m6A-centered Drug Response Information
General Information of the Drug (ID: M6ADRUG0099)
| Name |
Linsitinib
|
||||
|---|---|---|---|---|---|
| Synonyms |
Linsitinib; 867160-71-2; OSI-906; Linsitinib(OSI-906); OSI906; OSI 906; OSI-906 (Linsitinib); OSI-906AA; ASP-7487; UNII-15A52GPT8T; 3-[8-amino-1-(2-phenylquinolin-7-yl)imidazo[1,5-a]pyrazin-3-yl]-1-methylcyclobutan-1-ol; 15A52GPT8T; CHEMBL1091644; MMV676605; cis-3-[8-Amino-1-(2-phenyl-7-quinolinyl)imidazo[1,5-a]pyrazin-3-yl]-1-methylcyclobutanol; C26H23N5O; cis-3-(8-amino-1-(2-phenyl-7-quinolinyl)imidazo(1,5-a)pyrazin-3-yl)-1-methylcyclobutanol; (1r,3r)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidazo[1,5-a]pyrazin-3-yl)-1-methylcyclobutanol.; Kinome_3532; Linsitinib [USAN:INN]; Linsitinib; OSI-906; Linsitinib (USAN/INN); Linsitinib (OSI-906); MLS006010304; SCHEMBL400369; SCHEMBL400734; SCHEMBL400735; GTPL7423; CHEMBL1996234; SCHEMBL10255925; CHEBI:91402; EX-A719; QCR-128; BCPP000137; BDBM185150; DTXSID401007055; HMS3295G15; HMS3654C19; AOB87170; BCP01831; BDBM50315887; MFCD12912153; NSC756652; NSC800784; s1091; ZINC53239527; AKOS024464740; ZINC100071817; ZINC113742575; BCP9001035; CCG-264815; CS-0242; DB06075; NSC-756652; NSC-800784; PB22643; NCGC00250375-02; NCGC00250375-03; NCGC00250375-11; NCGC00250375-14; AC-26953; AS-16999; HY-10191; SMR004700744; FT-0670821; SW218150-2; X7473; D09925; SR-05000022538; J-520074; Q6554803; SR-05000022538-1; BRD-K07667918-001-01-3; Q27163260; 3-[8-amino-1-(2-phenyl-7-quinolinyl)-3-imidazo[1,5-a]pyrazinyl]-1-methyl-1-cyclobutanol; (1s,3r)-3-[8-amino-1-(2-phenylquinolin-7-yl)imidazo[1,5-a]pyrazin-3-yl]-1-methylcyclobutan-1-ol; (1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidazo[1,5-a]pyrazin-3-yl)-1-methylcyclobutanol; 1356958-77-4; 3-(8-amino-1-(2-phenylquinolin-7-yl)-2,3-dihydroimidazo[1,5-a]pyrazin-3-yl)-1-methylcyclobutanol; 3-[8-AMINO-1-(2-PHENYL-QUINOLIN-7-YL)-IMIDAZO[1,5-A]PYRAZIN-3-YL]-1-METHYL-CYCLOBUTANOL; 3-[8-Imino-1-(2-phenylquinolin-7-yl)-7,8-dihydroimidazo[1,5-a]pyrazin-3-yl]-1-methylcyclobutan-1-ol; Cis-3-(8-Amino-1-(2-phenylquinolin-7-yl)imidazo[1,5-a]pyrazin-3-yl)-1-methylcyclobutan-1-ol
Click to Show/Hide
|
||||
| Status | Phase 3 | [1] | |||
| Structure |
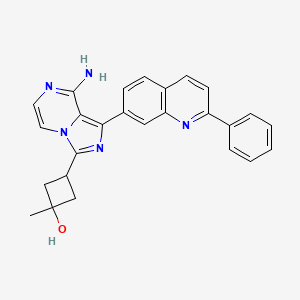 |
||||
| Formula |
C26H23N5O
|
||||
| InChI |
InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)
|
||||
| InChIKey |
PKCDDUHJAFVJJB-UHFFFAOYSA-N
|
||||
| PubChem CID | |||||
| TTD Drug ID | |||||
| DrugBank ID | |||||
Full List of m6A Targets Related to This Drug
Myc proto-oncogene protein (MYC)
| In total 3 item(s) under this target gene | ||||
| Experiment 1 Reporting the m6A-centered Drug Response by This Target Gene | [2] | |||
| Response Summary | The m6A reader YTHDF2 stabilized Myc proto-oncogene protein (MYC) mRNA specifically in cancer stem cells. Given the challenge of targeting MYC, YTHDF2 presents a therapeutic target to perturb MYC signaling in glioblastoma. The IGF1/IGF1R inhibitor linsitinib preferentially targeted YTHDF2-expressing cells, inhibiting GSC viability without affecting NSCs and impairing in vivo glioblastoma growth. YTHDF2 links RNA epitranscriptomic modifications and GSC growth, laying the foundation for the YTHDF2-MYC-IGFBP3 axis as a specific and novel therapeutic target in glioblastoma. | |||
| Responsed Disease | Glioma | ICD-11: 2A00.0 | ||
| Target Regulator | YTH domain-containing family protein 2 (YTHDF2) | READER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | RNA degradation | hsa03018 | ||
| Cell Process | RNA stability | |||
| In-vitro Model | () | |||
| HNP1 (A human neural progenitor cell) | ||||
| NHA (Normal human astrocytes) | ||||
| NSC11 (Pluripotent derived neural progenitor cell) | ||||
| In-vivo Model | For in vivo drug treatment studies, intracranial xenografts were generated by implanting 5000 patient-derived GSCs (387 and 4121) into the right cerebral cortex of NSG mice as described above. | |||
| Experiment 2 Reporting the m6A-centered Drug Response by This Target Gene | [2] | |||
| Response Summary | The m6A reader YTHDF2 stabilized Myc proto-oncogene protein (MYC) mRNA specifically in cancer stem cells. Given the challenge of targeting MYC, YTHDF2 presents a therapeutic target to perturb MYC signaling in glioblastoma. The IGF1/IGF1R inhibitor linsitinib preferentially targeted YTHDF2-expressing cells, inhibiting GSC viability without affecting NSCs and impairing in vivo glioblastoma growth. YTHDF2 links RNA epitranscriptomic modifications and GSC growth, laying the foundation for the YTHDF2-MYC-IGFBP3 axis as a specific and novel therapeutic target in glioblastoma. | |||
| Responsed Disease | Glioma | ICD-11: 2A00.0 | ||
| Target Regulator | Insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3) | READER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | RNA degradation | hsa03018 | ||
| Cell Process | RNA stability | |||
| In-vitro Model | () | |||
| HNP1 (A human neural progenitor cell) | ||||
| NHA (Normal human astrocytes) | ||||
| NSC11 (Pluripotent derived neural progenitor cell) | ||||
| In-vivo Model | For in vivo drug treatment studies, intracranial xenografts were generated by implanting 5000 patient-derived GSCs (387 and 4121) into the right cerebral cortex of NSG mice as described above. | |||
| Experiment 3 Reporting the m6A-centered Drug Response by This Target Gene | [2] | |||
| Response Summary | The IGF1/IGF1R inhibitor, linsitinib for further investigation based upon the role of the IGF pathway member, IGFBP3, as a downstream effector of YTHDF2-Myc proto-oncogene protein (MYC) axis in GSCs. Inhibiting glioblastoma stem cells viability without affecting NSCs and impairing in vivo glioblastoma growth. | |||
| Responsed Disease | Glioblastoma | ICD-11: 2A00.00 | ||
| Target Regulator | YTH domain-containing family protein 2 (YTHDF2) | READER | ||
| Target Regulation | Up regulation | |||
| In-vitro Model | NSC11 (Pluripotent derived neural progenitor cell) | |||
| NHA (Normal human astrocytes) | ||||
| HNP1 (A human neural progenitor cell) | ||||
| ENSA (A human embryonic stem derived neural progenitor cell) | ||||
| In-vivo Model | Implanting 5000 human derived GSCs into the right cerebral cortex of NSG mice at a depth of 3.5 mm under a University of California, San Diego Institutional Animal Care and Use Committee (IACUC) approved protocol. Brains were harvested and fixed in 4% formaldehyde, cryopreserved in 30% sucrose, and then cryosectioned. Hematoxylin and eosin (H&E) staining was performed on sections for histological analysis. In parallel survival experiments, mice were observed until the development of neurological signs. For in vivo drug treatment studies, intracranial xenografts were generated by implanting 5000 patient-derived GSCs (387 and 4121) into the right cerebral cortex of NSG mice as described above. Mice recovered for 7 days were randomly assigned into drug vs. treatment group by a blinded investigator. Mice were then treated daily with either vehicle (25 mM Tartaric acid) or 50 mg/kg linsitinib by oral gavage. | |||
References