m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05654
|
[1] | |||
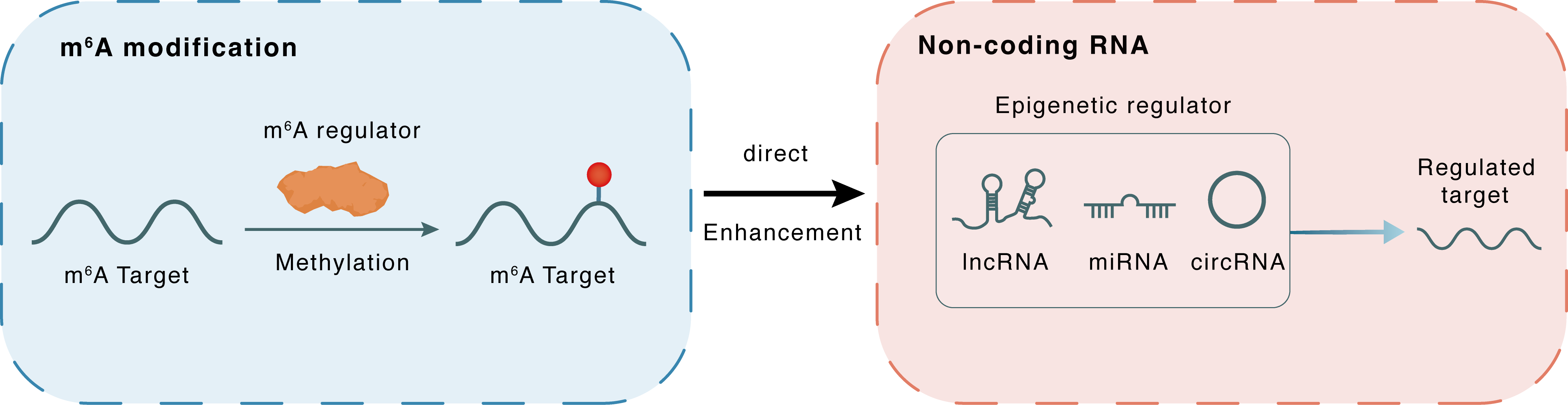 m6A modification
lncRNA4114
lncRNA4114
METTL3
Methylation
m6A modification
lncRNA4114
lncRNA4114
METTL3
Methylation
 : m6A sites
Direct
Enhancement
Non-coding RNA
lncRNA4114
Regulated Target
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
lncRNA4114
Regulated Target
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Long intergenic non-protein coding RNA 1638 (LINC01638) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | Long intergenic non-protein coding RNA 1638 (LINC01638) | LncRNA | View Details | ||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | Overexpression of methyltransferase like 3 (METTL3) promoted the osteogenic differentiation of PPDLSCs, while knocking down METTL3 showed an inhibitory effect. Furthermore, METTL3 overexpression promotes the stability of Long intergenic non-protein coding RNA 1638 (LINC01638) to upregulate the expression level. Moreover, lncRNA4114 overexpression promoted the osteogenic differentiation of PPDLSCs. | ||||
| Responsed Disease | Periodontitis | ICD-11: DA0C | |||
| Cell Process | Cell differentiation | ||||
In-vitro Model |
PPDLSCs (Porcine Periodontal Ligament Stem Cells) | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| DA0C: Periodontitis | 7 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| AlloDerm | Approved | [2] | ||
| Synonyms |
AlloCraft; Cymetra; GraftJacket; Repliform; Strattice; Graft tissue product, LifeCell
Click to Show/Hide
|
|||
| External Link | ||||
| Trafermin | Phase 3 | [3] | ||
| Synonyms |
Fiblast; Fiblast (TN); Trafermin (genetical recombination); Trafermin (USAN/INN); Trafermin (genetical recombination) (JAN)
Click to Show/Hide
|
|||
| External Link | ||||
| RhGDF-5 | Phase 2 | [4] | ||
| Synonyms |
MD-05; MD-06; MD-07; MD-08; RhGDF-5 (bone regeneration); MP52 (BCP carrier, bone regeneration), BioPharm/Scil; RhGDF-5 (bone regeneration), Scil/Medtronic; RhGDF-5 (implantable bone substitute), BioPharm/Scil; MP52 (beta-tricalciumphosphate carrier, bone regeneration), BioPharm/Scil
Click to Show/Hide
|
|||
| External Link | ||||
| PerioPatch | Phase 2 | [5] | ||
| External Link | ||||
| MSI-469 | Terminated | [6] | ||
| External Link | ||||
| Sharon-3000 | Investigative | [7] | ||
| External Link | ||||
| BT-301 | Investigative | [7] | ||
| Synonyms |
PRF-K
Click to Show/Hide
|
|||
| External Link | ||||
References