m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05646
|
[1] | |||
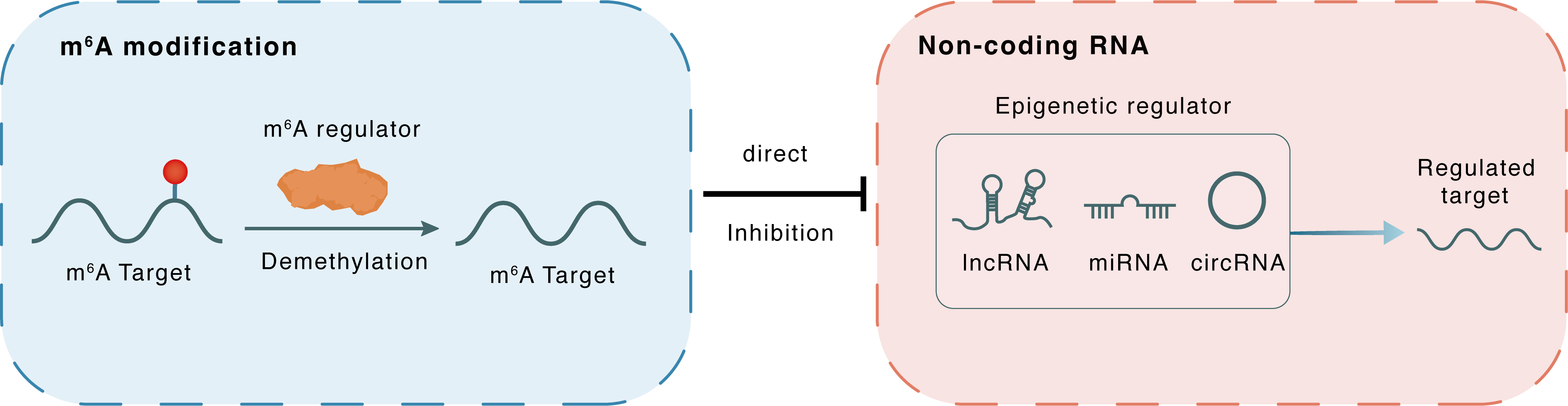 m6A modification
GAS5
GAS5
FTO
Demethylation
m6A modification
GAS5
GAS5
FTO
Demethylation
 : m6A sites
Direct
Inhibition
Non-coding RNA
GAS5
BRD4
lncRNA miRNA circRNA : m6A sites
Direct
Inhibition
Non-coding RNA
GAS5
BRD4
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | |||
| m6A Target | Growth arrest specific 5 (GAS5) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | Growth arrest specific 5 (GAS5) | LncRNA | View Details | ||
| Regulated Target | Bromodomain-containing protein 4 (BRD4) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Inhibition | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | LncRNA Growth arrest specific 5 (GAS5) regulated by FTO-mediated m6A demethylation promotes autophagic cell death in NSCLC by targeting UPF1/Bromodomain-containing protein 4 (BRD4) axis. | ||||
| Responsed Disease | Non-small cell lung cancer | ICD-11: 2C25.Y | |||
| Cell Process | mRNA stability | ||||
| Cell autophagy | |||||
In-vitro Model |
BEAS-2B | Normal | Homo sapiens | CVCL_0168 | |
| NCI-H322 | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_1556 | ||
| NCI-H650 | Lung adenocarcinoma | Homo sapiens | CVCL_1575 | ||
| HCC827 | Lung adenocarcinoma | Homo sapiens | CVCL_2063 | ||
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | ||
| NCI-H358 | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_1559 | ||
| NCI-H838 | Lung adenocarcinoma | Homo sapiens | CVCL_1594 | ||
| NCI-H23 | Lung adenocarcinoma | Homo sapiens | CVCL_1547 | ||
| In-vivo Model | Animal experiments were approved by the Hainan Affiliated Hospital of Hainan Medical University. Male BALB/c mice (Vital River, Beijing, China) were subcutaneously injected with H322 cells (1 × 106) transfected with ov-NC, ov-FTO, ov-GAS5 or ov-FTO + ov-GAS5 (n = 5/group). Tumor volume was measured every 7 days, and mice were sacrificed and the tumor tissues were removed for weighting and analyzing after 35 days. Also, tumor tissues were prepared into paraffin sections to carry out Ki67 immunohistochemistry (IHC) staining using SP Kit (Solarbio, Beijing, China) and TUNEL staining using Colorimetric TUNEL Apoptosis Assay Kit (Beyotime) basing on kit instructions. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Bromodomain-containing protein 4 (BRD4) | 60 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| CPI-0610 | Phase 3 | [2] | ||
| MOA | Modulator | |||
| Activity | IC50 = 39 nM | |||
| External Link | ||||
| PLX2853 | Phase 1/2 | [3] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| RVX-208 | Phase 1/2 | [2] | ||
| MOA | Modulator | |||
| External Link | ||||
| INCB57643 | Phase 1/2 | [3] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| OTX-015 | Phase 1/2 | [2] | ||
| Synonyms |
MK 8628
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | Ki = 5.7 nM | |||
| External Link | ||||
| TEN010 | Phase 1 | [2] | ||
| MOA | Modulator | |||
| External Link | ||||
| AZD5153 | Phase 1 | [3] | ||
| Synonyms |
RSMYFSPOTCDHHJ-GOSISDBHSA-N; AZD-5153; 1869912-39-9; UNII-C7C7U6YEAO; C7C7U6YEAO; SCHEMBL17477306; MolPort-044-561-768; AZD 5153 [WHO-DD]; AZD 5153; EX-A1317; BCP20057; ZINC575441177; AKOS030627661; SB18713; AS-35242; J3.544.368A; (3r)-4-[2-[4-[1-(3-methoxy-[1,2,4]triazolo[4,3-b]pyridazin-6-yl)-4-piperidyl]phenoxy]ethyl]-1,3-dimethyl-piperazin-2-one; XNH; 2-Piperazinone, 4-(2-(4-(1-(3-methoxy-1,2,4-triazolo(4,3-b)pyridazin-6-yl)-4-piperidinyl)phenoxy)ethyl)-1,3-dimethyl-, (3R)-; (R)-4-(2-(4-(1-(3-methoxy-[1,2,4]triazolo[4,3-b]pyridazin-6-yl)pipe
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 5 nM | |||
| External Link | ||||
| GSK525762 | Phase 1 | [2] | ||
| Synonyms |
GSK-525762
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | Ki = 23 nM | |||
| External Link | ||||
| ABBV-744 | Phase 1 | [3] | ||
| Synonyms |
OEDSFMUSNZDJFD-UHFFFAOYSA-N; 2138861-99-9; N-ethyl-4-(2-(4-fluoro-2,6-dimethylphenoxy)-5-(2-hydroxypropan-2-yl)phenyl)-6-methyl-7-oxo-6,7-dihydro-1H-pyrrolo[2,3-c]pyridine-2-carboxamide; ABBV 744; SCHEMBL19463409; EX-A2713; ACN-054460; HY-112090; CS-0043318
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 1.6 nM | |||
| External Link | ||||
| (+)-JQ1 | Phase 1 | [4] | ||
| Synonyms |
1268524-70-4; (+)-JQ-1; JQ1 compound; JQ-1; UNII-1MRH0IMX0W; (S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-triMethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate; (S)-JQ1; 1MRH0IMX0W; JQ1; CHEMBL1957266; AK109409; (S)-(+)-tert-Butyl 2-(4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate; (S)-(+)-tert-Butyl 2-(4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno(3,2-f)(1,2,4)triazolo(4,3-a)(1,4)diazepin-6-yl)acetate; (S)-tert-Butyl 2-(4-(4-chlorophenyl)-2,3
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 5.3 nM | |||
| External Link | ||||
| PMID26924192-Compound-23 | Patented | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Aminocyclopentenone compound 1 | Patented | [5] | ||
| Synonyms |
PMID26924192-Compound-93
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| Pyrazole and thiophene derivative 4 | Patented | [5] | ||
| Synonyms |
PMID26924192-Compound-92
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Benzothiazepine analog 12 | Patented | [5] | ||
| Synonyms |
PMID26924192-Compound-99
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 50 nM | |||
| External Link | ||||
| PMID26924192-Compound-20 | Patented | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID26924192-Compound-24 | Patented | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Aminocyclopentenone compound 2 | Patented | [5] | ||
| Synonyms |
PMID26924192-Compound-94
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| Pyrrolo-pyrrolone derivative 3 | Patented | [5] | ||
| Synonyms |
PMID26924192-Compound-36
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 11 nM | |||
| External Link | ||||
| Aminocyclopentenone compound 5 | Patented | [5] | ||
| Synonyms |
PMID26924192-Compound-97
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| Aminocyclopentenone compound 6 | Patented | [5] | ||
| Synonyms |
PMID26924192-Compound-98
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID26924192-Compound-22 | Patented | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID26924192-Compound-31 | Patented | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Pyrazole and thiophene derivative 2 | Patented | [5] | ||
| Synonyms |
PMID26924192-Compound-90
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID26924192-Compound-25 | Patented | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Pyrrolo-pyrrolone derivative 4 | Patented | [5] | ||
| Synonyms |
PMID26924192-Compound-37
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 11 nM | |||
| External Link | ||||
| Pyrazole and thiophene derivative 3 | Patented | [5] | ||
| Synonyms |
PMID26924192-Compound-91
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Aminocyclopentenone compound 3 | Patented | [5] | ||
| Synonyms |
PMID26924192-Compound-95
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| Aminocyclopentenone compound 4 | Patented | [5] | ||
| Synonyms |
PMID26924192-Compound-96
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| Pyrrolo-pyrrolone derivative 2 | Patented | [5] | ||
| Synonyms |
PMID26924192-Compound-35
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 11 nM | |||
| External Link | ||||
| Benzothiazepine analog 11 | Patented | [5] | ||
| Synonyms |
PMID26924192-Compound-101
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 50 nM | |||
| External Link | ||||
| Pyrazole and thiophene derivative 1 | Patented | [5] | ||
| Synonyms |
PMID26924192-Compound-89
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID26924192-Compound-21 | Patented | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Pyrrolo-pyrrolone derivative 5 | Patented | [5] | ||
| Synonyms |
PMID26924192-Compound-38
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 11 nM | |||
| External Link | ||||
| Benzothiazepine analog 10 | Patented | [5] | ||
| Synonyms |
PMID26924192-Compound-100
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 50 nM | |||
| External Link | ||||
| PMID26924192-Compound-30 | Patented | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID26924192-Compound-32 | Patented | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID26924192-Compound-33 | Patented | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Pyrrolo-pyrrolone derivative 1 | Patented | [5] | ||
| Synonyms |
PMID26924192-Compound-34
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 11 nM | |||
| External Link | ||||
| PMID26924192-Compound-104 | Patented | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID26924192-Compound-102 | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 41 nM | |||
| External Link | ||||
| PMID26924192-Compound-103 | Patented | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID26924192-Compound-105 | Patented | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| XD1 | Investigative | [6] | ||
| Synonyms |
colchicein; colchiceine; NSC33411
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID25703523C7d | Investigative | [7] | ||
| Synonyms |
43U; GTPL8304
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| isoxazole azepine compound 3 | Investigative | [8] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 26 nM | |||
| External Link | ||||
| PFI-1 | Investigative | [9] | ||
| Synonyms |
QCR-192; HY-16586; PF-6405761
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 220 nM | |||
| External Link | ||||
| MS436 | Investigative | [10] | ||
| MOA | Inhibitor | |||
| Activity | Ki < 85 nM | |||
| External Link | ||||
| CPI-203 | Investigative | [11] | ||
| Synonyms |
CPI203; CPI 203
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 92 nM | |||
| External Link | ||||
| BzT-7 | Investigative | [12] | ||
| Synonyms |
CHEMBL1958337; 8-Chloro-1,4-Dimethyl-6-Phenyl-4h-[1,2,4]triazolo[4,3-A][1,3,4]benzotriazepine; 3u5l; GTPL7515; SCHEMBL11468349; UYIVCPRWMLOCSB-UHFFFAOYSA-N; BDBM50365263; 8-chloro-1,4-dimethyl-6-phenyl-[1,2,4]triazolo[4,3-a][1,3,4]benzotriazepine; 8-chloro-1,4-dimethyl-6-phenyl-4H-s-triazolo(4,3-a)(1,3,4)benzotriazepine; 8-chloro-1,4-dimethyl-6-phenyl-4H-s-triazolo(4,3-a)(1,3,4 )benzotriazepine; 8-chloro-1,4-dimethyl-6-phenyl-4H-s-triazolo(4,3-a) (1,3,4)benzotriazepine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID23517011C9 | Investigative | [13] | ||
| Synonyms |
GTPL7517; BDBM50430836
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 371.54 nM | |||
| External Link | ||||
| MS417 | Investigative | [14] | ||
| Synonyms |
Methyl [(6s)-4-(4-Chlorophenyl)-2,3,9-Trimethyl-6h-Thieno[3,2-F][1,2,4]triazolo[4,3-A][1,4]diazepin-6-Yl]acetate; 916489-36-6; GGRCIHACOIMRKY-HNNXBMFYSA-N; 0S6; 4f3i; US9125915, compound 6; GTPL7512; CHEMBL3769755; SCHEMBL12228301; CHEBI:83406; BDBM179283; MS-417; ZINC95921068; HY-111139; CS-0034388; Methyl 2-((6S,Z)-4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate; methyl (S)-2-(4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 16.7 nM | |||
| External Link | ||||
| PMID25408830C3 | Investigative | [15] | ||
| Synonyms |
GSK-5959; 901245-65-6; GSK5959; GSK 5959; 4uye; GTPL7811; MolPort-004-921-361; HMS1821D17; EX-A2263; BCP16039; ZINC8594565; BDBM50032912; AKOS000481208; MCULE-6727694163; CS-4867; NCGC00107508-01; HY-18665; C301-5895; 9F9; N-[
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3162.28 nM | |||
| External Link | ||||
| PMID25408830C1 | Investigative | [15] | ||
| Synonyms |
BAS 08314227; 4uyd; AC1LLXEX; ChemDiv2_002770; MLS000100042; GTPL7809; MolPort-001-912-793; HMS2483G18; HMS1376N20; ZINC812404; BDBM50032913; AKOS001788033; MCULE-4972680634; SMR000081748; V1T
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 79432.82 nM | |||
| External Link | ||||
| PMID21851057C4d | Investigative | [16] | ||
| Synonyms |
GTPL7516; BDBM50353596
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4786.3 nM | |||
| External Link | ||||
| PMID25408830C2 | Investigative | [15] | ||
| Synonyms |
AC1LNW3E; MLS000663364; GTPL7810; MolPort-002-097-893; HMS2628P17; HMS3438L21; ZINC1063855; BDBM50032921; STL431755; AKOS000445117; MCULE-6603148725; CCG-129099; SMR000300929; SR-01000295962; SR-01000295962-1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2500 nM | |||
| External Link | ||||
| GW841819X | Investigative | [17] | ||
| Synonyms |
KB-75882
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 15.5 nM | |||
| External Link | ||||
| PMID24000170C36 | Investigative | [18] | ||
| Synonyms |
GTPL7521
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID24000170C38 | Investigative | [18] | ||
| Synonyms |
GTPL7522
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| I-BET151 | Investigative | [19] | ||
| Synonyms |
GSK1210151A
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 9 nM | |||
| External Link | ||||
| XD14 | Investigative | [6] | ||
| Synonyms |
1370888-71-3; XD 14; 4-acetyl-N-[5-(diethylsulfamoyl)-2-hydroxyphenyl]-3-ethyl-5-methyl-1H-pyrrole-2-carboxamide; CHEMBL3787231; 4-acetyl-N-(5-(N,N-diethylsulfamoyl)-2-hydroxyphenyl)-3-ethyl-5-methyl-1H-pyrrole-2-carboxamide; 4lyw; GTPL7524; SCHEMBL16181763; AOB6049; MolPort-010-365-022; XD-14; EX-A1864; ZINC59367920; BDBM50158909; AKOS025293503; NE41584; MCULE-3472066147; Z606699744
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2C25: Lung cancer | 52 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Adagrasib | Approved | [20] | ||
| Synonyms |
2326521-71-3; MRTX-849; UNII-8EOO6HQF8Y; 8EOO6HQF8Y; 2-((S)-4-(7-(8-Chloronaphthalen-1-yl)-2-(((S)-1-methylpyrrolidin-2-yl)methoxy)-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4-yl)-1-(2-fluoroacryloyl)piperazin-2-yl)acetonitrile; CHEMBL4594350; SCHEMBL20974691; GTPL10888; Kras G12C inhibitor MRTX849; BCP31538; EX-A3258; MRTX 849; MFCD32263433; s8884; compound 20 [PMID: 32250617]; BS-16211; HY-130149; CS-0105265; 2-Piperazineacetonitrile, 4-(7-(8-chloro-1-naphthalenyl)-5,6,7,8-tetrahydro-2-(((2S)-1-methyl-2-pyrrolidinyl)methoxy)pyrido(3,4-d)pyrimidin-4-yl)-1-(2-fluoro-1-oxo-2-propen-1-yl)-, (2S)-
Click to Show/Hide
|
|||
| External Link | ||||
| Amivantamab | Approved | [21] | ||
| External Link | ||||
| Mobocertinib | Approved | [22] | ||
| Synonyms |
1847461-43-1; TAK-788; TAK788; AP32788; UNII-39HBQ4A67L; 39HBQ4A67L; propan-2-yl 2-[4-{[2-(dimethylamino)ethyl](methyl)amino}-2-methoxy-5-(prop-2-enamido)anilino]-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Mobocertinib [INN]; Mobocertinib [USAN]; AP-32788; Mobocertinib (TAK788); Mobocertinib(TAK-788); SCHEMBL17373133; GTPL10468; BDBM368374; BCP31045; EX-A3392; US10227342, Example 10; MFCD32669806; NSC825519; s6813; TAK-788;AP32788; WHO 11183; NSC-825519; example 94 [WO2015195228A1]; HY-135815; CS-0114256; TAK-788;TAK 788; AP 32788; 5-Pyrimidinecarboxylic acid, 2-((4-((2-(dimethylamino)ethyl)methylamino)-2-methoxy-5-((1-oxo-2-propen-1-yl)amino)phenyl)amino)-4-(1-methyl-1H-indol-3-yl)-, 1-methylethyl ester; C(C=C)(=O)NC=1C(=CC(=C(C=1)NC1=NC=C(C(=N1)C1=CN(C2=CC=CC=C12)C)C(=O)OC(C)C)OC)N(C)CCN(C)C; Isopropyl 2-((5-acrylamido-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenyl)amino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Isopropyl 2-(5-acrylamido-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenylamino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Propan-2-yl 2-(5-(acryloylamino)-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyanilino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; propan-2-yl 2-[4-[2-(dimethylamino)ethyl-methylamino]-2-methoxy-5-(prop-2-enoylamino)anilino]-4-(1-methylindol-3-yl)pyrimidine-5-carboxylate
Click to Show/Hide
|
|||
| External Link | ||||
| Sugemalimab | Approved in China | [23] | ||
| External Link | ||||
| Sacituzumab govitecan | Approved | [24] | ||
| Synonyms |
1491917-83-9; 1535963-91-7; 1796566-95-4; CYSTEINYL CL2A-SN-38; DA64T2C2IO; DTXSID401335985; EX-A4354; F82944; GOVITECAN CYSTEINYL CONJUGATE; hRS 7SN38; hRS7-SN38; IMMU 132; IMMU-132; M9BYU8XDQ6; Sacituzumab govitecan; Sacituzumab govitecan [USAN]; sacituzumab-govitecan; Satralizumab linker; SN-38 CYSTEINYL CONJUGATE; UNII-M9BYU8XDQ6
Click to Show/Hide
|
|||
| External Link | ||||
| Atezolizumab | Approved | [25] | ||
| External Link | ||||
| Tepotinib | Approved | [26] | ||
| Synonyms |
1100598-32-0; EMD 1214063; UNII-1IJV77EI07; Tepotinib (EMD 1214063); EMD1214063; 1IJV77EI07; MSC-2156119J; Benzonitrile, 3-[1,6-dihydro-1-[[3-[5-[(1-methyl-4-piperidinyl)methoxy]-2-pyrimidinyl]phenyl]methyl]-6-oxo-3-pyridazinyl]-; C29H28N6O2; Tepotinib [INN]; Benzonitrile, 3-[1,6-Dihydro-1-[[3-[5-[(1-Methyl-4-Piperidinyl)Methoxy]-2-PyriMidinyl]Phenyl]Methyl]-6-Oxo-3-Pyridazinyl]; Benzonitrile, 3-(1,6-dihydro-1-((3-(5-((1-methyl-4-piperidinyl)methoxy)-2-pyrimidinyl)phenyl)methyl)-6-oxo
Click to Show/Hide
|
|||
| External Link | ||||
| Sotorasib | Approved | [27] | ||
| Synonyms |
AMG-510; AMG510; AMG-510 racemate; 2252403-56-6; AMG 510; Kras G12C inhibitor 9; 2296729-00-3; UNII-2B2VM6UC8G; 2B2VM6UC8G; CHEMBL4535757; 2296729-00-3 (racemate); 4-((S)-4-Acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(2-isopropyl-4-methylpyridin-3-yl)pyrido[2,3-d]pyrimidin-2(1H)-one; 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-[(2S)-2-methyl-4-prop-2-enoylpiperazin-1-yl]pyrido[2,3-d]pyrimidin-2-one; Sotorasib [INN]; 6-Fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-((2S)-2-methyl-4-prop-2-enoylpiperazin-1-yl)pyrido(2,3-d)pyrimidin-2-one; AMG510 racemate; Sotorasib [USAN]; AMG-510(racemate); Kras mutant-targeting AMG 510; SCHEMBL20560375; GTPL10678; AMG 510 pound>>AMG-510; AMY16918; BCP30452; BCP33368; EX-A3538; BDBM50514402; NSC818433; s8830; WHO 11370; DB15569; NSC-818433; BS-16684; HY-114277; CS-0081316; compound (R)-38 [PMID: 31820981]; (1m)-6-Fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-(propan-2-yl)pyridin-3-yl)-4-((2S)-2-methyl-4-(prop-2-enoyl)piperazin-1-yl)pyrido(2,3-d)pyrimidin-2(1H)-one; (1S)-4-((S)-4-Acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(2-isopropyl-4-methylpyridin-3-yl)pyrido[2,3-d]pyrimidin-2(1H)-one; 2296729-66-1; Pyrido(2,3-d)pyrimidin-2(1H)-one, 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-(1-methylethyl)-3-pyridinyl)-4-((2S)-2-methyl-4-(1-oxo-2-propen-1-yl)-1-piperazinyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| Entrectinib | Approved | [28] | ||
| Synonyms |
1108743-60-7; RXDX-101; UNII-L5ORF0AN1I; Entrectinib (RXDX-101); L5ORF0AN1I; Benzamide, N-[5-[(3,5-difluorophenyl)methyl]-1H-indazol-3-yl]-4-(4-methyl-1-piperazinyl)-2-[(tetrahydro-2H-pyran-4-yl)amino]-; Benzamide, N-(5-((3,5-difluorophenyl)methyl)-1H-indazol-3-yl)-4-(4-methyl-1-piperazinyl)-2-((tetrahydro-2H-pyran-4-yl)amino)-; Entrectinib [USAN:INN]; YMX; Kinome_2659; Entrectinib(rxdx-101); Entrectinib (USAN/INN); SCHEMBL3512601; GTPL8290; CHEMBL1983268; KS-00000TSK
Click to Show/Hide
|
|||
| External Link | ||||
| Repotrectinib | Approved | [29] | ||
| Synonyms |
FIKPXCOQUIZNHB-RRKGBCIJSA-N; SCHEMBL20438940; TPX 0005; BCP19778
Click to Show/Hide
|
|||
| External Link | ||||
| MYL-1402O | Phase 3 | [30] | ||
| Synonyms |
bevacizumab biosimilar
Click to Show/Hide
|
|||
| External Link | ||||
| AB154 | Phase 3 | [31] | ||
| Synonyms |
Domvanalimab
Click to Show/Hide
|
|||
| External Link | ||||
| Datopotamab deruxtecan | Phase 3 | [32] | ||
| External Link | ||||
| CS1001 | Phase 3 | [33] | ||
| External Link | ||||
| JDQ443 | Phase 3 | [34] | ||
| Synonyms |
(S)-JDQ-443; 1-(6-((4S)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl)-2-azaspiro[3.3]heptan-2-yl)prop-2-en-1-one; 1-[6-[(4R)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-inda zol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]- 2-Propen-1-one; 1-[6-[(4R)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]-2-propen-1-one; 1-[6-[4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methylindazol-5-yl)pyrazol-1-yl]-2-azaspiro[3.3]heptan-2-yl]prop-2-en-1-one; 1-{6-[(4M)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5- methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2- azaspiro[3.3]heptan-2-yl}prop-2-en-1-one; 1-{6-[(4M)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]heptan-2-yl}prop-2-en-1-one; 2653994-08-0; 2653994-10-4; 2-Propen-1-one, 1-[6-[(4R)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]-; AKOS040757949; AT36708; BDBM50579985; CHEMBL5077861; compound 5 [PMID: 35404998]; CS-0226220; CS-0311034; EX-A5693; example 1a [WO2021120890A1]; GLXC-25533; GTPL11715; HY-139612A; JDQ 443; JDQ 443 [WHO-DD]; JDQ443; JDQ-443; MS-29737; NSC846146; NSC-846146; NVP-JDQ443; NVP-JDQ-443; Opnurasib; opnurasib [INN]; -PROPEN-1-ONE, 1-(6-((4R)-4-(5-CHLORO-6-METHYL-1H-INDAZOL-4-YL)-5-METHYL-3-(1-METHYL-1H-INDAZOL-5-YL)-1H-PYRAZOL-1-YL)-2-AZASPIRO(3.3)HEPT-2-YL)-; Q3W0H3V1LQ; SCHEMBL23533580
Click to Show/Hide
|
|||
| External Link | ||||
| TRS003 | Phase 3 | [35] | ||
| External Link | ||||
| Tusamitamab ravtansine | Phase 3 | [36] | ||
| Synonyms |
SAR408701
Click to Show/Hide
|
|||
| External Link | ||||
| RG6058 | Phase 3 | [37] | ||
| Synonyms |
Tiragolumab
Click to Show/Hide
|
|||
| External Link | ||||
| GSK4069889 | Phase 2 | [38] | ||
| Synonyms |
TSR-022
Click to Show/Hide
|
|||
| External Link | ||||
| APL-101 | Phase 2 | [39] | ||
| Synonyms |
Bozitinib; PLB-1001; 1440964-89-5; Vebreltinib; Vebreltinib [USAN]; UNII-2WZP8A9VFN; 2WZP8A9VFN; Bozitinib (PLB-1001); SCHEMBL15594471; BDBM107096; CBI-3103; s6762; WHO 11677; HY-125017; CS-0088607; US9695175, 44; 1,2,4-Triazolo(4,3-b)pyridazine, 6-(1-cyclopropyl-1H-pyrazol-4-yl)-3-(difluoro(6- fluoro-2-methyl-2H-indazol-5-yl)methyl)-; 6-(1-Cyclopropyl-1H-pyrazol-4-yl)-3-(difluoro(6-fluoro-2-methyl-2H-indazol-5- yl)methyl)-1,2,4-triazolo(4,3-b)pyridazine
Click to Show/Hide
|
|||
| External Link | ||||
| SGN-LIV1A | Phase 2 | [40] | ||
| Synonyms |
Ladiratuzumab Vedotin
Click to Show/Hide
|
|||
| External Link | ||||
| BNT116 | Phase 2 | [41] | ||
| External Link | ||||
| AZD7789 | Phase 2 | [42] | ||
| External Link | ||||
| L-DOS47 | Phase 1/2 | [43] | ||
| External Link | ||||
| NC318 | Phase 2 | [44] | ||
| External Link | ||||
| Vorolanib | Phase 2 | [45] | ||
| Synonyms |
UNII-YP8G3I74EL; YP8G3I74EL; 1013920-15-4; (S,Z)-N-(1-(Dimethylcarbamoyl)pyrrolidin-3-yl)-5-((5-fluoro-2-oxoindolin-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide; Vorolanib [INN]; SCHEMBL2439528; CHEMBL4297587; N-((3S)-1-(dimethylcarbamoyl)pyrrolidin-3-yl)-5-((Z)-(5-fluoro-2-oxo-1H-indol-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide; s6843; DB15247; HY-109019; CS-0030517; Q27294638; 1H-Pyrrole-3-carboxamide, N-((3S)-1-((dimethylamino)carbonyl)-3-pyrrolidinyl)-5-((Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidene)methyl)-2,4-dimethyl-
Click to Show/Hide
|
|||
| External Link | ||||
| Xofigo | Phase 2 | [46] | ||
| External Link | ||||
| Bintrafusp alfa | Phase 2 | [47] | ||
| External Link | ||||
| Voyager-V1 | Phase 2 | [48] | ||
| Synonyms |
VSV-IFNBeta-NIS
Click to Show/Hide
|
|||
| External Link | ||||
| AB-106 | Phase 2 | [49] | ||
| Synonyms |
DS6051b; GTPL11198; AB106
Click to Show/Hide
|
|||
| External Link | ||||
| RO-5126766 | Phase 2 | [50] | ||
| Synonyms |
VS-6766; CH-5126766; Dual Raf/MEK protein kinase inhibitor (cancer), Roche
Click to Show/Hide
|
|||
| External Link | ||||
| TC-210 | Phase 1/2 | [51] | ||
| External Link | ||||
| EMB-01 | Phase 1/2 | [52] | ||
| External Link | ||||
| NM21-1480 | Phase 1/2 | [53] | ||
| External Link | ||||
| MRx0518 | Phase 1/2 | [54] | ||
| External Link | ||||
| DZD9008 | Phase 1/2 | [55] | ||
| External Link | ||||
| BGB-A425 | Phase 1/2 | [56] | ||
| External Link | ||||
| Rilvegostomig | Phase 1/2 | [57] | ||
| Synonyms |
AZD2936
Click to Show/Hide
|
|||
| External Link | ||||
| IK-007 | Phase 1/2 | [58] | ||
| Synonyms |
grapiprant
Click to Show/Hide
|
|||
| External Link | ||||
| IBI318 | Phase 1 | [59] | ||
| External Link | ||||
| GEN-011 | Phase 1 | [60] | ||
| External Link | ||||
| ENV-105 | Phase 1 | [61] | ||
| Synonyms |
Carotuximab
Click to Show/Hide
|
|||
| External Link | ||||
| AMG 160 | Phase 1 | [62] | ||
| External Link | ||||
| ADP-A2M10 | Phase 1 | [63] | ||
| External Link | ||||
| MEDI5752 | Phase 1 | [64] | ||
| External Link | ||||
| PF-07104091 | Phase 1 | [65] | ||
| External Link | ||||
| PF-06936308 | Phase 1 | [66] | ||
| External Link | ||||
| GEM3PSCA | Phase 1 | [67] | ||
| External Link | ||||
| PF-07257876 | Phase 1 | [68] | ||
| External Link | ||||
| Cosibelimab | Phase 1 | [69] | ||
| Synonyms |
CK-301/TG-1501
Click to Show/Hide
|
|||
| External Link | ||||
| Gemcitabine | Approved | [70] | ||
| External Link | ||||
| SMI-4a | Investigative | [71] | ||
| Synonyms |
438190-29-5; SMI 4a; TCS PIM-1 4a; (Z)-SMI-4a; (Z)-5-(3-(trifluoromethyl)benzylidene)thiazolidine-2,4-dione; (5Z)-5-[3-(trifluoromethyl)benzylidene]-1,3-thiazolidine-2,4-dione; CHEMBL183906; (5Z)-5-[[3-(TRIFLUOROMETHYL)PHENYL]METHYLENE]-2,4-THIAZOLIDINEDIONE; (5Z)-5-[[3-(trifluoromethyl)phenyl]methylidene]-1,3-thiazolidine-2,4-dione; 327033-36-3; C11H6F3NO2S; (Z)-5-(3-(Trifluoromethyl)benzylidene)-thiazolidine-2,4-dione; (5Z)-5-{[3-(trifluoromethyl)phenyl]methylidene}-1,3-thiazolidine-2,4-dione; Pim inhibitor 4a; 3vc4; SMI-4q; TCS PIM-1-4a; 5-(3-(Trifluoromethyl)benzylidene)thiazolidine-2,4-dione; 5-[3-(Trifluoromethyl)benzylidene]thiazolidine-2,4-dione; cc-717; thiazolidine-2,4-dione, 4a; SCHEMBL2541382; SCHEMBL2541388; BDBM26626; AOB6260; EX-A111; SYN1113; BDBM138364; HMS3229J21; 2720AH; HY-16576A; MFCD01152003; s8005; ZINC12576047; AKOS001314163; SMI-4a, >=98% (HPLC); CCG-265027; NCGC00345836-02; NCGC00345836-14; AC-32861; HY-15474; AB0165836; EC-000.2291; J3.561.866J; A11945; W-5256; US8877795, 12; Q27451064; 5-[[3-(trifluoromethyl)phenyl]methylene]-2,4-thiazolidinedione
Click to Show/Hide
|
|||
| External Link | ||||
References