m6A-centered Drug Response Information
General Information of the Drug (ID: M6APDG01116)
| Name |
RO-316233
|
||||
|---|---|---|---|---|---|
| Synonyms |
119139-23-0; bisindolylmaleimide iv; 3,4-di(1H-indol-3-yl)-1H-pyrrole-2,5-dione; Arcyriarubin A; 3,4-Bis(3-indolyl)maleimide; 3,4-Di-1H-indol-3-yl-1H-pyrrole-2,5-dione; UNII-MBK3OO5K8T; BIM IV; 3,4-bis(1H-indol-3-yl)pyrrole-2,5-dione; MBK3OO5K8T; CHEMBL266487; 3,4-bis(1H-indol-3-yl)-2,5-dihydro-1H-pyrrole-2,5-dione; DQYBRTASHMYDJG-UHFFFAOYSA-N; 2,3-bis(1H-Indol-3-yl)maleimide; 1H-Pyrrole-2,5-dione, 3,4-di-1H-indol-3-yl-; Ro-31-6233; AK-15401; 3,4-bis(3-indolyl)-1H-pyrrole-2,5-dione; Bisindoylmaleimide; Bisindolyl deriv. 3
Click to Show/Hide
|
||||
| Status |
Investigative
|
||||
| Structure |
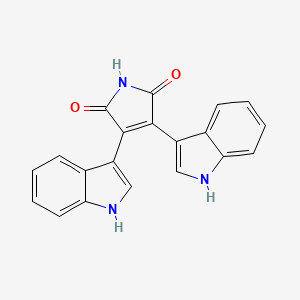 |
||||
| Formula |
C20H13N3O2
|
||||
| InChI |
1S/C20H13N3O2/c24-19-17(13-9-21-15-7-3-1-5-11(13)15)18(20(25)23-19)14-10-22-16-8-4-2-6-12(14)16/h1-10,21-22H,(H,23,24,25)
|
||||
| InChIKey |
DQYBRTASHMYDJG-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
Target Gene(s) and Their Upstream m6A Regulator, Together with the Effect of Target Gene(s) in Drug Response
The target genes involved in drug-target interaction (such as drug-metabolizing enzymes, drug transporters and therapeutic targets) and drug-mediated cell death signaling (including modulating DNA damage and repair capacity, escaping from drug-induced apoptosis, autophagy, cellular metabolic reprogramming, oncogenic bypass signaling, cell microenvironment, cell stemness, etc.) could be regulated by m6A regulator(s) and affected their corresponding drug response. You can browse detailed information on drug-related target gene(s) mediated by m6A regulators.
cAMP-dependent protein kinase A type I (PRKAR1A)
Fat mass and obesity-associated protein (FTO)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | cAMP-dependent protein kinase A type I (PRKAR1A) is a therapeutic target for RO-316233. The Fat mass and obesity-associated protein (FTO) has potential in affecting the response of RO-316233 through regulating the expression of cAMP-dependent protein kinase A type I (PRKAR1A). | [1], [2] | ||
Cyclin-dependent kinase 1 (CDK1)
Methyltransferase-like 3 (METTL3)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | Cyclin-dependent kinase 1 (CDK1) is a therapeutic target for RO-316233. The Methyltransferase-like 3 (METTL3) has potential in affecting the response of RO-316233 through regulating the expression of Cyclin-dependent kinase 1 (CDK1). | [3], [4] | ||
Protein virilizer homolog (VIRMA)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | Cyclin-dependent kinase 1 (CDK1) is a therapeutic target for RO-316233. The Protein virilizer homolog (VIRMA) has potential in affecting the response of RO-316233 through regulating the expression of Cyclin-dependent kinase 1 (CDK1). | [4], [5] | ||
Extracellular signal-regulated kinase 2 (ERK2)
Heterogeneous nuclear ribonucleoproteins A2/B1 (HNRNPA2B1)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | Extracellular signal-regulated kinase 2 (ERK2) is a therapeutic target for RO-316233. The Heterogeneous nuclear ribonucleoproteins A2/B1 (HNRNPA2B1) has potential in affecting the response of RO-316233 through regulating the expression of Extracellular signal-regulated kinase 2 (ERK2). | [6], [7] | ||
Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | Extracellular signal-regulated kinase 2 (ERK2) is a therapeutic target for RO-316233. The Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) has potential in affecting the response of RO-316233 through regulating the expression of Extracellular signal-regulated kinase 2 (ERK2). | [7], [8] | ||
YTH domain-containing family protein 3 (YTHDF3)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | Extracellular signal-regulated kinase 2 (ERK2) is a therapeutic target for RO-316233. The YTH domain-containing family protein 3 (YTHDF3) has potential in affecting the response of RO-316233 through regulating the expression of Extracellular signal-regulated kinase 2 (ERK2). | [7], [8] | ||
NAD-dependent deacetylase sirtuin-1 (SIRT1)
Fat mass and obesity-associated protein (FTO)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | NAD-dependent deacetylase sirtuin-1 (SIRT1) is a therapeutic target for RO-316233. The Fat mass and obesity-associated protein (FTO) has potential in affecting the response of RO-316233 through regulating the expression of NAD-dependent deacetylase sirtuin-1 (SIRT1). | [9], [10] | ||
Methyltransferase-like 14 (METTL14)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | NAD-dependent deacetylase sirtuin-1 (SIRT1) is a therapeutic target for RO-316233. The Methyltransferase-like 14 (METTL14) has potential in affecting the response of RO-316233 through regulating the expression of NAD-dependent deacetylase sirtuin-1 (SIRT1). | [10], [11] | ||
Methyltransferase-like 3 (METTL3)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | NAD-dependent deacetylase sirtuin-1 (SIRT1) is a therapeutic target for RO-316233. The Methyltransferase-like 3 (METTL3) has potential in affecting the response of RO-316233 through regulating the expression of NAD-dependent deacetylase sirtuin-1 (SIRT1). | [10], [12] | ||
Protein virilizer homolog (VIRMA)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | NAD-dependent deacetylase sirtuin-1 (SIRT1) is a therapeutic target for RO-316233. The Protein virilizer homolog (VIRMA) has potential in affecting the response of RO-316233 through regulating the expression of NAD-dependent deacetylase sirtuin-1 (SIRT1). | [10], [13] | ||
RAC-alpha serine/threonine-protein kinase (AKT1)
Heterogeneous nuclear ribonucleoproteins A2/B1 (HNRNPA2B1)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | RAC-alpha serine/threonine-protein kinase (AKT1) is a therapeutic target for RO-316233. The Heterogeneous nuclear ribonucleoproteins A2/B1 (HNRNPA2B1) has potential in affecting the response of RO-316233 through regulating the expression of RAC-alpha serine/threonine-protein kinase (AKT1). | [7], [14] | ||
Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | RAC-alpha serine/threonine-protein kinase (AKT1) is a therapeutic target for RO-316233. The Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) has potential in affecting the response of RO-316233 through regulating the expression of RAC-alpha serine/threonine-protein kinase (AKT1). | [7], [8] | ||
Methyltransferase-like 14 (METTL14)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | RAC-alpha serine/threonine-protein kinase (AKT1) is a therapeutic target for RO-316233. The Methyltransferase-like 14 (METTL14) has potential in affecting the response of RO-316233 through regulating the expression of RAC-alpha serine/threonine-protein kinase (AKT1). | [7], [15] | ||
Methyltransferase-like 3 (METTL3)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | RAC-alpha serine/threonine-protein kinase (AKT1) is a therapeutic target for RO-316233. The Methyltransferase-like 3 (METTL3) has potential in affecting the response of RO-316233 through regulating the expression of RAC-alpha serine/threonine-protein kinase (AKT1). | [7], [16] | ||
RNA demethylase ALKBH5 (ALKBH5)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | RAC-alpha serine/threonine-protein kinase (AKT1) is a therapeutic target for RO-316233. The RNA demethylase ALKBH5 (ALKBH5) has potential in affecting the response of RO-316233 through regulating the expression of RAC-alpha serine/threonine-protein kinase (AKT1). | [7], [17] | ||
YTH domain-containing family protein 1 (YTHDF1)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | RAC-alpha serine/threonine-protein kinase (AKT1) is a therapeutic target for RO-316233. The YTH domain-containing family protein 1 (YTHDF1) has potential in affecting the response of RO-316233 through regulating the expression of RAC-alpha serine/threonine-protein kinase (AKT1). | [7], [18] | ||
YTH domain-containing family protein 2 (YTHDF2)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | RAC-alpha serine/threonine-protein kinase (AKT1) is a therapeutic target for RO-316233. The YTH domain-containing family protein 2 (YTHDF2) has potential in affecting the response of RO-316233 through regulating the expression of RAC-alpha serine/threonine-protein kinase (AKT1). | [7], [19] | ||
YTH domain-containing family protein 3 (YTHDF3)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | RAC-alpha serine/threonine-protein kinase (AKT1) is a therapeutic target for RO-316233. The YTH domain-containing family protein 3 (YTHDF3) has potential in affecting the response of RO-316233 through regulating the expression of RAC-alpha serine/threonine-protein kinase (AKT1). | [7], [8] | ||
YTH domain-containing protein 2 (YTHDC2)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | RAC-alpha serine/threonine-protein kinase (AKT1) is a therapeutic target for RO-316233. The YTH domain-containing protein 2 (YTHDC2) has potential in affecting the response of RO-316233 through regulating the expression of RAC-alpha serine/threonine-protein kinase (AKT1). | [7], [20] | ||
Ribosomal protein S6 kinase beta-1 (S6K1)
Methyltransferase-like 3 (METTL3)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | Ribosomal protein S6 kinase beta-1 (S6K1) is a therapeutic target for RO-316233. The Methyltransferase-like 3 (METTL3) has potential in affecting the response of RO-316233 through regulating the expression of Ribosomal protein S6 kinase beta-1 (S6K1). | [7], [21] | ||
Stress-activated protein kinase 2a (p38 alpha)
Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | Stress-activated protein kinase 2a (p38 alpha) is a therapeutic target for RO-316233. The Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) has potential in affecting the response of RO-316233 through regulating the expression of Stress-activated protein kinase 2a (p38 alpha). | [8], [22] | ||
Methyltransferase-like 3 (METTL3)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | Stress-activated protein kinase 2a (p38 alpha) is a therapeutic target for RO-316233. The Methyltransferase-like 3 (METTL3) has potential in affecting the response of RO-316233 through regulating the expression of Stress-activated protein kinase 2a (p38 alpha). | [22], [23] | ||
YTH domain-containing family protein 3 (YTHDF3)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | Stress-activated protein kinase 2a (p38 alpha) is a therapeutic target for RO-316233. The YTH domain-containing family protein 3 (YTHDF3) has potential in affecting the response of RO-316233 through regulating the expression of Stress-activated protein kinase 2a (p38 alpha). | [8], [22] | ||
Stress-activated protein kinase JNK1 (JNK1)
Methyltransferase-like 3 (METTL3)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | Stress-activated protein kinase JNK1 (JNK1) is a therapeutic target for RO-316233. The Methyltransferase-like 3 (METTL3) has potential in affecting the response of RO-316233 through regulating the expression of Stress-activated protein kinase JNK1 (JNK1). | [7], [24] | ||
RNA demethylase ALKBH5 (ALKBH5)
| In total 1 mechanisms lead to this potential drug response | ||||
| Response Summary | Stress-activated protein kinase JNK1 (JNK1) is a therapeutic target for RO-316233. The RNA demethylase ALKBH5 (ALKBH5) has potential in affecting the response of RO-316233 through regulating the expression of Stress-activated protein kinase JNK1 (JNK1). | [7], [25] | ||
References