m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05974
|
[1] | |||
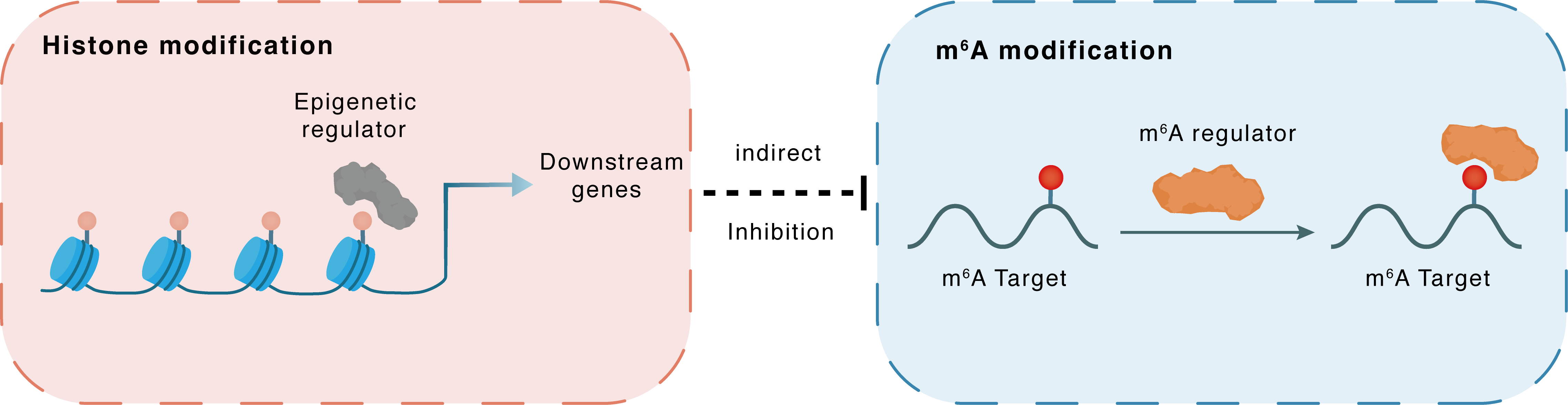 Histone modification
H3K4me3
KDM5A
WTAP
Indirect
Inhibition
m6A modification
LncRNA NORAD
LncRNA NORAD
YTHDF2
Histone modification
H3K4me3
KDM5A
WTAP
Indirect
Inhibition
m6A modification
LncRNA NORAD
LncRNA NORAD
YTHDF2
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | YTH domain-containing family protein 2 (YTHDF2) | READER | |||
| m6A Target | Long intergenic non-protein coding RNA 657 (NORAD) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Lysine-specific demethylase 5A (KDM5A) | ERASER | View Details | ||
| Regulated Target | Histone H3 lysine 4 trimethylation (H3K4me3) | View Details | |||
| Downstream Gene | WTAP | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Inhibition | |||
| Crosstalk Mechanism | Histone modification indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | LncRNA Long intergenic non-protein coding RNA 657 (NORAD) could be modified by m6A due to an increase in WTAP, which was regulated by KDM5A-mediated Histone H3 lysine 4 trimethylation (H3K4me3) modification of the promoter. YTHDF2-mediated decay of NORAD is enhanced in senescent NPCs, and then deficiency of NORAD results in less sequestraion of PUMILIO proteins, contributing to the augmented activity of PUM1 and PUM2, thus repressing the expression of target E2F3 mRNAs and promoting the cellular senescence. | ||||
| Responsed Disease | Intervertebral disc degeneration | ICD-11: FA80 | |||
In-vitro Model |
Nucleus pulposus cells (NPCs) (Nucleus pulposus cells) | ||||
| In-vivo Model | After 50g male NORAD-KO or C57 mice at age of 8 weeks were anesthetized with 3% (w/v) pentobarbital (2 ml/kg) and grouped randomly, investigators blinded to the group allocation performed the experiment. The disc levels in rat tail (Co6/7, 7/8, and 8/9) were located by palpation on the coccygeal vertebrae and confirmed by trial radiography. Needles (33-G) were used to puncture the annulus fibrosus layer though the tail skin, in parallel to the end plates. To ensure that the needle did not penetrate too deeply , the length of the needle was pre-determined according to the dimensions of annulus fibrosus and the NP , which were measured in a preliminary experiment and found to be approximately 4 mm. Five kinds of solution were prepared for intradisc injection, including AA V vector, AAV containing shPUM1, AAV containing shPUM2 for Norad KO mice, AAV vector, AAV containing shE2F3, AAVcontaining OE-E2F3 for WT mice. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Lysine-specific demethylase 5A (KDM5A) | 21 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| 6EP | Patented | [2] | ||
| Synonyms |
2-{5-[(4-Chloro-2-Methylphenyl)methoxy]-1h-Pyrazol-1-Yl}pyridine-4-Carboxylic Acid; 1613410-75-5; CHEMBL3786952; SCHEMBL15778339; BDBM191600; NCGC00390881-02; QC3611,QC-3611,QC 3611; 2-(5-((4-chloro-2-methylbenzyl)oxy)-1Hpyrazol-1-yl)isonicotinic acid (N19)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| BDBM50158803 | Patented | [3] | ||
| Synonyms |
CHEMBL3787438; SCHEMBL15792889
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| US10022354, Example 151 | Patented | [4] | ||
| Synonyms |
SCHEMBL19513974; CHEMBL4060968; BDBM281211
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.065 nM | |||
| External Link | ||||
| US9714230, 12 | Patented | [2] | ||
| Synonyms |
SCHEMBL15778399; LKBZHRSAENXIOI-UHFFFAOYSA-N; BDBM263942; 2-(5-p-tolyl-1H-pyrazol-1- yl)isonicotinic acid; 2-(5-p-tolyl-1H-pyrazol-1-yl)isonicotinic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 550 nM | |||
| External Link | ||||
| BDBM50158791 | Patented | [5] | ||
| Synonyms |
CHEMBL3786596; SCHEMBL15818867; SCHEMBL19646964
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 550 nM | |||
| External Link | ||||
| US10040779, Example 4 | Patented | [6] | ||
| Synonyms |
SCHEMBL15792083; BDBM277707; 3-[(5-chloro-1-methyl-1H-indazol-3-yl)amino]pyridine-4-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 0.1nM | |||
| External Link | ||||
| US9611221, Example 9 | Patented | [7] | ||
| Synonyms |
3-[(biphenyl-4-ylmethyl)amino]pyridine-4-carboxylic acid; SCHEMBL15286753; RNBCOBWQQCESLL-UHFFFAOYSA-N; BDBM314105
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| US9714230, 46 | Patented | [8] | ||
| Synonyms |
SCHEMBL15778753; MITOFELVTHNBGA-UHFFFAOYSA-N; BDBM263981; 2-(5-(4-bromophenyl)-1H-pyrazol- 1-yl)isonicotinic acid; 2-[5-(4-bromophenyl)-1H-pyrazol-1-yl]pyridine-4-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 10nM | |||
| External Link | ||||
| BDBM50158794 | Patented | [6] | ||
| Synonyms |
CHEMBL3785470; SCHEMBL15792416
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 0.1nM | |||
| External Link | ||||
| BDBM50158703 | Patented | [2] | ||
| Synonyms |
CHEMBL3785832; SCHEMBL15777940
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| AKOS020330656 | Patented | [9] | ||
| Synonyms |
CHEMBL3774545; SCHEMBL15820618; RFUZGPWCXINBNW-UHFFFAOYSA-N; BDBM50153334; ZINC123452149; 3-{[(5-methylthiophen-2-yl)methyl]amino}pyridine-4-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| BDBM50158755 | Patented | [10] | ||
| Synonyms |
CHEMBL3786579; SCHEMBL15778210
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| US10174026, Example 99 | Patented | [11] | ||
| Synonyms |
SCHEMBL16157351; BDBM320432; 2-(pyrrolidin-1-ylcarbonyl)-1H- pyrrolo[3,2-b]pyridine-7- carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| AKOS020330481 | Patented | [7] | ||
| Synonyms |
3-[(4-methoxybenzyl)amino]pyridine-4-carboxylic acid; SCHEMBL4855920; US9611221, Example 7; XKZFNTZMCLZYHZ-UHFFFAOYSA-N; BDBM314103; 3[(4-methoxybenzyl)amino]pyridine-4-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 550 nM | |||
| External Link | ||||
| US10022354, Example 5 | Patented | [4] | ||
| Synonyms |
SCHEMBL17682496; CHEMBL4062756; BDBM281065
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7.4 nM | |||
| External Link | ||||
| NCGC00381656-01 | Patented | [12] | ||
| Synonyms |
CHEMBL4100530; SCHEMBL16157407; BDBM320423; US10174026, Example 88; 2-[(2-chlorophenyl)-propoxy- methyl]-1H-pyrrolo[3,2-b]- pyridine-7-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100nM | |||
| External Link | ||||
| US10174026, Example 2 | Patented | [11] | ||
| Synonyms |
SCHEMBL16149258; FEZIKLVLFANZBD-UHFFFAOYSA-N; BDBM320362; 2-phenyl-1H-pyrrolo[3,2-b]pyridine-7-carboxylic acid; 2-phenyl-1H-pyrrolo[3,2-b] pyridine-7-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 550 nM | |||
| External Link | ||||
| US10022354, Example 152 | Patented | [4] | ||
| Synonyms |
CHEMBL4059597; SCHEMBL17682668; BDBM281212
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.083 nM | |||
| External Link | ||||
| US10040779, Example 1 | Patented | [6] | ||
| Synonyms |
SCHEMBL15792304; BDBM277704; 3-[(1-methyl-1H-indazol-3-yl)amino]pyridine-4-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 0.1nM | |||
| External Link | ||||
| 1190312-92-5 | Patented | [11] | ||
| Synonyms |
3-chloro-1H-pyrrolo[3,2-b]pyridine-7-carboxylic acid; 3-CHLORO-4-AZAINDOLE-7-CARBOXYLIC ACID; SCHEMBL16157363; US10174026, Example 1; UAFNSWUBMGTOQA-UHFFFAOYSA-N; BDBM320361; ZINC44713035; 3-chloro-1H-pyrrolo[3,2-b] pyridine-7-carboxylic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5500 nM | |||
| External Link | ||||
| PBIT | Investigative | [13] | ||
| Synonyms |
2514-30-9; 2-(4-methylphenyl)-1,2-benzisothiazol-3(2H)-one; 2-(4-methylphenyl)-1,2-benzothiazol-3-one; MLS000583746; 2-(p-Tolyl)benzo[d]isothiazol-3(2H)-one; 2-(p-tolyl)-1,2-benzothiazol-3-one; SMR000200989; 2-(4-methylphenyl)-1,2-benzothiazol-3(2H)-one; 1,2-Benzisothiazol-3(2H)-one, 2-(4-methylphenyl)-; 2-(4-methylphenyl)-2,3-dihydro-1,2-benzothiazol-3-one; ChemDiv3_007090; AC1LIP69; cid_935415; SCHEMBL2443755; GTPL7026; CHEMBL1336959; CTK0J4356; BDBM34737; AOB6896; DTXSID10359056; MolPort-002-285-696; HMS2576N21
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| FA80: Intervertebral disc degeneration | 3 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Rexlemestrocel-L | Phase 3 | [14] | ||
| Synonyms |
MPC-150-M
Click to Show/Hide
|
|||
| External Link | ||||
| CybroCell | Phase 1/2 | [15] | ||
| External Link | ||||
| IDCT | Phase 1/2 | [16] | ||
| Synonyms |
rebonuputemcel
Click to Show/Hide
|
|||
| External Link | ||||
References