m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05566
|
[1] | |||
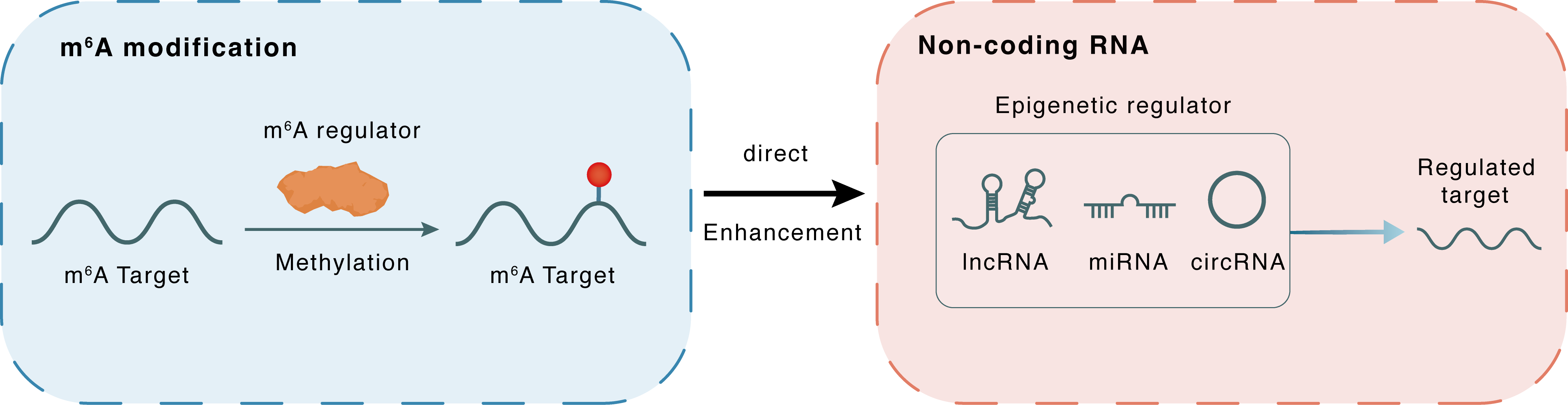 m6A modification
hsa_circ_0000677
hsa_circ_0000677
METTL3
Methylation
m6A modification
hsa_circ_0000677
hsa_circ_0000677
METTL3
Methylation
 : m6A sites
Direct
Enhancement
Non-coding RNA
circ_0000677
ABCC1
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
circ_0000677
ABCC1
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | hsa_circ_0000677 | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | hsa_circ_0000677 (Circ_ABCC1) | circRNA | View Details | ||
| Regulated Target | ATP binding cassette subfamily C member 1 (ABCC1 blood group) (ABCC1) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | hsa_circ_0000677 and its downstream target ATP binding cassette subfamily C member 1 (ABCC1 blood group) (ABCC1) were upregulated in CRC cells, induced by the METTL3-mediated m6 A modification of circ_0000677 and SUMO1-mediated SUMOylation of METTL3. This work provided a new strategy for the therapeutic treatment of CRC. | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Cell Process | Cell proliferation | ||||
In-vitro Model |
NCM460 | Normal | Homo sapiens | CVCL_0460 | |
| HCT 116 | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
| HCT 15 | Colon adenocarcinoma | Homo sapiens | CVCL_0292 | ||
| LoVo | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | ||
| SW480 | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| Caco-2 | Colon adenocarcinoma | Homo sapiens | CVCL_0025 | ||
| In-vivo Model | For the tumor xenograft, mice were randomly divided into three groups with four mice for each group. Then, 1 × 106 cells after indicated treatment were harvested and resuspended in 50 ul of PBS. Then the cells were subcutaneously injected into the right front flank of each mouse. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| ATP binding cassette subfamily C member 1 (ABCC1 blood group) (ABCC1) | 7 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Sulfinpyrazone | Approved | [2] | ||
| Synonyms |
Anturan; Anturane; Anturanil; Anturano; Anturen; Anturidin; Diphenylpyrazone; Enturan; Enturen; Novopyrazone; Sulfinpirazona; Sulfinpyrazine; Sulfinpyrazon; Sulfinpyrazonum; Sulfoxyphenylpyrazolidin; Sulfoxyphenylpyrazolidine; Sulphinpyrazone; Apo Sulfinpyrazone; Apotex Brand of Sulfinpyrazone; Novartis Brand of Sulfinpyrazone; Nu Pharm Brand of Sulfinpyrazone; Nu Sulfinpyrazone; G 28315; Anturane (TN); Apo-Sulfinpyrazone; G 28,315; Nu-Pharm Brand of Sulfinpyrazone; Nu-Sulfinpyrazone; Sulfinpirazona [INN-Spanish]; Sulfinpyrazone (SPZ); Sulfinpyrazonum [INN-Latin]; USAF GE-13; Sulfinpyrazone [USAN:INN:JAN]; Sulfinpyrazone (JP15/USP/INN); (+/-)-SULFINPYRAZONE; 1,2-Diphenyl-3,5-dioxo-4-(2'-phenyl-sulfinyl-aethyl)-pyrazolidin; 1,2-Diphenyl-3,5-dioxo-4-(2'-phenyl-sulfinyl-aethyl)-pyrazolidin [German]; 1,2-Diphenyl-3,5-dioxo-4-(2-phenylsulfinylethyl) pyrazolidine; 1,2-Diphenyl-3,5-dioxo-4-(2-phenylsulfinylethyl)pyrazolidine; 1,2-Diphenyl-4-(2'-phenylsulfinethyl)-3,5-pyrazolidinedione; 1,2-Diphenyl-4-(2-(phenylsulfinyl)ethyl)-3,5-pyrazolidinedione; 1,2-Diphenyl-4-(phenylsulfinylethyl)-3,5-pyrazolidinedione; 1,2-Diphenyl-4-[2-(phenylsulfinyl)ethyl-3,5-pyrazolidinedione; 1,2-diphenyl-4-[2-(phenylsulfinyl)ethyl]pyrazolidine-3,5-dione; 4-(2-Benzenesulfinylethyl)-1,2-diphenylpyrazolidine-3,5-dione; 4-(Phenylsulfoxyethyl)-1,2-diphenyl-3,5-pyrazolidinedione; 4-[2-(benzenesulfinyl)ethyl]-1,2-diphenylpyrazolidine-3,5-dione
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Benzbromarone | Approved | [3] | ||
| Synonyms |
Acifugan; Azubromaron; Benzbromaron; Benzbromaronratiopharm; Benzbromaronum; Benzobromarona; Besuric; Desuric; Exurate; Harolan; Hipurik; Minuric; Narcaricin; Normurat; Uricovac; Urinorm; Uroleap; Aliud Brand of Benzbromarone; Benzbromaron AL; Benzbromaron ratiopharm; Benzbromarone Aliud Brand; Benzbromarone Heumann Brand; Benzbromarone Sanfer Brand; Benzbromarone ratiopharm Brand; Heumann Brand of Benzbromarone; Ratiopharm Brand of Benzbromarone; Sanfer Brand of Benzbromarone; Sanofi Winthrop Brand of Benzbromarone; L 2214; L2214; MJ 10061; NCI85433; AL, Benzbromaron; Benzbromaron-ratiopharm; Benzbromaronum [INN-Latin]; Benzobromarona [INN-Spanish]; L 2214-Labaz; L-2214; Uroleap (TN); Benzbromarone [USAN:INN:BAN]; Methanone, (3; Benzbromarone (JP15/USAN/INN); KETONE, 3,5-DIBROMO-4-HYDROXYPHENYL 2-ETHYL-3-BENZOFURANYL; (2-Ethyl-3-benzofuranyl)-(3,5-dibrom-4-hydroxyphenyl)keton; (3,5-Dibromo-4-hydroxyphenyl)(2-ethyl-1-benzofuran-3-yl)methanone; (3,5-dibromo-4-hydroxyphenyl)-(2-ethyl-1-benzofuran-3-yl)methanone; 2-Ethyl-3-(3,5-dibrom-4-hydroxybenzoyl)benzofuran; 3, 5-Dibromo-4-hydroxyphenyl-2-ethyl-3-benzofuranyl ketone; 3,5-Dibromo-4-hydroxyphenyl-2-ethyl-3-benzofuranyl ketone; 3-(3,5-Dibromo-4-hydroxybenzoyl)-2-ethylbenzofuran; 3-[3,5-DIBROMO-4-HYDROXYBENZOYL]-2-ETHYLBENZOFURAN
Click to Show/Hide
|
|||
| MOA | Stimulator | |||
| Activity | Ki = 190 nM | |||
| External Link | ||||
| Pyrrolopyrimidine | Investigative | [4] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| XR-9456 | Investigative | [5] | ||
| Synonyms |
CHEMBL346292; SCHEMBL7170174; BDBM50375810
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| XR-9504 | Investigative | [5] | ||
| Synonyms |
CHEMBL258896; SCHEMBL7168501
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| XR-9544 | Investigative | [5] | ||
| Synonyms |
CHEMBL444075; SCHEMBL14290581; BDBM50375811
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| XR-9577 | Investigative | [5] | ||
| Synonyms |
CHEMBL428402
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2B91: Colorectal cancer | 25 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Retifanlimab | Approved | [6] | ||
| Synonyms |
INCMGA0012; Retifanlimab
Click to Show/Hide
|
|||
| External Link | ||||
| Aflibercept | Approved | [7] | ||
| Synonyms |
Ziv-Aflibercept; Zaltrap (TN); VEGF Trap; VEGF Trap-Eye
Click to Show/Hide
|
|||
| External Link | ||||
| Regorafenib | Approved | [8] | ||
| Synonyms |
755037-03-7; BAY 73-4506; Regorafenibum; Stivarga; 4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide; BAY73-4506; Regorafenib (BAY 73-4506); UNII-24T2A1DOYB; 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide; BAY-73-4506; 24T2A1DOYB; CHEMBL1946170; CHEBI:68647; Stivarga (TN); BAY73-4506 hydrochloride; Regorafenib [USAN:INN]
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [9] | ||
| Synonyms |
Bevacizumab (ophthalmic slow-release tissue tablet)
Click to Show/Hide
|
|||
| External Link | ||||
| SYM-004 | Phase 3 | [9] | ||
| Synonyms |
Chimeric IgG1 antibody 1024 (cancer), Symphogen; Chimeric IgG1 antibody 992 (cancer), Symphogen; Chimeric IgG1 antibodies992 + 1024 (cancer), Symphogen
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab + Erlotinib | Phase 3 | [10] | ||
| External Link | ||||
| CPI-613 | Phase 3 | [9] | ||
| Synonyms |
95809-78-2; 6,8-bis(benzylthio)octanoic acid; CPI 613; MLS006010202; SCHEMBL1062218; 6,8-Bis(benzylsulfanyl)octanoic acid; Octanoic acid, 6,8-bis((phenylmethyl)thio)-; Octanoic acid, 6,8-bis[(phenylMethyl)thio]-; 6,8-Bis[(phenylmethyl)thio]octanoic acid; CPI613; CHEMBL3186849; QCR-193; AOB1058; MolPort-023-219-128; HMS3656L06; C22H28O2S2; BCP04663; EX-A2043; s2776; AKOS025142095; BCP9000552; DB12109; RL06062; CS-0961; NCGC00344764-01; SMR004701300; AS-16613; BC261916; AK174899; HY-15453; BCP0726000030; KB-293127; AB0035874
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [7] | ||
| External Link | ||||
| AlloStim | Phase 2/3 | [11] | ||
| Synonyms |
AlloStim (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Sibrotuzumab | Phase 2 | [12] | ||
| External Link | ||||
| CV301 | Phase 2 | [13] | ||
| External Link | ||||
| Efatutazone | Phase 2 | [14] | ||
| Synonyms |
Inolitazone; 223132-37-4; 5-[[4-[[6-(4-amino-3,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-yl]methoxy]phenyl]methyl]-2,4-Thiazolidinedione; Efatutazone [INN]; RS5444; CS-7017; SCHEMBL3246054; CHEMBL3545280; JCYNMRJCUYVDBC-UHFFFAOYSA-N; Efatutazone;CS-7017;RS5444; BCP07478; AKOS030526729; DB11894; CS-0778; KB-77905; DA-07988; HY-14792; QC-10456; 4CA-1384; FT-0737589; 5-[4-[6-(4-amino-3 ,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-ylmethoxy]benzyl]thiazolidine-2,4-dione
Click to Show/Hide
|
|||
| External Link | ||||
| LOR-2040 | Phase 2 | [15] | ||
| External Link | ||||
| RG7221 | Phase 2 | [16] | ||
| External Link | ||||
| PEG-SN38 | Phase 2 | [17] | ||
| Synonyms |
EZN-2208
Click to Show/Hide
|
|||
| External Link | ||||
| MEGF0444A | Phase 2 | [18] | ||
| External Link | ||||
| Encapsulated cell therapy | Phase 1/2 | [19] | ||
| External Link | ||||
| AB928 | Phase 1/2 | [20] | ||
| External Link | ||||
| MGD007 | Phase 1 | [16] | ||
| External Link | ||||
| BNC-101 | Phase 1 | [21] | ||
| External Link | ||||
| Navicixizumab | Phase 1 | [9] | ||
| External Link | ||||
| RG7160 | Discontinued in Phase 2 | [22] | ||
| External Link | ||||
| Nimesulide | Terminated | [23] | ||
| Synonyms |
51803-78-2; N-(4-Nitro-2-phenoxyphenyl)methanesulfonamide; Mesulid; Flogovital; Sulidene; Nimed; R-805; 4-NITRO-2-PHENOXYMETHANESULFONANILIDE; Nisulid; Nimesulidum [INN-Latin]; Nimesulida [INN-Spanish]; R 805; UNII-V4TKW1454M; 4-Nitro-2-phenoxy-methanesulfonanilide; 4'-Nitro-2'-phenoxymethanesulfonanilide; Methanesulfonamide, N-(4-nitro-2-phenoxyphenyl)-; EINECS 257-431-4; 4'-Nitro-2'-phenoxymethansulfonanilid; BRN 2421175; CHEMBL56367; MLS000069680; V4TKW1454M; Methanesulfonanilide, 4'-nitro-2'-phenoxy-; CHEBI:44445; Dulanermin
Click to Show/Hide
|
|||
| External Link | ||||
| Saracatinib | Phase 2 | [24] | ||
| External Link | ||||
| G3139 + Irinotecan | Investigative | [25] | ||
| External Link | ||||
References