m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05824
|
[1] | |||
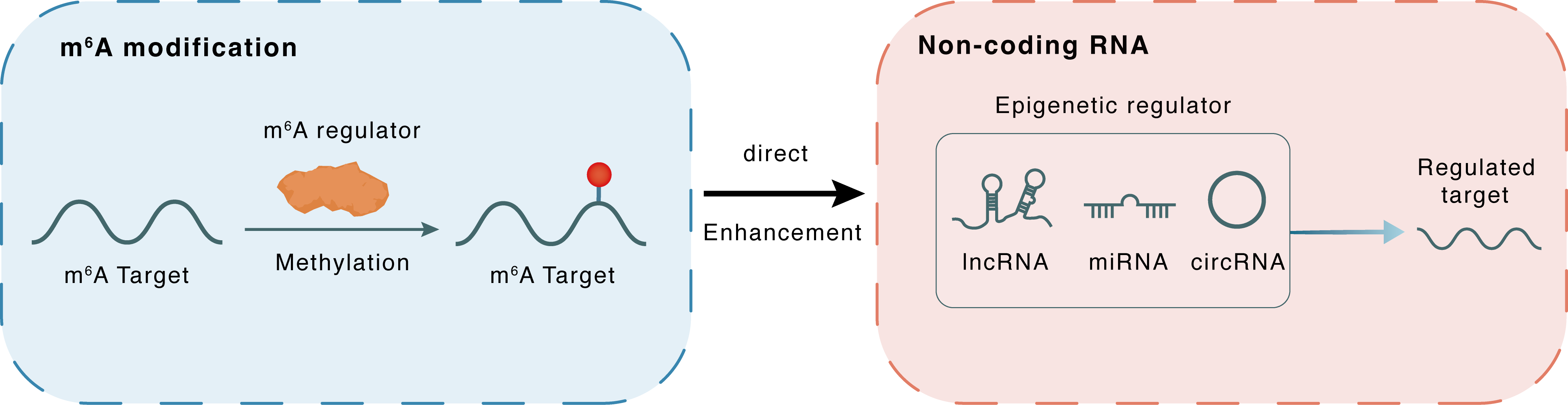 m6A modification
hsa_circ_0136959
hsa_circ_0136959
METTL3
Methylation
m6A modification
hsa_circ_0136959
hsa_circ_0136959
METTL3
Methylation
 : m6A sites
Direct
Enhancement
Non-coding RNA
hsa_circ_0136959
Regulated Target
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
hsa_circ_0136959
Regulated Target
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | hsa_circ_0136959 | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | hsa_circ_0136959 | circRNA | View Details | ||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | METTL3 was notably reduced in PTC samples and was positively correlated with hsa_circ_0136959. Mechanistically, METTL3 enhanced hsa_circ_0136959 expression through m6A modification. Our results demonstrate that METTL3-mediated m6A modification elevated hsa_circ_0136959 expression and subsequently restricted the tumor characteristics of PTC by accelerating ferroptosis. | ||||
| Responsed Disease | Papillary thyroid cancer | ICD-11: 2D10.1 | |||
| Pathway Response | Ferroptosis | hsa04216 | |||
| Cell Process | Ferroptosis | ||||
In-vitro Model |
TPC-1 | Thyroid gland papillary carcinoma | Homo sapiens | CVCL_6298 | |
| Nthy-ori 3-1 | Normal | Homo sapiens | CVCL_2659 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| 2D10: Thyroid Cancer | 12 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Lenvatinib | Approved | [2] | ||
| Synonyms |
E 7080; E-7080, E7080; 4-[3-chloro-4-(cyclopropylcarbamoylamino)phenoxy]-7-methoxy-quinoline-6-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| Cabozantinib | Approved | [3] | ||
| Synonyms |
Cabometyx; Cometriq
Click to Show/Hide
|
|||
| External Link | ||||
| Thyrotropin Alfa | Approved | [4] | ||
| External Link | ||||
| Selpercatinib | Approved | [5] | ||
| Synonyms |
MFOVQWYFURMVKU-IWAAJCSBSA-N; 2222755-14-6; LOXO292; ARRY-192; LOXO 292; EX-A2636
Click to Show/Hide
|
|||
| External Link | ||||
| Selumetinib | Phase 3 | [6] | ||
| Synonyms |
AZD-6244; ARRY142886; AZD6244; AZD 6244; 6UH91I579U; ARRY 142886; ARRY-142886; AZD6244 (Selumetinib); AZD6244(Selumetinib); CHEBI:90227; CHEMBL1614701; MEK inhibitors; Selumetinib (AZD6244); UNII-6UH91I579U
Click to Show/Hide
|
|||
| External Link | ||||
| QGE-031 | Phase 2 | [7] | ||
| External Link | ||||
| GI-6207 | Phase 2 | [8] | ||
| External Link | ||||
| AIC100 | Phase 1 | [9] | ||
| External Link | ||||
| CYTO-403 | Phase 1 | [10] | ||
| External Link | ||||
| Demogastrin | Phase 1 | [11] | ||
| Synonyms |
Technetium-99m-Demogastrin 2; Cholecystokinin-2 receptor/gastrin-R binding radiolabeled minigastrinanalogue (medullary thyroid cancer), Biomedica Life Science; 99mTc-Demogastrin 2; 99mTc-N4-Gly-(D)Glu-(Glu)5-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2
Click to Show/Hide
|
|||
| External Link | ||||
| Recombinant TSH superagonists | Investigative | [12] | ||
| Synonyms |
Recombinant TSH superagonists (thyroid cancer); TR-1401; TR-1402; Recombinant TSH superagonists (thyroid cancer), Trophogen; TSH superagonist (thyroid cancer/goiter), Trophogen; Thyroid stimulating hormone superagonist (thyroid cancer/goiter), Trophogen
Click to Show/Hide
|
|||
| External Link | ||||
| ITRI-305 | Investigative | [13] | ||
| Synonyms |
RET tyrosine kinase inhibitors, Industrial Technology Research Institute
Click to Show/Hide
|
|||
| External Link | ||||
References