTAATCCCAGCACTTTGGGAGGCTGAG | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000594991.5; ENST00000434726.6; rmsk_5014520; ENST00000412532.6; ENST00000593713.1; ENST00000542575.6 | ||
| External Link | RMBase: m5C_site_25038 | ||
N6-methyladenosine (m6A)
| In total 61 m6A sequence/site(s) in this target gene | |||
| mod ID: M6ASITE041632 | Click to Show/Hide the Full List | ||
| mod site | chr19:46774921-46774922:- | [5] | |
| Sequence | ACCTGCTGTCACTCCAGAGGACATTTTTTTTAGCAATAAAA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HEK293T; A549 | ||
| Seq Type List | DART-seq; m6A-seq | ||
| Transcript ID List | ENST00000542575.6; ENST00000434726.6; ENST00000594991.5 | ||
| External Link | RMBase: m6A_site_445566 | ||
| mod ID: M6ASITE041633 | Click to Show/Hide the Full List | ||
| mod site | chr19:46774947-46774948:- | [5] | |
| Sequence | ACTCTGGGGAGAGGCTGAGGACAAATACCTGCTGTCACTCC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HEK293T; hESC-HEK293T; A549 | ||
| Seq Type List | DART-seq; MAZTER-seq; m6A-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000434726.6; ENST00000542575.6; ENST00000412532.6; ENST00000594991.5 | ||
| External Link | RMBase: m6A_site_445567 | ||
| mod ID: M6ASITE041634 | Click to Show/Hide the Full List | ||
| mod site | chr19:46774967-46774968:- | [6] | |
| Sequence | TAACAGAAACACTCCCAGGGACTCTGGGGAGAGGCTGAGGA | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | A549 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000542575.6; ENST00000594991.5; ENST00000412532.6; ENST00000434726.6 | ||
| External Link | RMBase: m6A_site_445568 | ||
| mod ID: M6ASITE041635 | Click to Show/Hide the Full List | ||
| mod site | chr19:46774977-46774978:- | [5] | |
| Sequence | CCCTCCACAATAACAGAAACACTCCCAGGGACTCTGGGGAG | ||
| Motif Score | 2.506922619 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000412532.6; ENST00000542575.6; ENST00000594991.5; ENST00000434726.6 | ||
| External Link | RMBase: m6A_site_445569 | ||
| mod ID: M6ASITE041637 | Click to Show/Hide the Full List | ||
| mod site | chr19:46774979-46774980:- | [5] | |
| Sequence | TTCCCTCCACAATAACAGAAACACTCCCAGGGACTCTGGGG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HEK293T; A549 | ||
| Seq Type List | DART-seq; m6A-seq | ||
| Transcript ID List | ENST00000594991.5; ENST00000542575.6; ENST00000434726.6; ENST00000412532.6 | ||
| External Link | RMBase: m6A_site_445570 | ||
| mod ID: M6ASITE041638 | Click to Show/Hide the Full List | ||
| mod site | chr19:46774985-46774986:- | [7] | |
| Sequence | CCCCTATTCCCTCCACAATAACAGAAACACTCCCAGGGACT | ||
| Motif Score | 2.168095238 | ||
| Cell/Tissue List | hESC-HEK293T; AML | ||
| Seq Type List | MAZTER-seq; miCLIP | ||
| Transcript ID List | ENST00000434726.6; ENST00000542575.6; ENST00000412532.6; ENST00000594991.5 | ||
| External Link | RMBase: m6A_site_445571 | ||
| mod ID: M6ASITE041639 | Click to Show/Hide the Full List | ||
| mod site | chr19:46774991-46774992:- | [7] | |
| Sequence | CCAGATCCCCTATTCCCTCCACAATAACAGAAACACTCCCA | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000542575.6; ENST00000412532.6; ENST00000594991.5; ENST00000434726.6 | ||
| External Link | RMBase: m6A_site_445572 | ||
| mod ID: M6ASITE041640 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775027-46775028:- | [5] | |
| Sequence | TGACCTCCTGTCCCCATGGTACGTCCCACCCTGTCCCCAGA | ||
| Motif Score | 2.830077381 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000412532.6; ENST00000594991.5; ENST00000434726.6; ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445573 | ||
| mod ID: M6ASITE041641 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775091-46775092:- | [5] | |
| Sequence | GTGGGGGGATGTGTGTGTGCACGTGTGTGTGTGTGTGTGTG | ||
| Motif Score | 2.80452381 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000542575.6; ENST00000594991.5; ENST00000434726.6; ENST00000412532.6 | ||
| External Link | RMBase: m6A_site_445574 | ||
| mod ID: M6ASITE041642 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775143-46775144:- | [7] | |
| Sequence | CCCACTGGGGGGATGTTACAACACCATGCTGGTTATTTTGG | ||
| Motif Score | 2.173910714 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000594991.5; ENST00000434726.6; ENST00000542575.6; ENST00000412532.6 | ||
| External Link | RMBase: m6A_site_445575 | ||
| mod ID: M6ASITE041643 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775146-46775147:- | [5] | |
| Sequence | GCCCCCACTGGGGGGATGTTACAACACCATGCTGGTTATTT | ||
| Motif Score | 2.07285119 | ||
| Cell/Tissue List | HEK293T; hESC-HEK293T | ||
| Seq Type List | DART-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000434726.6; ENST00000542575.6; ENST00000594991.5; ENST00000412532.6 | ||
| External Link | RMBase: m6A_site_445576 | ||
| mod ID: M6ASITE041644 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775267-46775268:- | [8] | |
| Sequence | CCCCAACTCAAGGCTAGAAAACAGCAAGATGGAGAAATAAT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HepG2; A549; HEK293T; HeLa; MM6; peripheral-blood; iSLK; TREX; MSC; TIME; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000412532.6; ENST00000542575.6; ENST00000594991.5; ENST00000434726.6 | ||
| External Link | RMBase: m6A_site_445577 | ||
| mod ID: M6ASITE041645 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775295-46775296:- | [5] | |
| Sequence | CCTCAAAACCCCCAGTTCTCACTCATGTCCCCAACTCAAGG | ||
| Motif Score | 2.469291667 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000412532.6; ENST00000542575.6; ENST00000594991.5; ENST00000434726.6 | ||
| External Link | RMBase: m6A_site_445578 | ||
| mod ID: M6ASITE041646 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775308-46775309:- | [9] | |
| Sequence | CACAAGGGTTACTCCTCAAAACCCCCAGTTCTCACTCATGT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; HepG2; A549; HEK293T; U2OS; H1A; H1B; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000434726.6; ENST00000412532.6; ENST00000542575.6; ENST00000594991.5 | ||
| External Link | RMBase: m6A_site_445579 | ||
| mod ID: M6ASITE041648 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775327-46775328:- | [5] | |
| Sequence | CTGCTGGAGTACATGTGTTCACAAGGGTTACTCCTCAAAAC | ||
| Motif Score | 2.047297619 | ||
| Cell/Tissue List | HEK293T; hESC-HEK293T | ||
| Seq Type List | DART-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000542575.6; ENST00000412532.6; ENST00000434726.6; ENST00000594991.5 | ||
| External Link | RMBase: m6A_site_445580 | ||
| mod ID: M6ASITE041649 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775337-46775338:- | [5] | |
| Sequence | GGATGCCTGGCTGCTGGAGTACATGTGTTCACAAGGGTTAC | ||
| Motif Score | 2.856142857 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000434726.6; ENST00000412532.6; ENST00000594991.5; ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445581 | ||
| mod ID: M6ASITE041650 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775369-46775370:- | [9] | |
| Sequence | GGGCCCCAGCACCCTCCAGGACAGGAGATCTGGGATGCCTG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; hESC-HEK293T; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; DART-seq; MAZTER-seq; miCLIP | ||
| Transcript ID List | ENST00000542575.6; ENST00000434726.6; ENST00000594991.5; ENST00000412532.6 | ||
| External Link | RMBase: m6A_site_445582 | ||
| mod ID: M6ASITE041651 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775406-46775407:- | [7] | |
| Sequence | GGCTCTGGGGGTCTGCCTGCACACTCTGGGGAGCCAGGGGC | ||
| Motif Score | 2.830589286 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000412532.6; ENST00000434726.6; ENST00000542575.6; ENST00000594991.5 | ||
| External Link | RMBase: m6A_site_445583 | ||
| mod ID: M6ASITE041652 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775464-46775465:- | [9] | |
| Sequence | CTGCTGGGGGTGCTCTTTGGACACTGGATTATGAGGAATGG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; hESC-HEK293T; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; CD8T; H1299; MM6; Huh7; Jurkat; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000542575.6; ENST00000434726.6; ENST00000412532.6; ENST00000594991.5 | ||
| External Link | RMBase: m6A_site_445584 | ||
| mod ID: M6ASITE041653 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775496-46775497:- | [9] | |
| Sequence | TCATGTAAACCCCGGGAGGGACCTTCCCTGCCCTGCTGGGG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000542575.6; ENST00000434726.6; ENST00000594991.5; ENST00000412532.6 | ||
| External Link | RMBase: m6A_site_445585 | ||
| mod ID: M6ASITE041654 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775508-46775509:- | [9] | |
| Sequence | AGAAGGAATCAGTCATGTAAACCCCGGGAGGGACCTTCCCT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000542575.6; ENST00000434726.6; ENST00000412532.6; ENST00000594991.5 | ||
| External Link | RMBase: m6A_site_445586 | ||
| mod ID: M6ASITE041655 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775569-46775570:- | [9] | |
| Sequence | GAAGGAAACCCCCTCCTCAAACACTATCGGGGGCCCGCAGG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HEK293T; hESC-HEK293T; HepG2; U2OS; hESCs; fibroblasts; A549; MM6; Huh7; HEK293A-TOA; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000434726.6; ENST00000542575.6; ENST00000412532.6; ENST00000594991.5 | ||
| External Link | RMBase: m6A_site_445587 | ||
| mod ID: M6ASITE041656 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775582-46775583:- | [9] | |
| Sequence | AGTCCCCACTGAGGAAGGAAACCCCCTCCTCAAACACTATC | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; HEK293T; HepG2; U2OS; hESCs; fibroblasts; A549; MM6; Huh7; HEK293A-TOA; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000542575.6; ENST00000434726.6; ENST00000594991.5; ENST00000412532.6 | ||
| External Link | RMBase: m6A_site_445588 | ||
| mod ID: M6ASITE041657 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775638-46775639:- | [7] | |
| Sequence | AGCACAGAGCCTGAGTTGATACAAGTGAAGAGTGAGCTGCC | ||
| Motif Score | 2.110482143 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000594991.5; ENST00000412532.6; ENST00000434726.6; ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445589 | ||
| mod ID: M6ASITE041659 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775675-46775676:- | [10] | |
| Sequence | ACTCCTCCAAAATTACGTGGACCGTACGGAGTCGAGAAGCA | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HepG2; MM6; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000412532.6; ENST00000542575.6; ENST00000434726.6; ENST00000594991.5 | ||
| External Link | RMBase: m6A_site_445590 | ||
| mod ID: M6ASITE041660 | Click to Show/Hide the Full List | ||
| mod site | chr19:46775695-46775696:- | [10] | |
| Sequence | GGTGACGCTCTGGGGGCAGGACTCCTCCAAAATTACGTGGA | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HepG2; Huh7; peripheral-blood | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000412532.6; ENST00000594991.5; ENST00000542575.6; ENST00000434726.6 | ||
| External Link | RMBase: m6A_site_445591 | ||
| mod ID: M6ASITE041661 | Click to Show/Hide the Full List | ||
| mod site | chr19:46776983-46776984:- | [10] | |
| Sequence | CTCCTTGATCCTGGCTGTGGACTGGCTAGTGTGAGTGTGGG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HepG2; Huh7; peripheral-blood | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000594991.5; ENST00000434726.6; ENST00000412532.6; ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445594 | ||
| mod ID: M6ASITE041662 | Click to Show/Hide the Full List | ||
| mod site | chr19:46777234-46777235:- | [10] | |
| Sequence | GCTCAGCCAGCAGTCCTTGGACTTCGTAAAGATCATCACCA | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HepG2; Huh7; peripheral-blood | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000412532.6; ENST00000542575.6; ENST00000593713.1; ENST00000434726.6; ENST00000594991.5 | ||
| External Link | RMBase: m6A_site_445595 | ||
| mod ID: M6ASITE041663 | Click to Show/Hide the Full List | ||
| mod site | chr19:46777306-46777307:- | [7] | |
| Sequence | GCCCATCGGCGCCACCGTCAACATGGACGGTGCCGCGCTCT | ||
| Motif Score | 2.173910714 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000594991.5; ENST00000434726.6; ENST00000542575.6; ENST00000412532.6; ENST00000593713.1 | ||
| External Link | RMBase: m6A_site_445596 | ||
| mod ID: M6ASITE041664 | Click to Show/Hide the Full List | ||
| mod site | chr19:46777345-46777346:- | [7] | |
| Sequence | GAATAATGGCGTGGCCAAGCACATCAGCCGTTTCATCCTGC | ||
| Motif Score | 2.830589286 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000593713.1; ENST00000594991.5; ENST00000434726.6; ENST00000412532.6; ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445597 | ||
| mod ID: M6ASITE041665 | Click to Show/Hide the Full List | ||
| mod site | chr19:46778684-46778685:- | [11] | |
| Sequence | CGCTGGCCACTGCCTTTGGGACCTCTTCCAGGTACGGCCTT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HepG2; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000542575.6; ENST00000594991.5; ENST00000593713.1; ENST00000412532.6; ENST00000434726.6 | ||
| External Link | RMBase: m6A_site_445598 | ||
| mod ID: M6ASITE041666 | Click to Show/Hide the Full List | ||
| mod site | chr19:46778737-46778738:- | [12] | |
| Sequence | CTTCCTCTTCACCCGCAAAAACCCCTACCGCTTCCTGTGGG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000593713.1; ENST00000594991.5; ENST00000434726.6; ENST00000542575.6; ENST00000412532.6 | ||
| External Link | RMBase: m6A_site_445599 | ||
| mod ID: M6ASITE041668 | Click to Show/Hide the Full List | ||
| mod site | chr19:46778818-46778819:- | [7] | |
| Sequence | CTTTGCCCGCCTTGGCAAGTACATTCTGTGCTGCCTGCTGG | ||
| Motif Score | 2.856142857 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000434726.6; ENST00000594991.5; ENST00000593713.1; ENST00000542575.6; ENST00000412532.6 | ||
| External Link | RMBase: m6A_site_445600 | ||
| mod ID: M6ASITE041669 | Click to Show/Hide the Full List | ||
| mod site | chr19:46782517-46782518:- | [9] | |
| Sequence | GCAGGAGGTGGAGGGGATGAACATCCTGGGCTTGGTAGTGT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HepG2; hESC-HEK293T | ||
| Seq Type List | m6A-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000598022.1; ENST00000594991.5; ENST00000542575.6; ENST00000593713.1; ENST00000412532.6; ENST00000434726.6 | ||
| External Link | RMBase: m6A_site_445601 | ||
| mod ID: M6ASITE041670 | Click to Show/Hide the Full List | ||
| mod site | chr19:46784107-46784108:- | [13] | |
| Sequence | AAGAGAGGAATATCACCGGAACCAGGGTGAAGGTGAGAGCA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000542575.6; ENST00000598022.1; ENST00000593713.1; ENST00000412532.6; ENST00000594991.5; ENST00000434726.6 | ||
| External Link | RMBase: m6A_site_445602 | ||
| mod ID: M6ASITE041671 | Click to Show/Hide the Full List | ||
| mod site | chr19:46784615-46784616:- | [5] | |
| Sequence | ACGCCAACCCAGCCTCTCAGACAATGCTGCCCTCCCACTAT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HEK293T; peripheral-blood; MM6 | ||
| Seq Type List | DART-seq; m6A-seq | ||
| Transcript ID List | ENST00000434726.6; ENST00000412532.6; ENST00000598022.1; ENST00000593713.1; ENST00000542575.6; ENST00000594991.5 | ||
| External Link | RMBase: m6A_site_445603 | ||
| mod ID: M6ASITE041672 | Click to Show/Hide the Full List | ||
| mod site | chr19:46784806-46784807:- | [9] | |
| Sequence | GCCAGCATTCCTCACCCCGAACCCACAATGCCTCAGGGCTA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; peripheral-blood | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000412532.6; ENST00000542575.6; ENST00000594991.5; ENST00000434726.6 | ||
| External Link | RMBase: m6A_site_445604 | ||
| mod ID: M6ASITE041673 | Click to Show/Hide the Full List | ||
| mod site | chr19:46787302-46787303:- | [10] | |
| Sequence | TTGGGGCCCCTGGAGCAGAGACTTCCCTCTAGAGTCTTAGG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HepG2; HEK293T; NB4; MM6 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000412532.6; ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445605 | ||
| mod ID: M6ASITE041674 | Click to Show/Hide the Full List | ||
| mod site | chr19:46787335-46787336:- | [10] | |
| Sequence | TGGCGGGAGTGGTCACGTGGACAGGACGGGGCTTTGGGGCC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HepG2; HEK293T; TREX; NB4; MM6 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000542575.6; ENST00000412532.6 | ||
| External Link | RMBase: m6A_site_445606 | ||
| mod ID: M6ASITE041675 | Click to Show/Hide the Full List | ||
| mod site | chr19:46787606-46787607:- | [9] | |
| Sequence | CGGCGGCGCCGCCAGCCTGGACCCCGGCGCGCTCGGCCGTC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; A549; HEK293T; U2OS; H1A; H1B; MM6; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TREX; TIME; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445607 | ||
| mod ID: M6ASITE041676 | Click to Show/Hide the Full List | ||
| mod site | chr19:46787758-46787759:- | [9] | |
| Sequence | GTGGCCGGCGTGGCGCTGGGACTGGGGGTGTCGGGGGCCGG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445608 | ||
| mod ID: M6ASITE041677 | Click to Show/Hide the Full List | ||
| mod site | chr19:46787793-46787794:- | [7] | |
| Sequence | CCAACCTGCTTGTGCTGCTGACAGTGGTGGCCGTGGTGGCC | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | hESC-HEK293T; AML | ||
| Seq Type List | MAZTER-seq; miCLIP | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445609 | ||
| mod ID: M6ASITE041679 | Click to Show/Hide the Full List | ||
| mod site | chr19:46787837-46787838:- | [9] | |
| Sequence | CGGCTACTGCGGTTCCCGGGACCAGGTGCGCCGCTGCCTTC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445610 | ||
| mod ID: M6ASITE041680 | Click to Show/Hide the Full List | ||
| mod site | chr19:46787876-46787877:- | [9] | |
| Sequence | GGCGCTGGCCTCCATCGAGGACCAAGGCGCGGCAGCAGGCG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445612 | ||
| mod ID: M6ASITE041681 | Click to Show/Hide the Full List | ||
| mod site | chr19:46787942-46787943:- | [9] | |
| Sequence | GGTGGCCGATCCTCCTCGAGACTCCAAGGGGCTCGCAGCGG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; CD8T; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445613 | ||
| mod ID: M6ASITE041682 | Click to Show/Hide the Full List | ||
| mod site | chr19:46787982-46787983:- | [9] | |
| Sequence | CAGCTTCCAGGCGCTAAGAAACCCCGGTGCTTCCCATCATG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445614 | ||
| mod ID: M6ASITE041683 | Click to Show/Hide the Full List | ||
| mod site | chr19:46788103-46788104:- | [7] | |
| Sequence | AACTTCAGTGCTGTGAACTCACAACTCTAAGGAGCCCTCCA | ||
| Motif Score | 2.047297619 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445619 | ||
| mod ID: M6ASITE041685 | Click to Show/Hide the Full List | ||
| mod site | chr19:46788107-46788108:- | [9] | |
| Sequence | AAGGAACTTCAGTGCTGTGAACTCACAACTCTAAGGAGCCC | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445620 | ||
| mod ID: M6ASITE041686 | Click to Show/Hide the Full List | ||
| mod site | chr19:46788122-46788123:- | [9] | |
| Sequence | CAGCTTCCAAGAGCCAAGGAACTTCAGTGCTGTGAACTCAC | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445621 | ||
| mod ID: M6ASITE041687 | Click to Show/Hide the Full List | ||
| mod site | chr19:46788148-46788149:- | [9] | |
| Sequence | GCCCAGCCTGGGGAATTTAAACACTCCAGCTTCCAAGAGCC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; hESC-HEK293T; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445622 | ||
| mod ID: M6ASITE041688 | Click to Show/Hide the Full List | ||
| mod site | chr19:46788196-46788197:- | [7] | |
| Sequence | CCAGCTTTCGGACATCTGGCACACGGGGCAGAGCAGAGAAG | ||
| Motif Score | 2.830589286 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445623 | ||
| mod ID: M6ASITE041689 | Click to Show/Hide the Full List | ||
| mod site | chr19:46788205-46788206:- | [9] | |
| Sequence | TCCGAACTCCCAGCTTTCGGACATCTGGCACACGGGGCAGA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; kidney; A549; hESC-HEK293T; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-REF-seq; MAZTER-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445624 | ||
| mod ID: M6ASITE041690 | Click to Show/Hide the Full List | ||
| mod site | chr19:46788220-46788221:- | [9] | |
| Sequence | AAGGAACCCGGGCGCTCCGAACTCCCAGCTTTCGGACATCT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445625 | ||
| mod ID: M6ASITE041691 | Click to Show/Hide the Full List | ||
| mod site | chr19:46788235-46788236:- | [9] | |
| Sequence | CCAGCTCCTAGGGCCAAGGAACCCGGGCGCTCCGAACTCCC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; miCLIP | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445626 | ||
| mod ID: M6ASITE041692 | Click to Show/Hide the Full List | ||
| mod site | chr19:46788267-46788268:- | [9] | |
| Sequence | CCCACGTCCCACCCAGAGAAACTCTCGTATTCCCAGCTCCT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445627 | ||
| mod ID: M6ASITE041693 | Click to Show/Hide the Full List | ||
| mod site | chr19:46788330-46788331:- | [9] | |
| Sequence | CAGAACCTAGCCTCCTGCAGACCTCCGCCATCTGGGGGCAC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445628 | ||
| mod ID: M6ASITE041694 | Click to Show/Hide the Full List | ||
| mod site | chr19:46788346-46788347:- | [9] | |
| Sequence | CCGGGATCTGCGCCACCAGAACCTAGCCTCCTGCAGACCTC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445629 | ||
| mod ID: M6ASITE041696 | Click to Show/Hide the Full List | ||
| mod site | chr19:46788406-46788407:- | [9] | |
| Sequence | CCCAGCCCTCAGTGTCCAAGACCCAGGCAGCCCGGGTCCCC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; GM12878; LCLs; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445631 | ||
| mod ID: M6ASITE041697 | Click to Show/Hide the Full List | ||
| mod site | chr19:46788487-46788488:- | [9] | |
| Sequence | CCCGCATCCCACCCTCCCGGACCTAAGAGCCTGGGTCCCCT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; MT4; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445636 | ||
| mod ID: M6ASITE041698 | Click to Show/Hide the Full List | ||
| mod site | chr19:46788531-46788532:- | [9] | |
| Sequence | CCGCTACTCCCGGACACCAGACCACCGCCTTCCGTACACAG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; MT4; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445637 | ||
| mod ID: M6ASITE041699 | Click to Show/Hide the Full List | ||
| mod site | chr19:46788538-46788539:- | [9] | |
| Sequence | CTGGTAACCGCTACTCCCGGACACCAGACCACCGCCTTCCG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; MT4; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000542575.6 | ||
| External Link | RMBase: m6A_site_445638 | ||
References