m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05897
|
[1] | |||
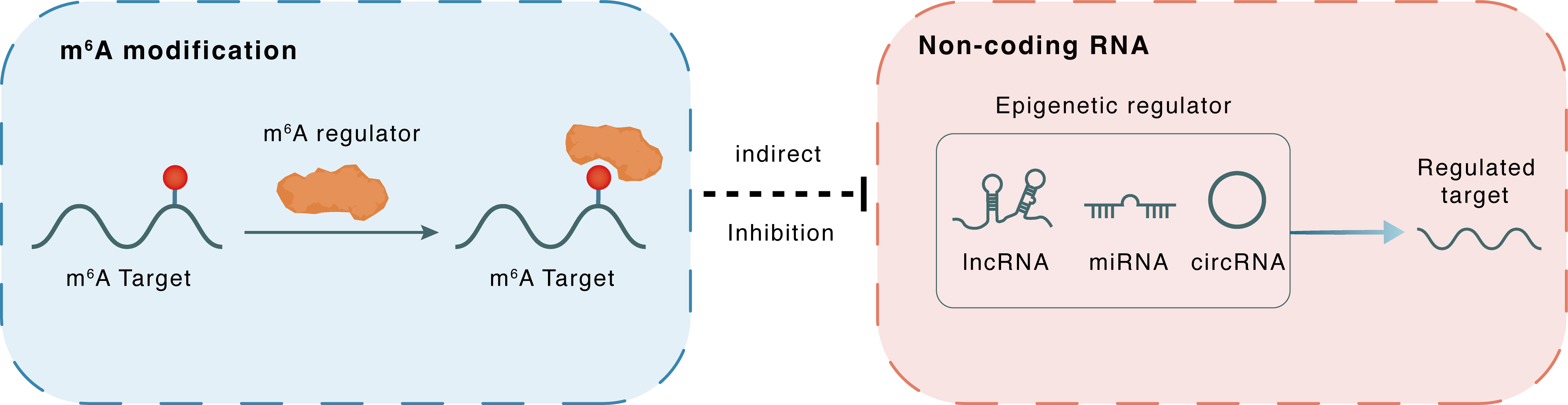 m6A modification
Circ_ASXL1
Circ_ASXL1
IGF2BP1
m6A modification
Circ_ASXL1
Circ_ASXL1
IGF2BP1
 : m6A sites
Indirect
Inhibition
Non-coding RNA
miR-320d
RACGAP1
lncRNA miRNA circRNA : m6A sites
Indirect
Inhibition
Non-coding RNA
miR-320d
RACGAP1
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) | READER | |||
| m6A Target | Circ_ASXL1 | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | hsa-miR-320d | microRNA | View Details | ||
| Regulated Target | Rac GTPase-activating protein 1 (RACGAP1) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Inhibition | |||
| Crosstalk Mechanism | m6A regulators indirectly modulate the functionality of ncRNAs through downstream signaling pathways | ||||
| Crosstalk Summary | METTL3/IGF2BP1-mediated m6A modification maintained Circ_ASXL1 stability and upregulated its expression. CircASXL1 was a ceRNA that sequestrated hsa-miR-320d from Rac GTPase-activating protein 1 (RACGAP1), leading to increased RACGAP1 expression. CircASXL1 promoted OC cell proliferation, migration and invasion via the miR-320d/RACGAP1 axis. | ||||
| Responsed Disease | Ovarian cancer | ICD-11: 2C73 | |||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | |||
| Cell Process | Cell proliferation, Cell migration | ||||
| Cell invasion | |||||
In-vitro Model |
SK-OV-3 | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| OVCAR-3 | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | ||
| ES-2 | Ovarian clear cell adenocarcinoma | Homo sapiens | CVCL_3509 | ||
| HEY | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0297 | ||
| A2780 | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | ||
| COV362 | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_2420 | ||
| In-vivo Model | SKOV3 cells were treated with sh-NC or sh-circASXL1. 100 μL of PBS containing 1 × 106 cells were implanted into mice. Tumor volume was measured every 5 days. After 30 days, mice were euthanized. The tumor was excised and weighed. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| 2C73: Ovarian cancer | 198 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Atezolizumab | Approved | [2] | ||
| External Link | ||||
| Carboplatin | Approved | [3] | ||
| Synonyms |
Azanide; Carbopaltin; Carboplatine; Carboplatino; Carboplatinum; Cbdca; Ercar; Paraplatin; Carboplatine [French]; Carboplatino [Spanish]; Carboplatinum [Latin]; C 2538; JM 8; Carboplatin (USAN); IUPAC: Azane; JM-8; Paraplatin (TN); Paraplatin, Carboplatin; Paraplatin-AQ; Cis-Diammine(cyclobutanedicarboxylato)platinumII; Platinum(+2) Cation; Carboplatin (JAN/USP/INN); Carboplatin [USAN:INN:BAN:JAN]; Cyclobutane-1,1-dicarboxylate; Cyclobutane-1,1-dicarboxylic acid; Diammine-1,1-cyclobutane dicarboxylate platinum II; Cis-Diamine[1,1-cyclobutanedicarboxylato]platinum(II); Cis-Diammine(1,1-cyclobutanedicarboxylato) platinum; Cis-Diammine(1,1-cyclobutanedicarboxylato)platinum; Cis-Diammine[1,1-cyclobutane-dicarboxylato] platinum; Diammine(1,1-cyclobutanedicarboxylato)platinum (II); Platinum, {diammine[1,1-cyclobut; Cis-(1,1-Cyclobutanedicarboxylato)diammineplatinum(II); Cis-Diamine(1,1-cyclobutanedicarboxylato)platinum(II); Cis-Diammine(1,1-cyclobutanedicarboxylato)platinum(II); Platinum(II), (1, 1-cyclobutanedicar; Diammine[cyclobutane-1,1-dicarboxylato(2-)-k2O1,O1]platinum; Diammine(cyclobutane-1,1-dicarboxylato(2-)-O,O')platinum; Platinum, diammine(1,1-cyclobutanedicarboxylato(2-)-O,O')-, (SP-4-2); (SP-4-2)-diammine[cyclobutane-1,1-dicarboxylato(2-)-kappa(2)O,O']platinum; 1,1-Cyclobutanedicarboxylate diammine platinum (II); 1,1-Cyclobutanedicarboxylate diammine platinum(II)
Click to Show/Hide
|
|||
| External Link | ||||
| Lurbinectedin | Phase 3 | [4] | ||
| Synonyms |
UNII-2CN60TN6ZS; 497871-47-3; 2CN60TN6ZS; Lurbinectedin [INN]; SCHEMBL16152477; DTXSID30198065; DB12674; CS-6323; HY-16293; J3.652.626B
Click to Show/Hide
|
|||
| External Link | ||||
| Mirvetuximab soravtansine | Approved | [2] | ||
| Synonyms |
ZOHXWSHGANNQGO-QRVRWUFNSA-N; DB12489
Click to Show/Hide
|
|||
| External Link | ||||
| Lenvatinib | Approved | [5] | ||
| Synonyms |
E 7080; E-7080, E7080; 4-[3-chloro-4-(cyclopropylcarbamoylamino)phenoxy]-7-methoxy-quinoline-6-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| Catumaxomab | Phase 2 | [6] | ||
| External Link | ||||
| Avelumab | Approved | [2] | ||
| External Link | ||||
| Olaparib | Approved | [2] | ||
| Synonyms |
AZD 2281; AZD2281; AZD-2281; Acylpiperazine analogue, 47; KU-0059436; KU-59436; Olaparib, KU-0059436, AZD2281,KU0059436, AZD2281; 4-[(3-{[4-Cyclopropylcarbonyl)piperazin-4-yl]carbonyl}-4-fluorophenyl)methyl]phtalazin-1(2H)-one; 4-[3-(4-Cyclopropanecarbonyl-piperazine-1-carbonyl)-4-fluoro-benzyl]-2H-phthalazin-1-one
Click to Show/Hide
|
|||
| External Link | ||||
| Cabozantinib | Approved | [7] | ||
| Synonyms |
Cabometyx; Cometriq
Click to Show/Hide
|
|||
| External Link | ||||
| Taxol | Approved | [8] | ||
| Synonyms |
C47H51NO14; weekly paclitaxel; Micellar Paclitaxel; Paclitaxel [USAN:INN:BAN]; SCHEMBL15000506; Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, (2aR-(2aalpha,4beta,4abeta,6beta,9alpha(alphaR*,betaS*),11alpha,12alpha,12aalpha,12balpha))-
Click to Show/Hide
|
|||
| External Link | ||||
| Tisotumab vedotin | Phase 2 | [9] | ||
| External Link | ||||
| KU-0058948 | Approved | [10] | ||
| Synonyms |
CHEMBL380648; 4-[3-(1,4-diazepan-1-ylcarbonyl)-4-fluorobenzyl]phthalazin-1(2H)-one; 4-(3-(1,4-diazepane-1-carbonyl)-4-fluorobenzyl)phthalazin-1(2H)-one; Homopiperazine analogue, 14; SCHEMBL864319; BDBM27533; HGEPGGJUGUMFHT-UHFFFAOYSA-N; ZINC3821234; DB08058; NCGC00386677-01; KU-58948; FT-0670691; TL80090044; 4-[3-([1,4]diazepane-1-carbonyl)-4 -fluorobenzyl]-2H-phthalazin-1-one; 4-[3-([1,4]diazepane-1-carbonyl)-4-fluorobenzyl]-2H-phthalazin-1-one
Click to Show/Hide
|
|||
| External Link | ||||
| MVax | Approved | [11] | ||
| Synonyms |
MVax (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Intedanib | Phase 2 | [12] | ||
| Synonyms |
Nintedanib; Vargatef; 656247-17-5; BIBF-1120; BIBF 1120; 928326-83-4; BIBF1120; Nintedanib (BIBF 1120); OFEV; UNII-G6HRD2P839; (Z)-methyl 3-((4-(N-methyl-2-(4-methylpiperazin-1-yl)acetamido)phenylamino)(phenyl)methylene)-2-oxoindoline-6-carboxylate; G6HRD2P839; CHEBI:85164; 1160294-26-7; Methyl (3z)-3-{[(4-{methyl[(4-Methylpiperazin-1-Yl)acetyl]amino}phenyl)amino](Phenyl)methylidene}-2-Oxo-2,3-Dihydro-1h-Indole-6-Carboxylate
Click to Show/Hide
|
|||
| External Link | ||||
| Fluzone QIV | Approved | [11] | ||
| Synonyms |
Seasonal influenza vaccine (quadrivalent)
Click to Show/Hide
|
|||
| External Link | ||||
| Pembrolizumab | Approved | [2] | ||
| External Link | ||||
| Plicamycin | Approved | [13] | ||
| Synonyms |
A-2371; NSC24559; PA-144; MTM A; AC1O3EQZ; CHEMBL413720; SCHEMBL14066059; NSC-24559; AKOS030213136; EC-7071; CCG-208236; NCGC00160390-01; NCI60_004287; AB01273958-01
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [2] | ||
| Synonyms |
Bevacizumab (ophthalmic slow-release tissue tablet)
Click to Show/Hide
|
|||
| External Link | ||||
| Trabectedin | Approved | [2] | ||
| Synonyms |
Ecteinascidin; Ecteinascidin-743; Et-743; Yondelis (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Topotecan | Approved | [14] | ||
| Synonyms |
Hycamptamine; Hycamptin; Hycamtamine; Topotecane; Topotecanum; Topotecan lactone; SKF 104864; SKF-S 104864; TOPOTECAN, HYCAMTIN; Topotecan (BAN); Topotecan [INN:BAN]; Topotecane [INN-French]; Topotecanum [INN-Latin]; Topotecan Monohydrochloride, (S)-Isomer; (4S)-10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione; (S)-10-((Dimethylamino)methyl)-4-ethyl-4,9-dihydroxy-1H-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4H,12H)-dione; (S)-10-[(DIMETHYLAMINO)METHYL]-4-ETHYL-4,9-DIHYDROXY-1H-PYRANO[3',4':6,7]INOLIZINO[1,2-B]-QUINOLINE-3,14(4H,12H)-DIONE; (S)-10-[(Dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]-quinoline-3,14(4H,12H)-dione; (S)-10-[(Dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione; 9-Dimethylaminomethyl-10-hydroxycamptothecin
Click to Show/Hide
|
|||
| External Link | ||||
| Altretamine | Approved | [15] | ||
| Synonyms |
Altretamina; Altretaminum; HEXAMETHYLMELAMINE; HMM; HTM; HXM; Hemel; Hexalen; Hexastat; Hexinawas; Altretamine Bellon Brand; Altretamine Chiesi Brand; Altretamine Wassermann Brand; Bellon Brand of Altretamine; Chiesi Brand of Altretamine; MGI Pharma Brand of Altretamine; Rhone Poulenc Rorer Brand of Altretamine; Wassermann Brand of Altretamine; A 8723; ENT 50852; NC 195; Altretamina [INN-Spanish]; Altretaminum [INN-Latin]; Hexalen (TN); Hexalen, Altretamine; KB-913; Rhone-Poulenc Rorer Brand of Altretamine; Altretamine (USP/INN); Altretamine [USAN:INN:BAN]; No-s-triazine; N,N,N',N',N'',N''-hexamethyl-1,3,5-triazine-2,4,6-triamine; N~2~,N~2~,N~4~,N~4~,N~6~,N~6~-Hexamethyl-1,3,5-triazine-2,4,6-triamine; 2,4, 6-Tris(dimethylamino)-1,3,5-triazine; 2,4,6-Tris(dimethylami; 2,4,6-Tris(dimethylamino)-1,3,5-triazine; 2,4,6-Tris(dimethylamino-s-triazine; 2-N,2-N,4-N,4-N,6-N,6-N-hexamethyl-1,3,5-triazine-2,4,6-triamine
Click to Show/Hide
|
|||
| External Link | ||||
| Rucaparib | Approved | [16] | ||
| Synonyms |
283173-50-2; Rubraca; AG-14447; UNII-8237F3U7EH; 8237F3U7EH; AK317822; 8-Fluoro-1,3,4,5-tetrahydro-2-[4-[(methylamino)methyl]phenyl]-6H-pyrrolo[4,3,2-ef][2]benzazepin-6-one; 8-FLUORO-2-(4-((METHYLAMINO)METHYL)PHENYL)-4,5-DIHYDRO-1H-AZEPINO[5,4,3-CD]INDOL-6(3H)-ONE; 6H-Pyrrolo[4,3,2-ef][2]benzazepin-6-one,8-fluoro-1,3,4,5-tetrahydro-2-[4-[(methylamino)methyl]phenyl]-; 8-fluoro-2-(4-methylaminomethyl-phenyl)-1,3,4,5-tetrahydro-azepino[5,4,3-cd]indol-6-one
Click to Show/Hide
|
|||
| External Link | ||||
| Melphalan flufenamide | Approved | [17] | ||
| Synonyms |
J-1; Dipeptide-conjugated melphalan prodrug (iv formulation, ovary tumor), Oncopeptides
Click to Show/Hide
|
|||
| External Link | ||||
| Nivolumab | Approved | [2] | ||
| External Link | ||||
| Ofranergene obadenovec | Phase 3 | [18] | ||
| Synonyms |
VB-111
Click to Show/Hide
|
|||
| External Link | ||||
| Batiraxcept | Phase 3 | [19] | ||
| Synonyms |
AVB-S6-500
Click to Show/Hide
|
|||
| External Link | ||||
| Trebananib | Phase 3 | [20] | ||
| External Link | ||||
| Nemvaleukin alfa | Phase 3 | [21] | ||
| External Link | ||||
| RRx-001 | Phase 2 | [2] | ||
| Synonyms |
925206-65-1; 2-BROMO-1-(3,3-DINITROAZETIDIN-1-YL)ETHAN-1-ONE; UNII-7RPW6SU9SC; 2-Bromo-1-(3,3-dinitroazetidin-1-yl)ethanone; 7RPW6SU9SC; RRx001; Ethanone, 2-bromo-1-(3,3-dinitro-1-azetidinyl)-; SCHEMBL2249018; CHEMBL3526802; MolPort-044-559-098; EX-A2006; BCP19228; 1-bromoacetyl-3,3-dinitroazetidine; ZINC34805177; s8405; DB12060; CS-5286; HY-16438; AS-53015
Click to Show/Hide
|
|||
| External Link | ||||
| Picoplatin | Phase 3 | [22] | ||
| Synonyms |
AMD 473; JM-473; ZD0473; (SP-4-3)-Amminedichloro(2-methylpyridine)platinium; Platinum, amminedichloro(2-methylpyridine)-, (SP-4-3)-; Picoplatin [INN:BAN]; UNII-B5TAN0L720; ZD 0473; AC1L42P3; B5TAN0L720; DB04874; Amminedichloro(2-methylpyridine)platinium; AN-30599; LS-184068; J-011604; azane; 2-methylpyridine; dichloride
Click to Show/Hide
|
|||
| External Link | ||||
| VB-111 | Phase 3 | [2] | ||
| External Link | ||||
| Upifitamab rilsodotin | Phase 3 | [23] | ||
| External Link | ||||
| ABT-888 | Phase 3 | [2] | ||
| Synonyms |
Veliparib; 912444-00-9; ABT 888; ABT-888 (Veliparib); Veliparib (ABT-888); ABT888; UNII-01O4K0631N; 2-[(2R)-2-Methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide; CHEBI:62880; 2-[(R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide; (R)-2-(2-methylpyrrolidin-2-yl)-1H-benzo[d]imidazole-4-carboxamide; 01O4K0631N; 2-[(2R)-2-Methylpyrrolidin-2-yl]-1H-benimidazole-4-; (2r)-2-(7-Carbamoyl-1h-Benzimidazol-2-Yl)-2-Methylpyrrolidinium; Veliparib dihydrochloride
Click to Show/Hide
|
|||
| External Link | ||||
| Rubraca rucaparib | Phase 3 | [2] | ||
| External Link | ||||
| Masitinib | Phase 3 | [2] | ||
| Synonyms |
790299-79-5; AB1010; Masatinib; Masitinib (AB1010); Masivet; AB-1010; AB 1010; UNII-M59NC4E26P; Masitinib [INN]; M59NC4E26P; 4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-thiazolyl]amino]phenyl]benzamide; CHEMBL1908391; CHEBI:63450; Masitinib (INN); N-(4-Methyl-3-((4-(pyridin-3-yl)thiazol-2-yl)amino)phenyl)-4-((4-methylpiperazin-1-yl)methyl)benzamide; Q-201339; C28H30N6OS; N-(4-methyl-3-(4-(pyridin-3-yl)thiazol-2-ylamino)phenyl)-4-((4-methylpiperazin-1-yl)methyl)benzamide
Click to Show/Hide
|
|||
| External Link | ||||
| Galinpepimut-S | Phase 3 | [24] | ||
| External Link | ||||
| SNDX-275 | Phase 3 | [2] | ||
| Synonyms |
Entinostat; Histone Deacetylase Inhibitor I; IN1470; MS 275; SNDX 275; MS 27-275; Ms-275; Entinostat (USAN/INN); MS-27-275; Pyridin-3-ylmethyl 4-(2-aminophenylcarbamoyl)benzylcarbamate; Pyridin-3-ylmethyl N-[[4-[(2-aminophenyl)carbamoyl]phenyl]methyl]carbamate; Pyridin-3-ylmethyl {4-[(2-aminophenyl)carbamoyl]benzyl}carbamate; Pyridin-3-ylmethyl{4-[(2-aminophenyl)carbamoyl]benzyl}carbamate; Carbamic acid, [[4-[[(2-aminophenyl)amino]carbonyl]phenyl] methyl]-, 3-pyridinylmethyl ester; Carbamic acid, [[4-[[(2-aminophenyl)carbaonyl]phenyl]methyl]-, 3-pyridinylmethyl ester; Entinostat, SNDX-275, MS-27-275, MS-275; N-(2-Aminophenyl)-4-[N-(pyridin-3-yl-methoxycarbonyl)aminomethyl]benzamide; N-(2-aminophenyl)-4-(N-(pyridin-3-ylmethoxycarbonyl)aminomethyl)benzamide; Carbamic acid, ((4-(((2-aminophenyl)amino)carbonyl)phenyl)methyl)-, 3-pyridinylmethyl ester; 3-Pyridinylmethyl ((4-(((2-aminophenyl)amino)carbonyl)phenyl)methyl)carbamate
Click to Show/Hide
|
|||
| External Link | ||||
| acelarin | Phase 2 | [2] | ||
| Synonyms |
NUC-1031
Click to Show/Hide
|
|||
| External Link | ||||
| Glufosfamide | Phase 3 | [5] | ||
| Synonyms |
Glucosylifostamide mustard; D 19575; D-19575; Glc-IPM; Glucosyl-ifosfamide mustard; Beta-D-Glucopyranose 1-(N,N'-bis(2-chloroethyl)phosphorodiamidate; Beta-D-Glucopyranose, 1-(N,N'-bis(2-chloroethyl)phosphorodiamidate); (2S,3R,4S,5S,6R)-2-bis(2-chloroethylamino)phosphoryloxy-6-(hydroxymethyl)oxane-3,4,5-triol
Click to Show/Hide
|
|||
| External Link | ||||
| PM1183 | Phase 3 | [8] | ||
| External Link | ||||
| Amonafide | Phase 3 | [25] | ||
| Synonyms |
Quinamed (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Etirinotecan pegol | Phase 2 | [8] | ||
| Synonyms |
NKTR-102
Click to Show/Hide
|
|||
| External Link | ||||
| INCB24360 | Phase 2 | [2] | ||
| Synonyms |
Epacadostat
Click to Show/Hide
|
|||
| External Link | ||||
| AL3818 | Phase 1/2 | [2] | ||
| Synonyms |
Anlotinib; 1058156-90-3; UNII-GKF8S4C432; GKF8S4C432; AL-3818; SCHEMBL2063386; GTPL9601; KSMZEXLVHXZPEF-UHFFFAOYSA-N; MolPort-044-567-604; ZINC117924202; AKOS030526233; DB11885; CS-5396; AL 3818; HY-19716; 1-[[4-[(4-fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxyquinolin-7-yl]oxymethyl]cyclopropan-1-amine; 1-((4-(4-fluoro-2-methyl-1h-indol-5-yloxy)-6-methoxyquinolin-7-yloxy)methyl)cyclopropan-amine; Cyclopropanamine, 1-(((4-((4-fluoro-2-methyl-1H-indol-5-yl)oxy)-6-methoxy-7-quinolinyl)oxy)methyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| Sapacitabine | Phase 3 | [2] | ||
| Synonyms |
CYC682
Click to Show/Hide
|
|||
| External Link | ||||
| Pelareorep | Phase 2 | [8] | ||
| External Link | ||||
| Opdivo + Yervoynivolumab + ipilimumab | Phase 1/2 | [2] | ||
| External Link | ||||
| Epothilon | Phase 3 | [5] | ||
| External Link | ||||
| Oregovomab | Phase 3 | [26] | ||
| External Link | ||||
| Farletuzumab | Phase 3 | [27] | ||
| External Link | ||||
| DCVax-Ovarian | Phase 3 | [2] | ||
| Synonyms |
DCVax-L; Dendritic cell-based immunotherapy (ovarian cancer), Northwest Biotherapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| MK-8109 | Phase 3 | [28] | ||
| External Link | ||||
| Karenitecin | Phase 3 | [29] | ||
| Synonyms |
Karenitecin (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| TRC105 | Phase 2 | [30] | ||
| External Link | ||||
| SNS-595 | Phase 3 | [5] | ||
| Synonyms |
Voreloxin; Vosaroxin; AG 7352; SNS 595; SPC 595; AG 7352 (TN); AG-7352; SNS 595 (TN); SPC 595 (TN); SPC-595; Voreloxin (TN); Voreloxin (USAN); SNS-595 (TN); 1,4-Dihydro-7-(3-methoxy-4-methylamino-1-pyrrolidinyl)-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid
Click to Show/Hide
|
|||
| External Link | ||||
| MK-4827 | Phase 3 | [31] | ||
| Synonyms |
1038915-60-4; (S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-carboxamide; UNII-HMC2H89N35; HMC2H89N35; CHEMBL1094636; 2-{4-[(3s)-piperidin-3-yl]phenyl}-2h-indazole-7-carboxamide; MK4827; MK 4827; Niraparib [USAN:INN]; 2-{4-[(3S)-piperidin-3-yl]phenyl}indazole-7-carboxamide; 2-[4-[(3S)-piperidin-3-yl]phenyl]indazole-7-carboxamide; MK 4827 (Base); Niraparib (USAN); Zejula (TN); MK-4827(Niraparib); SCHEMBL1421875; GTPL8275; CTK8B9123; EX-A290; DTXSID50146129; MolPort-023-219-142; ZINC43206370; BDBM50316226
Click to Show/Hide
|
|||
| External Link | ||||
| EC20 | Phase 3 | [32] | ||
| Synonyms |
Technetium Tc-99m etarfolatide
Click to Show/Hide
|
|||
| External Link | ||||
| Abagovomab | Phase 2/3 | [33] | ||
| External Link | ||||
| Mecbotamab vedotin | Phase 2 | [34] | ||
| Synonyms |
HTBA3011; HTBA3012
Click to Show/Hide
|
|||
| External Link | ||||
| GEN-1 | Phase 2 | [2] | ||
| External Link | ||||
| Stenoparib | Phase 2 | [35] | ||
| External Link | ||||
| DKN-01 | Phase 2 | [2] | ||
| External Link | ||||
| EP-100 | Phase 2 | [2] | ||
| External Link | ||||
| EP0057 | Phase 2 | [36] | ||
| Synonyms |
CRLX101
Click to Show/Hide
|
|||
| External Link | ||||
| BNT141 | Phase 2 | [37] | ||
| External Link | ||||
| TJ004309 | Phase 2 | [38] | ||
| External Link | ||||
| Maveropepimut-S | Phase 2 | [39] | ||
| External Link | ||||
| PF-07901801 | Phase 2 | [40] | ||
| Synonyms |
maplirpacept
Click to Show/Hide
|
|||
| External Link | ||||
| SPL-108 | Phase 2 | [2] | ||
| External Link | ||||
| VS-6063 | Phase 2 | [2] | ||
| Synonyms |
Defactinib hydrochloride; 1073160-26-5; Defactinib (hydrochloride); UNII-L2S469LM49; Defactinib hydrochloride [USAN]; L2S469LM49; Defactinib hydrochloride (USAN); Benzamide, N-methyl-4-[[4-[[[3-[methyl(methylsulfonyl)amino]-2-pyrazinyl]methyl]amino]-5-(trifluoromethyl)-2-pyrimidinyl]amino]-, hydrochloride; Defactinib HCl; Benzamide, N-methyl-4-((4-(((3-(methyl(methylsulfonyl)amino)-2-pyrazinyl)methyl)amino)-5-(trifluoromethyl)-2-pyrimidinyl)amino)-, hydrochloride (1:1); Benzamide, N-methyl-4-[[4-[[[3-[methyl(methylsu
Click to Show/Hide
|
|||
| External Link | ||||
| PTX-200 | Phase 2 | [2] | ||
| Synonyms |
Plant-derived antiparkinsonian, Phytrix
Click to Show/Hide
|
|||
| External Link | ||||
| Mirvetuximab soratansine | Phase 2 | [8] | ||
| External Link | ||||
| Ovapuldencel-T | Phase 2 | [2] | ||
| External Link | ||||
| MUC-1 cancer vaccine | Phase 2 | [41] | ||
| Synonyms |
CVac (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| FANG vaccine | Phase 3 | [42] | ||
| Synonyms |
FANG (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Ispinesib | Phase 2 | [43] | ||
| Synonyms |
SB-715992; Ispinesib (SB-715992 /CK0238273); SB-715992, CK0238273,Ispinesib; N-(3-aminopropyl)-N-[(1R)-1-(3-benzyl-7-chloro-4-oxoquinazolin-2-yl)-2-methylpropyl]-4-methylbenzamide
Click to Show/Hide
|
|||
| External Link | ||||
| EGEN-001 | Phase 2 | [44] | ||
| External Link | ||||
| CRLX101 | Phase 2 | [2] | ||
| External Link | ||||
| Folate binding protein vaccine | Phase 2 | [45] | ||
| External Link | ||||
| RO-5126766 | Phase 2 | [46] | ||
| Synonyms |
VS-6766; CH-5126766; Dual Raf/MEK protein kinase inhibitor (cancer), Roche
Click to Show/Hide
|
|||
| External Link | ||||
| TPIV200 | Phase 2 | [24] | ||
| External Link | ||||
| BBI503 | Phase 2 | [47] | ||
| External Link | ||||
| Varlilumab | Phase 2 | [2] | ||
| External Link | ||||
| Kevetrin | Phase 2 | [2] | ||
| External Link | ||||
| OTL38 | Phase 2 | [8] | ||
| External Link | ||||
| NanoPac | Phase 2 | [2] | ||
| External Link | ||||
| Sagopilone | Phase 2 | [48] | ||
| Synonyms |
ZK-EPO; 305841-29-6; UNII-KY72JU32FO; KY72JU32FO; DE-03757; ZK-219477; EPO-477; SH-Y03757A; ZK epothilone; Sagopilone [USAN:INN]; Sagopilone (USAN/INN); SCHEMBL423579; CHEMBL2304041; SH Y03757A; Bay 86-5302; ZINC3964449; DB12391; SH-Y-03757; BAY-86-5302; AN-28960; DE 03757; ZK 219477; D09721; 841S296; (1S,3S,7S,10R,11S,12S,16R)-10-allyl-7,11-dihydroxy-8,8,12,16-tetramethyl-3-(2-methyl-1,3-benzothiazol-5-yl)-4,17-dioxabicyclo[14.1.0]heptadecane-5,9-dione
Click to Show/Hide
|
|||
| External Link | ||||
| PMID27841036-Compound-37 | Phase 2 | [49] | ||
| Synonyms |
2X-121
Click to Show/Hide
|
|||
| External Link | ||||
| BGB-290 | Phase 2 | [50] | ||
| Synonyms |
Pamiparib
Click to Show/Hide
|
|||
| External Link | ||||
| TLPLDC | Phase 2 | [24] | ||
| External Link | ||||
| Anetumab ravtansine | Phase 2 | [2] | ||
| Synonyms |
Mesothelin-ADC
Click to Show/Hide
|
|||
| External Link | ||||
| OGX-427 | Phase 2 | [51] | ||
| External Link | ||||
| ZW25 | Phase 1 | [2] | ||
| Synonyms |
Zanidatamab
Click to Show/Hide
|
|||
| External Link | ||||
| CDX-1401 | Phase 2 | [2] | ||
| External Link | ||||
| CA4P | Phase 2 | [8] | ||
| Synonyms |
Fosbretabulin disodium; 168555-66-6; Combretastatin A4 disodium phosphate; CA4DP; Fosbretabulin (disodium); Zybrestat; UNII-702RHR475O; Combretastatin A4 Phosphate Disodium Salt; Fosbretabulin disodium [USAN]; 702RHR475O; Combretastatin A-4 phosphate; Fosbretabulin disodium (USAN); CHEMBL289351; Fosbretabulin (Combretastatin A4 Phosphate (CA4P)) Disodium; AC1OCF9S; Fosbretabulin Disodium Salt; CA-4P; CA 4P; SCHEMBL321426; C18H19O8P.2Na; MolPort-006-823-175; ACT03122; 3559AH; s7204; AKOS027327946; BCP9000542; CS-1484; NSC-752293; Fosbr
Click to Show/Hide
|
|||
| External Link | ||||
| Dendritic cell vaccine | Phase 2 | [52] | ||
| Synonyms |
Dendritic cell vaccine, University of Bonn; Human telomerase reverse transcriptase (hTERT)-pulsed dendritic cells (cancer), University of Bonn
Click to Show/Hide
|
|||
| External Link | ||||
| LY2606368 | Phase 2 | [2] | ||
| Synonyms |
prexasertib; 1234015-52-1; UNII-820NH671E6; LY-2606368; 820NH671E6; Prexasertib [USAN]; 5-((5-(2-(3-aminopropoxy)-6-methoxyphenyl)-1H-pyrazol-3-yl)amino)pyrazine-2-carbonitrile; 5-({5-[2-(3-aminopropoxy)-6-methoxyphenyl]-1H-pyrazol-3-yl}amino)pyrazine-2-carbonitrile; 5-[[5-[2-(3-aminopropoxy)-6-methoxyphenyl]-1H-pyrazol-3-yl]amino]pyrazine-2-carbonitrile; SCHEMBL1975451; GTPL9549; SCHEMBL19457660; SCHEMBL18989301; CHEMBL3544911; EX-A758; DOTGPNHGTYJDEP-UHFFFAOYSA-N; AOB87325; ZINC95837013
Click to Show/Hide
|
|||
| External Link | ||||
| GSK3377794 | Phase 2 | [2] | ||
| External Link | ||||
| Melphalan | Approved | [53] | ||
| External Link | ||||
| MEDI-546 | Phase 2 | [54] | ||
| External Link | ||||
| RG-7599 | Phase 2 | [55] | ||
| Synonyms |
DNIB-0600A; Antibody-drug conjugate (NSCLC/ovarian cancer), Genentech
Click to Show/Hide
|
|||
| External Link | ||||
| EP-201 | Phase 2 | [24] | ||
| Synonyms |
Chissonox 201; Unox epoxide 201; Unox 201; Epoxide-201; EP 201; 141-37-7; CCRIS 279; NSC 61273; BRN 0246264; MLS002638023; CHEBI:82463; 3,4-epoxy-6-methylcyclohexylmethyl-3,4-epoxy-6-methylcyclohexanecarboxylate; Epoxide 201; 6-Methyl-3,4-epoxycyclohexylmethyl 6-methyl-3,4-epoxycyclohexane carboxylate; 3,4-Epoxy-6-methylcyclohexylmethyl-3,4-epoxy-6-methylcyclohexane carboxylate; 3,4-Epoxy-6-methylcyclohexylmethyl-3',4'-epoxy-6'-methylcyclohexane carboxylate; 3,4-Epoxy-6-methylcyclohexylmethyl 3,4-epoxy-6-methylcyclohexanec
Click to Show/Hide
|
|||
| External Link | ||||
| APR-246 | Phase 2 | [2] | ||
| Synonyms |
Eprenetapopt
Click to Show/Hide
|
|||
| External Link | ||||
| Tigatuzumab | Phase 2 | [56] | ||
| External Link | ||||
| CRS-207 | Phase 2 | [24] | ||
| External Link | ||||
| MK-1775 | Phase 2 | [57] | ||
| Synonyms |
955365-80-7; Adavosertib; MK1775; MK 1775; UNII-K2T6HJX3I3; AZD 1775; AZD-1775; AZD1775; 2-allyl-1-(6-(2-hydroxypropan-2-yl)pyridin-2-yl)-6-(4-(4-methylpiperazin-1-yl)phenylamino)-1H-pyrazolo[3,4-d]pyrimidin-3(2H)-one; K2T6HJX3I3; 1-[6-(2-hydroxypropan-2-yl)pyridin-2-yl]-6-[4-(4-methylpiperazin-1-yl)anilino]-2-prop-2-enylpyrazolo[3,4-d]pyrimidin-3-one; 2-Allyl-1-(6-(2-hydroxypropan-2-yl)pyridin-2-yl)-6-((4-(4-methylpiperazin-1-yl)phenyl)amino)-1H-pyrazolo[3,4-d]pyrimidin-3(2H)-one; AK-99219
Click to Show/Hide
|
|||
| External Link | ||||
| CP-547632 | Phase 2 | [58] | ||
| Synonyms |
BFF; CP 547632; CP-547,632; 3-(4-Bromo-2,6-difluorobenzyloxy)-5-(3-(4-pyrrolidin-1-ylbutyl)ureido)isothiazole-4-carboxylic acid amide
Click to Show/Hide
|
|||
| External Link | ||||
| Motolimid | Phase 2 | [8] | ||
| External Link | ||||
| TC-210 | Phase 1/2 | [59] | ||
| External Link | ||||
| REGN5668 | Phase 1/2 | [60] | ||
| External Link | ||||
| TC-510 | Phase 1/2 | [61] | ||
| External Link | ||||
| XMT-1592 | Phase 1/2 | [62] | ||
| External Link | ||||
| GL-ONC1 | Phase 2 | [63] | ||
| Synonyms |
olvimulogene nanivacirepvec
Click to Show/Hide
|
|||
| External Link | ||||
| LY2940680 | Phase 1/2 | [2] | ||
| Synonyms |
1258861-20-9; Taladegib; LY-2940680; UNII-QY8BWX1LJ5; 4-Fluoro-N-methyl-N-(1-(4-(1-methyl-1H-pyrazol-5-yl)phthalazin-1-yl)piperidin-4-yl)-2-(trifluoromethyl)benzamide; QY8BWX1LJ5; Taladegib (LY2940680); LY 2940680; 4-fluoro-n-methyl-n-(1-(4-(1-methyl-1h-pyrazol-5-yl)-1-phthalazinyl)-4-piperidinyl)-2-(trifluoromethyl)benzamide; 4-Fluoro-N-Methyl-N-{1-[4-(1-Methyl-1h-Pyrazol-5-Yl)phthalazin-1-Yl]piperidin-4-Yl}-2-(Trifluoromethyl)benzamide; Taladegib [USAN:INN]
Click to Show/Hide
|
|||
| External Link | ||||
| Her-2/neu vaccine | Phase 1/2 | [64] | ||
| External Link | ||||
| NY-ESO-TCR | Phase 1/2 | [65] | ||
| External Link | ||||
| GALE-302 | Phase 1/2 | [2] | ||
| External Link | ||||
| DPX-survivac cancer vaccine | Phase 1/2 | [66] | ||
| External Link | ||||
| REGN4018 | Phase 1/2 | [67] | ||
| External Link | ||||
| GALE-301 + GALE-302 | Phase 1/2 | [24] | ||
| External Link | ||||
| Anti-C-met CAR-T cells | Phase 1/2 | [68] | ||
| External Link | ||||
| CDX-014 | Phase 1/2 | [2] | ||
| External Link | ||||
| O-Vax | Phase 1/2 | [69] | ||
| Synonyms |
AC vaccine (ovarian cancer), AVAX
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-HER2 CAR-T | Phase 1/2 | [70] | ||
| External Link | ||||
| NT-501 CNTF | Phase 1/2 | [71] | ||
| External Link | ||||
| Phenoxodiol | Phase 1/2 | [72] | ||
| Synonyms |
Dehydroequol; Idronoxil; Haginin E; NV 06; NV-06; Idronoxil (USAN/INN); 2H-1-benzopyran-7-0,1,3-(4-hydroxyphenyl); 3-(4-Hydroxyphenyl)-2H-1-benzopyran-7-ol; 3-(4-Hydroxyphenyl)-2H-chromen-7-ol; 7-hydroxy-3-hydroxyphenyl-1H-benzopyran
Click to Show/Hide
|
|||
| External Link | ||||
| GALE-301 | Phase 2 | [24] | ||
| External Link | ||||
| IL-2/gene-modified lymphocytes | Phase 1/2 | [73] | ||
| External Link | ||||
| Ovarian dendritic cell-based vaccine | Phase 1/2 | [74] | ||
| External Link | ||||
| Anti-mesothelin CAR transduced PBL | Phase 1/2 | [75] | ||
| External Link | ||||
| ONCOS-102 | Phase 1/2 | [2] | ||
| Synonyms |
CGTG-102
Click to Show/Hide
|
|||
| External Link | ||||
| BNT115 | Phase 1 | [76] | ||
| External Link | ||||
| MEDI3617 | Phase 1 | [77] | ||
| External Link | ||||
| SL-172154 | Phase 1 | [78] | ||
| External Link | ||||
| INKmune | Phase 1 | [79] | ||
| External Link | ||||
| DeTIL-0255 | Phase 1 | [80] | ||
| External Link | ||||
| DS-6000 | Phase 1 | [81] | ||
| External Link | ||||
| MCY-M11 | Phase 1 | [82] | ||
| External Link | ||||
| IMT1012 | Phase 1 | [83] | ||
| External Link | ||||
| mRNA-2416 | Phase 1 | [84] | ||
| Synonyms |
mRNA-OX40L
Click to Show/Hide
|
|||
| External Link | ||||
| TG4050 | Phase 1 | [85] | ||
| External Link | ||||
| GRN-300 | Phase 1 | [86] | ||
| External Link | ||||
| ITIL-306 | Phase 1 | [87] | ||
| External Link | ||||
| STRO-002 | Phase 1 | [88] | ||
| External Link | ||||
| Ipafricept | Phase 1 | [89] | ||
| External Link | ||||
| IMT-1012 immunotherapeutic vaccine | Phase 1 | [90] | ||
| External Link | ||||
| STM 434 | Phase 1 | [8] | ||
| External Link | ||||
| Mibefradil | Withdrawn from market | [91] | ||
| Synonyms |
Mibefradil (Cytostatic Checkpoint Therapy, ovarian cancer); Mibefradil (Cytostatic Checkpoint Therapy, ovarian cancer), Tau Therapeutics; Calcium T-channel inhibitor (Cytostatic Checkpoint Therapy, ovarian cancer), Tau Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| RG7882 | Phase 1 | [92] | ||
| External Link | ||||
| MOv19-BBz CAR T cells | Phase 1 | [93] | ||
| External Link | ||||
| SC-003 | Phase 1 | [2] | ||
| External Link | ||||
| KHK-2866 | Phase 1 | [94] | ||
| External Link | ||||
| PUMVC3-hIGFBP-2 | Phase 1 | [95] | ||
| Synonyms |
IGFBP-2 DNA plasmid vaccine (intradermal, ovarian cancer); IGFBP-2 DNA plasmid vaccine (intradermal, ovarian cancer), Fred Hutchinson
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-hCD70 CAR transduced PBL | Phase 1 | [96] | ||
| External Link | ||||
| Prolanta | Phase 1 | [2] | ||
| External Link | ||||
| JCAR020 | Phase 1 | [65] | ||
| External Link | ||||
| COTI-2 | Phase 1 | [2] | ||
| Synonyms |
UNII-2BTA1O65BR; 2BTA1O65BR; 1039455-84-9; ZINC114475331; CS-8156; HY-19896; 1-Piperazinecarbothioic acid, 4-(2-pyridinyl)-, 2-(6,7-dihydro-8(5H)-quinolinylidene)hydrazide
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-meso-CAR vector transduced T cells | Phase 1 | [97] | ||
| External Link | ||||
| Cantrixil | Phase 1 | [2] | ||
| Synonyms |
UNII-5DVS457HEG; 5DVS457HEG; SCHEMBL19410294; TRX-E-002-1; cis-4-(p-hydroxyphenyl)-7,4'-dihydroxy-3',5'-dimethoxy-8-methylisoflavan; (+)-cis-4-(Para-hydroxyphenyl)-7,4-dihydroxy-3,5-dimethoxy-8-methylisoflavan; 1803036-93-2
Click to Show/Hide
|
|||
| External Link | ||||
| Iboctadekin + Doxil | Phase 1 | [98] | ||
| External Link | ||||
| FATE-NK100 | Phase 1 | [2] | ||
| External Link | ||||
| Iboctadekin | Phase 1 | [99] | ||
| External Link | ||||
| Anti-CD133-CAR vector-transduced T cells | Phase 1 | [100] | ||
| External Link | ||||
| Hu-CART meso cells | Phase 1 | [101] | ||
| External Link | ||||
| PTC596 | Phase 1 | [2] | ||
| External Link | ||||
| SOR-C13 | Phase 1 | [102] | ||
| External Link | ||||
| AE-O | Phase 1 | [103] | ||
| Synonyms |
Ovarian vaccine, Generex
Click to Show/Hide
|
|||
| External Link | ||||
| E 7974 | Phase 1 | [5] | ||
| Synonyms |
26510-52-1; ethyl 3-oxo-3-(pyridin-2-yl)propanoate; Ethyl picolinoylacetate; ethyl 3-oxo-3-(2-pyridyl)propionate; ethyl 3-oxo-3-pyridin-2-ylpropanoate; ethyl 3-oxo-3-(2-pyridinyl)propanoate; 3-Oxo-3-(2-pyridyl)propionic acid ethyl ester; Ethyl picolinoylacetate, 95%; ethyl 3-oxo-3-(2-pyridyl)propanoate; PubChem11088; ethyl-2-pyridoyl acetate; AC1MC5MX; AC1Q34ND; SCHEMBL601821; CHEMBL2035673; CTK4F8033; DTXSID30371376; MolPort-001-769-152; FQHXWZMJALFSJJ-UHFFFAOYSA-N; ZINC153764; Ethyl picolinoylacetate, AldrichCPR; STL131872
Click to Show/Hide
|
|||
| External Link | ||||
| DMUC-5754A | Phase 1 | [104] | ||
| External Link | ||||
| Alvespimycin hydrochloride | Phase 1 | [105] | ||
| Synonyms |
Alvespimycin hydrochloride (USAN); 17-DMAG; 17-Desmethoxy-17-N,N-dimethylaminoethylamino-geldanamycin, HCl; 17-N,N-Dimethylaminoethylamino-17-demethoxy-geldanamycin, HCl; 17DMAG; 17DMAG, Alvespimycin, KOS-1022, NSC 707545, 17-DMAG
Click to Show/Hide
|
|||
| External Link | ||||
| Navicixizumab | Phase 1 | [65] | ||
| External Link | ||||
| AR-A014418 | Patented | [106] | ||
| Synonyms |
487021-52-3; GSK-3beta Inhibitor VIII; 1-(4-methoxybenzyl)-3-(5-nitrothiazol-2-yl)urea; 1-[(4-methoxyphenyl)methyl]-3-(5-nitro-1,3-thiazol-2-yl)urea; A Inhibitor VIII; N-(4-METHOXYBENZYL)-N'-(5-NITRO-1,3-THIAZOL-2-YL)UREA; UNII-87KSH90Q6D; AR-AO 14418; SN 4521; AR-A 014418; CHEMBL259850; 87KSH90Q6D; N-[(4-Methoxyphenyl)methyl]-N'-(5-nitro-2-thiazolyl)urea; AK175829; C12H12N4O4S; N-(4-Methoxybenzyl)-N& -(5-nitro-1,3-thiazol-2-yl)urea; AR 014418; GSK 3be
Click to Show/Hide
|
|||
| External Link | ||||
| R1549 | Discontinued in Phase 3 | [107] | ||
| External Link | ||||
| Tanomastat | Discontinued in Phase 3 | [108] | ||
| Synonyms |
Tanomastat (USAN/INN); (2S)-4-[4-(4-chlorophenyl)phenyl]-4-oxo-2-(phenylsulfanylmethyl)butanoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| IDM-1 | Discontinued in Phase 3 | [109] | ||
| Synonyms |
Osidem
Click to Show/Hide
|
|||
| External Link | ||||
| Lurtotecan | Discontinued in Phase 2 | [110] | ||
| Synonyms |
OSI-211; Lurtotecan [INN]; 149882-10-0; UNII-4J1L80T08I; OSI 211; NX 211; GG 211; Gi 147211; 4J1L80T08I; C28H30N4O6; CHEMBL341028; 11H-1,4-Dioxino(2,3-g)pyrano(3',4':6,7)indolizino(1,2-b)quinoline-9,12(8H,14H)-dione, 8-ethyl-2,3-dihydro-8-hydroxy-15-((4-methyl-1-piperazinyl)methyl)-, (8S)-; GI-147211C; lurtotecan liposome; AC1L1U8C; SCHEMBL19208; CHEMBL305666; DTXSID30164422; GG-211; ZINC22010625; NX-211; GW-211; BDBM50036130; DB12222; LS-173358; Lurtotecan dihydrochloride; GI 147211C; GG-147211C; GI-147211; GI-147211A; GL-147211C; Liposomal lurtotecan
Click to Show/Hide
|
|||
| External Link | ||||
| AGS-8M4 | Discontinued in Phase 2 | [111] | ||
| Synonyms |
AGS-8 MAb, Agensys, AGS8M4, ASP-6183
Click to Show/Hide
|
|||
| External Link | ||||
| Biricodar | Discontinued in Phase 2 | [112] | ||
| Synonyms |
Incel; Biricodar [INN]; Vx 710; Incel (TN); 1,7-dipyridin-3-ylheptan-4-yl (2S)-1-[2-oxo-2-(3,4,5-trimethoxyphenyl)acetyl]piperidine-2-carboxylate
Click to Show/Hide
|
|||
| External Link | ||||
| Onyvax-O | Discontinued in Phase 1 | [113] | ||
| External Link | ||||
| RG7458 | Discontinued in Phase 1 | [114] | ||
| External Link | ||||
| RG7600 | Discontinued in Phase 1 | [115] | ||
| External Link | ||||
| S-8184 | Preclinical | [116] | ||
| External Link | ||||
| 111In-OC-125 F(ab1/2)-DTPA | Terminated | [117] | ||
| Synonyms |
Indimacis 125; Indium-111-OC-125 F(ab1/2)-DTPA
Click to Show/Hide
|
|||
| External Link | ||||
| HumaRAD-OV | Investigative | [118] | ||
| Synonyms |
HumaRAD88BV59; KSB-310
Click to Show/Hide
|
|||
| External Link | ||||
| FOLIGO 002 | Investigative | [118] | ||
| External Link | ||||
| HS-210 | Investigative | [118] | ||
| Synonyms |
HS-01; Endoplasmin modulator (ovary tumor, HeatShock/fusion protein/antigen), Heat Biologics; Gp-96-Ig + unspecified tumorantigen secreting live cell vaccine (ovary tumor, HeatShock), Heat Biologics
Click to Show/Hide
|
|||
| External Link | ||||
| CGEN-991 | Investigative | [118] | ||
| Synonyms |
Anti-CGEN-991 antibody (ovarian cancer); CGEN-991 (ovarian cancer), Compugen; Anti-CGEN-991 antibody (ovarian cancer), Compugen
Click to Show/Hide
|
|||
| External Link | ||||
| FabOvar | Investigative | [118] | ||
| Synonyms |
Alpha-folate receptor-targeting I131-labeled mAb fragment (ip, ovarian carcinoma), Advanced Accelerator Applications; Alpha-folate receptor-targeting I131-labeled mAb fragment (ip, ovarian carcinoma), Dompe
Click to Show/Hide
|
|||
| External Link | ||||
| ALVAC-CEA/hB7.1 | Investigative | [118] | ||
| External Link | ||||
| MX-35 | Investigative | [118] | ||
| Synonyms |
Monoclonal antibody (ovarian cancer), Recepta
Click to Show/Hide
|
|||
| External Link | ||||
| 6-(4-CHLOROPHENYL)-7-(2,4-DICHLOROPHENYL)-N-(HYDROXYMETHYL)-1,2,2-TRIMETHYL-1,2,3,4-TETRAHYDRO-1,8-NAPHTHYRIDINE-4-CARBOXAMIDE (ENANTIOMERIC MIX) | Investigative | [119] | ||
| Synonyms |
CHEMBL1082330; BDBM50320180
Click to Show/Hide
|
|||
| External Link | ||||
| ET-006 | Investigative | [118] | ||
| External Link | ||||
| AB-3D3 | Investigative | [118] | ||
| Synonyms |
AB-0447a; KAAG1-targeting mAb (ovarian cancer), Alethia; KAAG1-targeting monoclonal antibody (ovarian cancer), Alethia; Kidney associated antigen 1-targeting mAb (ovarian cancer), Alethia; Kidney associated antigen 1-targeting monoclonal antibody (ovarian cancer), Alethia; AB-0447-targeting monoclonal antibodies (cancer), Alethia
Click to Show/Hide
|
|||
| External Link | ||||
| RAP-701 | Investigative | [118] | ||
| Synonyms |
Small stabilized receptor active peptide (ovarian cancer), RAPID Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-folate receptor 1 humanized mab | Investigative | [118] | ||
| Synonyms |
Anti-folate receptor 1 humanized mAb (cancer)
Click to Show/Hide
|
|||
| External Link | ||||
| Disorazol Z-LHRH conjugates | Investigative | [118] | ||
| Synonyms |
Disorazol Z-LHRH conjugates (ovarian cancer)
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-CGEN-153 mab | Investigative | [118] | ||
| Synonyms |
CGEN-153; Anti-CGEN-153 mAb (ovarian cancer); CGEN-153 (Ovarian cancer), Compugen; Anti-CGEN-153 mAb (ovarian cancer), Compugen
Click to Show/Hide
|
|||
| External Link | ||||
| OGX-427 + Paclitaxel | Investigative | [120] | ||
| External Link | ||||
References