m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05414
|
[1] | |||
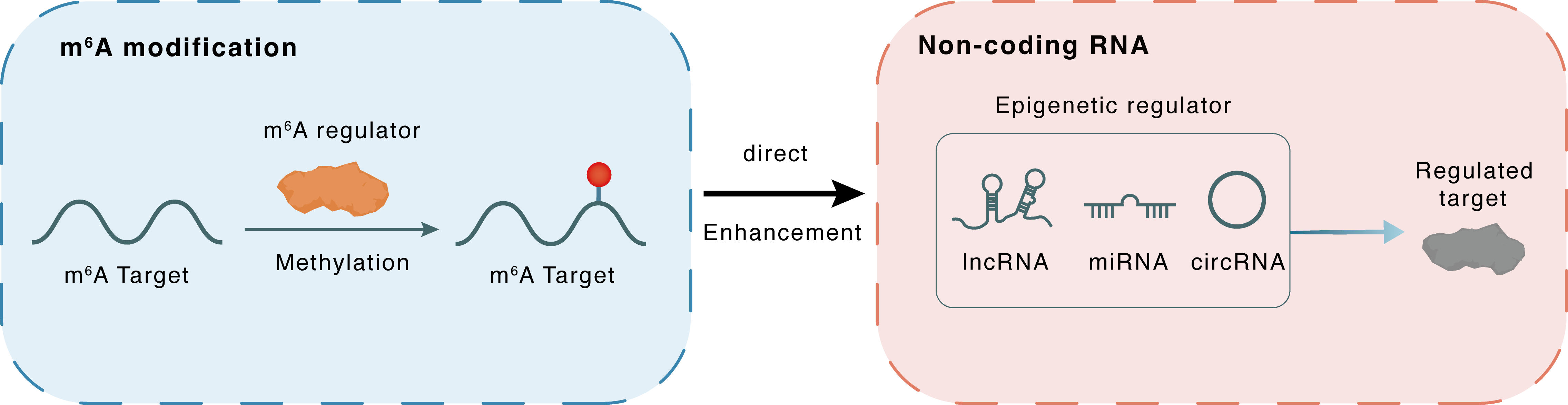 m6A modification
CDR1as
CDR1as
IGF2BP3
m6A modification
CDR1as
CDR1as
IGF2BP3
 : m6A sites
Direct
Enhancement
Non-coding RNA
CDR1as
IGF2BP3
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
CDR1as
IGF2BP3
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3) | READER | |||
| m6A Target | long intergenic non-protein coding RNA 632 (LINC00632) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | Long intergenic non-protein coding RNA 632 (LINC00632) | LncRNA | View Details | ||
| Regulated Target | Insulin like growth factor 2 mRNA binding protein 3 (IGF2BP3) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | CDR1 antisense RNA (CDR1-AS) a regulator of miR-7, as a hallmark of melanoma progression. CDR1as depletion results from epigenetic silencing of LINC00632, its originating long non-coding RNA (lncRNA) and promotes invasion in vitro and metastasis in vivo through a miR-7-independent, IGF2BP3-mediated mechanism. IGF2BP3 interacts with CDR1as and mediates invasion induced by CDR1as depletion. CDR1asHigh melanoma cell lines were strikingly more sensitive to three different GPX4 inhibitors, which are known to elicit ferroptotic cell death. | ||||
| Responsed Disease | Esophageal cancer | ICD-11: 2B70 | |||
| Responsed Drug | Cisplatin | ||||
| Pathway Response | Apoptosis | hsa04210 | |||
| Cell Process | Cellular Processes | ||||
| Cell growth and death | |||||
| Cell apoptosis | |||||
In-vitro Model |
KYSE-150 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1348 | |
| Eca-9706 (Esophageal carcinoma cell line) | |||||
| In-vivo Model | Used 1 × 106 SNHG3 knocked down KY-SE150 cells and NC lentivirus to inject into the right flank of mice to generate xenografts. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| 2B70: Esophageal cancer | 15 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Pembrolizumab | Approved | [2] | ||
| External Link | ||||
| Nivolumab | Approved | [2] | ||
| External Link | ||||
| Claudiximab | Phase 3 | [3] | ||
| Synonyms |
IMAB-362; Anti-GC182 mAbs (cancer), Ganymed; Anti-GC182 monoclonal antibodies (cancer), Ganymed; Anti-CLDN18-2 mAbs (cancer), Ganymed; Anti-CLDN18-2 monoclonal antibodies (cancer), Ganymed
Click to Show/Hide
|
|||
| External Link | ||||
| Golnerminogene pradenovac | Phase 3 | [4] | ||
| Synonyms |
TNFerade (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| DKN-01 | Phase 2 | [5] | ||
| External Link | ||||
| Pegamotecan | Phase 2 | [6] | ||
| Synonyms |
Prothecan; EZ-246; PEG-camptothecin; PEG-camptothecin, Enzon; Polyethylene glycol-camptothecin, Enzon
Click to Show/Hide
|
|||
| External Link | ||||
| APR-246 | Phase 2 | [2] | ||
| Synonyms |
Eprenetapopt
Click to Show/Hide
|
|||
| External Link | ||||
| Opdivo + Yervoy | Phase 3 | [2] | ||
| External Link | ||||
| Anti-NY-ESO-1 CAR-T cells | Phase 1/2 | [7] | ||
| External Link | ||||
| Anti-MUC1 CAR-T cells | Phase 1/2 | [8] | ||
| External Link | ||||
| CAR-T Cells targeting EpCAM | Phase 1/2 | [9] | ||
| External Link | ||||
| PCA062 | Phase 1 | [2] | ||
| External Link | ||||
| Cellspan esophageal implant | Clinical trial | [2] | ||
| External Link | ||||
| PKI166 | Discontinued in Phase 2 | [10] | ||
| Synonyms |
PKI-166; CGP-75166; 187724-61-4; NVP-PKI166; CHEMBL1914653; AC1OCFE0; UNII-9RIE5HW38P; 9RIE5HW38P; SCHEMBL177814; GTPL7642; CHEMBL1963502; ZINC23255; AOB1619; PKI-75166; BDBM50358046; NCGC00387215-02; AS-16676; KB-275097; PKI-166, > 4-[4-[[(1R)-1-phenylethyl]amino]-7H-pyrrolo[4,5-e]pyrimidin-6-yl]phenol
Click to Show/Hide
|
|||
| External Link | ||||
| Ramorelix | Discontinued in Phase 1 | [11] | ||
| Synonyms |
Hoe-013
Click to Show/Hide
|
|||
| External Link | ||||
References