m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05976
|
[1], [2] | |||
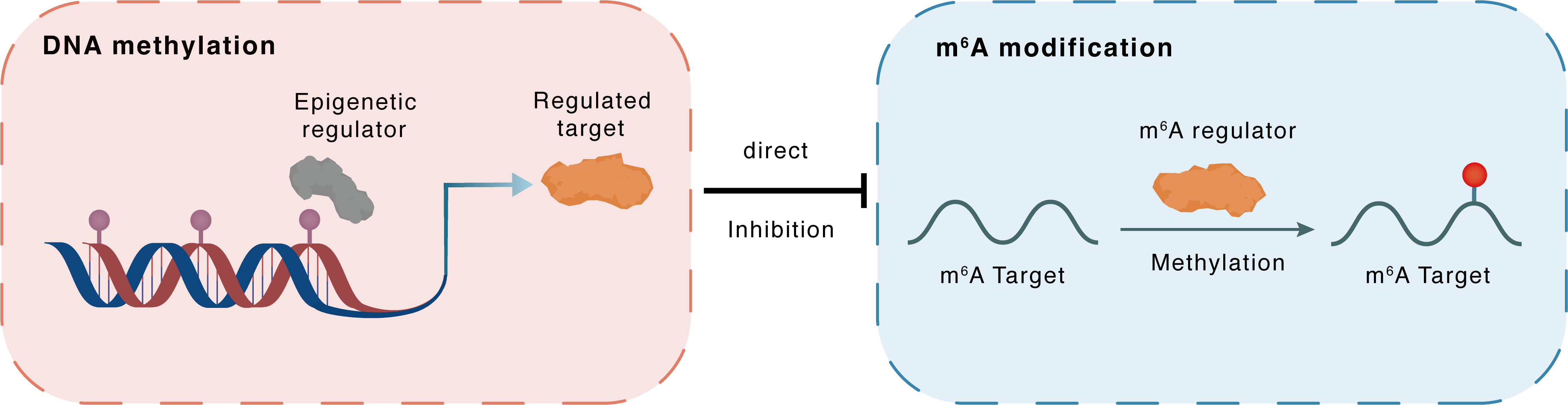 DNA methylation
DNMT3B
METTL14
Direct
Inhibition
m6A modification
CALCOCO1
CALCOCO1
METTL14
Methylation
DNA methylation
DNMT3B
METTL14
Direct
Inhibition
m6A modification
CALCOCO1
CALCOCO1
METTL14
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 14 (METTL14) | WRITER | |||
| m6A Target | Calcium-binding and coiled-coil domain-containing protein 1 (CALCOCO1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | DNA methylation (DNAMeth) | ||||
| Epigenetic Regulator | DNA (cytosine-5)-methyltransferase 3B (DNMT3B) | WRITER | View Details | ||
| Regulated Target | Methyltransferase-like protein 14 (METTL14) | View Details | |||
| Crosstalk Relationship | DNA methylation → m6A | Inhibition | |||
| Crosstalk Mechanism | DNA methylation directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | lncRNA UCA1 recruited DNA methyltransferase (DNMT1, DNMT3A, and DNMT3B) to the METTL14 promoter region to inhibit METTL14 expression in breast cancer. METTL3/METTL14 increased ER-phagy machinery formation by promoting m6A modification of the ER-phagy regulators Calcium-binding and coiled-coil domain-containing protein 1 (CALCOCO1) and p62, thus enhancing their mRNA stability. The chemotherapeutic drug paclitaxel (PTX) could induce ER stress and increase .the combination of METTL3/METTL14 inhibitors with PTX demonstrated a significant synergistic therapeutic effect in both BC cells and xenograft mice. | ||||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | |||
| Responsed Drug | STM2457 | ||||
In-vitro Model |
HEK293T | Normal | Homo sapiens | CVCL_0063 | |
| In-vivo Model | The experimental mice were housed in an SPF-grade animal laboratory at a temperature of approximately 25 ° C, a humidity range of 50%, and an average daylight duration of 12 h. BALB/C female mice at 5-6 weeks of age were taken and prepared for tumor-bearing. After the mice were sacrificed, their tissues were collected, weighed and immediately snap-frozen in liquid nitrogen. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| DNA (cytosine-5)-methyltransferase 3B (DNMT3B) | 22 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Curcumin | Phase 3 | [3] | ||
| Synonyms |
458-37-7; Diferuloylmethane; Natural yellow 3; Turmeric yellow; Turmeric; Curcuma; Kacha haldi; Gelbwurz; Indian saffron; Curcumin I; Souchet; Halud; Halad; Haidr; Haldar; Merita earth; Yellow Ginger; Terra Merita; Yellow Root; Safran d'Inde; Yo-Kin; Golden seal; Curcuma oil; Orange Root; Oils, curcuma; CI Natural Yellow 3; Curcumine; Hydrastis; Indian turmeric; Yellow puccoon; Turmeric extract; Diferaloylmethane; Kurkumin [Czech]; (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione; Tumeric yellow; Turmeric oil
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27376512-Compound-MTC-424 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1940 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-427 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 295 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-422 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1430 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-423 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 363 nM | |||
| External Link | ||||
| NSC-622444 | Investigative | [3] | ||
| Synonyms |
NSC622444; CHEMBL116347; AC1Q3LXD; AC1L7GK5; SCHEMBL9755151; dichlorinated diacylmethane fragment; ZINC1616868; BDBM50048522; 5,3'-dicarboxy-4,4'-dihydrodiphenylmethane; 5,5''-methylenebis(3-chloro-2-hydroxybenzoic acid); 5,5'-Methylenebis(3-chloro-2-hydroxybenzoic acid); 3,3'-methanediylbis(5-chloro-6-hydroxybenzoic acid); 5-(3-carboxy-5-chloro-4-hydroxybenzyl)-3-chloro-2-hydroxybenzoic acid; 3',3-Dichloro-4',4-dimethoxy-5',5-bis(methoxycarbonyl)-1,1-diphenylmethane
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-138419 | Investigative | [3] | ||
| Synonyms |
NSC138419; n-[4-(methylamino)benzoyl]glutamic acid; AC1Q5SG8; AC1L5YW4; SCHEMBL5925511; CHEMBL591443; CTK1H0013; 2-[(4-methylaminobenzoyl)amino]pentanedioic acid; A816490; 2-[[4-(methylamino)benzoyl]amino]pentanedioic acid; 2-[[4-(methylamino)phenyl]carbonylamino]pentanedioic acid; 2-[[[4-(methylamino)phenyl]-oxomethyl]amino]pentanedioic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-319745 | Investigative | [3] | ||
| Synonyms |
61629-60-5; HB 093; BRN 2168571; 4-(2-((5-Chloro-2-methoxybenzoyl)amino)ethyl)hydrocinnamic acid; 3-[4-[2-[(5-CHLORO-2-METHOXY-BENZOYL)AMINO]ETHYL]PHENYL]PROPANOIC ACID; 3-(4-(2-(5-Chlor-2-methoxy-benzamido)-aethyl)phenyl)-propionsaeure [German]; 3-[4-[2-[(5-chloro-2-methoxybenzoyl)amino]ethyl]phenyl]propanoic acid; HYDROCINNAMIC ACID, 4-(2-((5-CHLORO-2-METHOXYBENZOYL)AMINO)ETHYL)-; AC1L2AFL; CHEMBL597112; SCHEMBL11481071; CTK5B3505; DTXSID00210642; AIEFQKOARQRACO-UHFFFAOYSA-N; ZINC1572211; HB-093; NSC319745
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-348926 | Investigative | [3] | ||
| Synonyms |
NSC348926; 2-phthalimidoadipic acid; AC1L7IP1; SCHEMBL9741723; CHEMBL599367; 2-(1,3-dioxoisoindol-2-yl)hexanedioic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-401077 | Investigative | [3] | ||
| Synonyms |
NSC401077; MLS000757170; DNA Methyltransferase Inhibitor; CHEMBL383475; 32675-71-1; 2-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-3-(1H-indol-3-yl)-propionic acid; 2-(1,3-dioxo-2,3-dihydro-1H-isoindol-2-yl)-3-(1H-indol-3-yl)propanoic acid; 2-(1,3-Dioxo-1,3-dihydro-2H-isoindol-2-yl)-3-(1H-indol-3-yl)propanoic acid; 2-(1,3-Dioxo-1,3-dihydro-2H-isoindol-2-yl)-3-(1H-indol-3-yl)propionic acid; SMR000413613; AC1Q71QA; Oprea1_475901; Oprea1_410805; MLS000777218; MLS006011919; SCHEMBL562060
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| S-tubercidinylhomocysteine | Investigative | [5] | ||
| Synonyms |
CHEMBL552309; 57344-98-6; AC1L3YAS; AC1Q5QMO; (S)-7-(5-S-(3-amino-3-carboxypropyl)-5-thio-beta-D-ribofuranosyl)-7H-pyrrolo(2,3-d)pyrimidin-4-amine; (2s)-2-amino-4-({[(2s,3s,4r,5r)-5-(4-amino-7h-pyrrolo[2,3-d]pyrimidin-7-yl)-3,4-dihydroxytetrahydrofuran-2-yl]methyl}sulfanyl)butanoic acid(non-preferred name); BDBM50294482; (2S)-2-amino-4-[[(2S,3S,4R,5R)-5-(4-aminopyrrolo[2,3-d]pyrimidin-7-yl)-3,4-dihydroxyoxolan-2-yl]methylsulfanyl]butanoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 300 nM | |||
| External Link | ||||
| NSC-106084 | Investigative | [3] | ||
| Synonyms |
CHEMBL597113; NSC106084; AC1L6H8Q; CTK7J5419; ZINC1868549; BDBM50308983; {4-[5-bromo-2-(carboxymethoxy)benzoyl]phenoxy}acetic acid; 2-(4-bromo-2-(4-(carboxymethoxy)benzoyl)phenoxy)acetic acid; 2-[4-[5-bromo-2-(carboxymethyloxy)benzoyl]phenoxy]acetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-154957 | Investigative | [3] | ||
| Synonyms |
NSC154957; AC1L6EF2; CHEMBL586418; 3-benzhydrylsulfanyl-2-formamidopropanoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-54162 | Investigative | [3] | ||
| Synonyms |
NSC54162; AC1Q5WTA; AC1L6CM2; CHEMBL611994; 2,2'-{[(2-hydroxyphenyl)methanediyl]disulfanediyl}diacetic acid; ZINC1685025; Acetic acid, (salicylidenedithio)di-; 4265-51-4; Acetic acid, [(o-hydroxybenzylidene)dithio]di-; Acetic acid,2'-[[(2-hydroxyphenyl)methylene]bis(thio)]bis-; 2-[carboxymethylsulfanyl-(2-hydroxyphenyl)methyl]sulfanylacetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-57893 | Investigative | [3] | ||
| Synonyms |
MLS002667915; 7399-94-2; 4-[(1h-benzimidazol-2-ylmethyl)(formyl)amino]benzoic acid; NSC57893; AC1L6GFK; AC1Q5TWY; NCIOpen2_002368; CHEMBL599366; 4-[1H-benzimidazol-2-ylmethyl(formyl)amino]benzoic acid; CTK5D9099; DTXSID30288854; HMS3089M13; ZINC1688755; AKOS030547711
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-56071 | Investigative | [3] | ||
| Synonyms |
32230-52-7; NSC56071; AC1L6EJV; AC1Q7ES0; NCIOpen2_007380; CHEMBL596910; CTK4G8394; DTXSID80288485; ZINC1686711; 2,2'-[piperazine-1,4-diylbis(carbonothioylsulfanediyl)]diacetic acid; AKOS030574801; Acetic acid,2,2'-[1,4-piperazinediylbis(carbonothioylthio)]bis- (9CI); 2-[4-(carboxymethylsulfanylcarbothioyl)piperazine-1-carbothioyl]sulfanylacetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-137546 | Investigative | [3] | ||
| Synonyms |
NSC137546; CHEMBL591202; AC1L5Y49; AKOS008984447; 2-[(2,6-dichlorobenzoyl)amino]pentanedioic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-623548 | Investigative | [3] | ||
| Synonyms |
2581-36-4; NSC 408488; o-Cresotic acid, 5,5'-methylenedi-; 2,3-CRESOTIC ACID, 5,5'-METHYLENEDI-; UNII-S3D8KC88KC; 5,5'-Methylenedi-2,3-cresotic acid; NSC 623548; BRN 3433298; S3D8KC88KC; CHEMBL113835; 5,5'-Methylenedi-o-cresotic acid; NSC623548; NSC408488; 5,5'-Methylenebis(2-hydroxy-3-methylbenzoic acid); 2, 5,5'-methylenedi-; AC1L29YK; Oprea1_231968; 2-10-00-00398 (Beilstein Handbook Reference); SCHEMBL9755153; CTK4F6504; DTXSID90180466; o-Cresotic acid,5'-methylenedi-; MolPort-000-698-522; ZINC4028795; STL511095
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-345763 | Investigative | [3] | ||
| Synonyms |
7-(8-hydroxyquinolin-5-yl)-4,7-dioxoheptanoic acid; NSC345763; AC1L7HSU; CHEMBL597114
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-158324 | Investigative | [3] | ||
| Synonyms |
Acediasulfone; UNII-30YP2YHH8W; 30YP2YHH8W; CHEMBL48396; N-[4-[(4-AMINOPHENYL)SULPHONYL]PHENYL]GLYCINE; 2-[4-(4-aminophenyl)sulfonylanilino]acetic acid; Acediasulfonum; N-(4-((4-Aminophenyl)sulphonyl)phenyl)glycine; EINECS 201-243-7; AC1L25EF; ZINC862; SCHEMBL143660; CTK5E7379; DTXSID00229991; CHEBI:135300; BDBM50099670; AKOS027327086; DB08926; Glycine,N-[4-[(4-aminophenyl)sulfonyl]phenyl]-; {4-[(4-aminophenyl)sulfonyl]anilino}acetic acid; 2-(4-(4-aminophenylsulfonyl)phenylamino)acetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (L-)-S-adenosyl-L-homocysteine | Investigative | [6] | ||
| Synonyms |
S-Adenosyl-L-homocysteine; S-adenosylhomocysteine; 979-92-0; AdoHcy; S-(5'-adenosyl)-L-homocysteine; adenosylhomocysteine; Formycinylhomocysteine; Adenosyl-L-homocysteine; S-(5'-deoxyadenosin-5'-yl)-L-homocysteine; 2-S-adenosyl-L-homocysteine; 5'-Deoxy-S-adenosyl-L-homocysteine; S-adenosyl-homocysteine; S-Adenosyl Homocysteine; L-S-Adenosylhomocysteine; L-Homocysteine, S-(5'-deoxyadenosin-5'-yl)-; adenosylhomo-cys; adenosyl-homo-cys; UNII-8K31Q2S66S; (S)-5'-(S)-(3-Amino-3-carboxypropyl)-5'-thioadenosine; BRN 5166233; SAH
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 200 nM | |||
| External Link | ||||
| NSC-622445 | Investigative | [3] | ||
| Synonyms |
5,5'-Methylenedisalicylic acid; 122-25-8; 5,5'-Methylenebis(2-hydroxybenzoic acid); Methylenebis(salicylic acid); 5,5-Methylenebis(salicylic acid); UNII-2KF4FVV76N; 5,5-Methylenedisalicylic acid; 5-(3-Carboxy-4-hydroxybenzyl)salicylic acid; 4,4'-Dihydroxy-3,3'-dicarboxydiphenylmethane; 3,3'-Dicarboxy-4,4'-dihydroxydiphenylmethane; NSC 14778; 2KF4FVV76N; 4,4'-Dihydroxydiphenylmethane-3,3'-dicarboxylic acid; 3,3'-Methylenebis(6-hydroxybenzoic acid); CHEMBL115145; Benzoic acid, 3,3'-methylenebis[6-hydroxy-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 17000 nM | |||
| External Link | ||||
| 2C60: Breast cancer | 2 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Entrectinib | Approved | [7] | ||
| Synonyms |
1108743-60-7; RXDX-101; UNII-L5ORF0AN1I; Entrectinib (RXDX-101); L5ORF0AN1I; Benzamide, N-[5-[(3,5-difluorophenyl)methyl]-1H-indazol-3-yl]-4-(4-methyl-1-piperazinyl)-2-[(tetrahydro-2H-pyran-4-yl)amino]-; Benzamide, N-(5-((3,5-difluorophenyl)methyl)-1H-indazol-3-yl)-4-(4-methyl-1-piperazinyl)-2-((tetrahydro-2H-pyran-4-yl)amino)-; Entrectinib [USAN:INN]; YMX; Kinome_2659; Entrectinib(rxdx-101); Entrectinib (USAN/INN); SCHEMBL3512601; GTPL8290; CHEMBL1983268; KS-00000TSK
Click to Show/Hide
|
|||
| External Link | ||||
| Everolimus | Approved | [8] | ||
| External Link | ||||
References