m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05373
|
[1] | |||
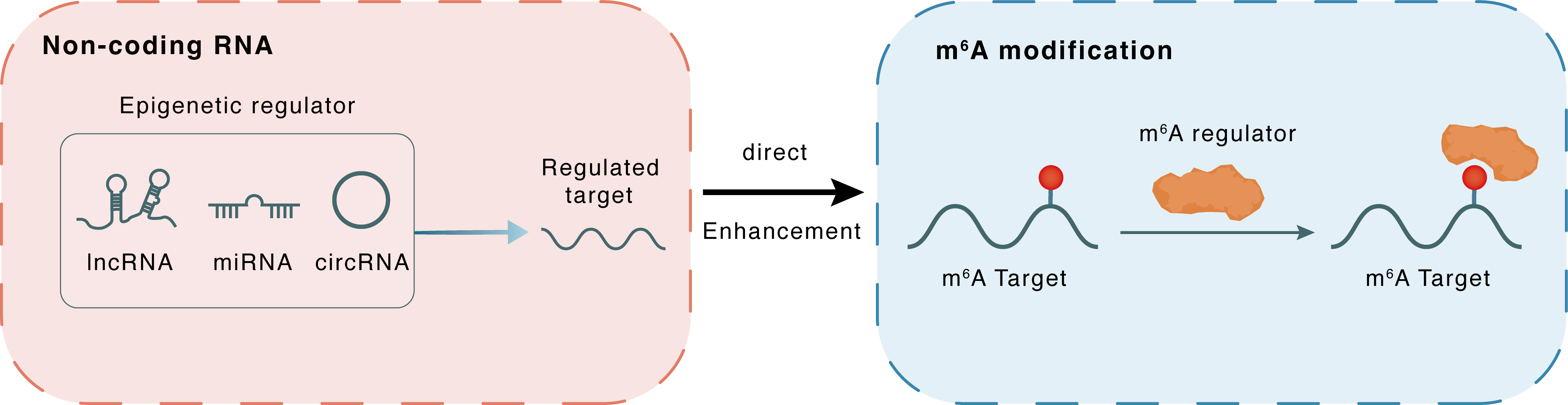 Non-coding RNA
MIAT
YTHDF2
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
CPT1A
CPT1A
Ythdf2
Non-coding RNA
MIAT
YTHDF2
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
CPT1A
CPT1A
Ythdf2
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | YTH domain-containing family protein 2 (YTHDF2) | READER | |||
| m6A Target | Carnitine O-palmitoyltransferase 1, liver isoform (CPT1A) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | Myocardial infarction associated transcript (MIAT) | LncRNA | View Details | ||
| Regulated Target | YTH domain-containing family protein 2 (YTHDF2) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Enhancement | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | YTHDF2 function was a downstream of MIAT in cardiac hypertrophy. Finally, we found that MIAT was a necessary regulator of cardiac hypertrophy due to its regulation of the Ythdf2/PPARalpha/Carnitine O-palmitoyltransferase 1, liver isoform (CPT1A) axis. This study indicated a new hypertrophic signaling pathway: MIAT/Ythdf2/PPARalpha/CPT-1a. The results provided a new understanding of the MIAT and m6A RNA methylation reading protein, Ythdf2, function and mechanism in cardiac hypertrophy and highlighted the potential therapeutic benefits in the heart. | ||||
| Responsed Disease | Heart failure | ICD-11: BD1Z | |||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| BD1Z: Heart failure | 25 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Digitoxin | Approved | [2] | ||
| Synonyms |
Acedoxin; Asthenthilo; Cardidigin; Cardigin; Cardiolanata; Carditalin; Carditoxin; Coramedan; Cristapurat; Crystodigin; Digicor; Digilanid; Digilong; Digimed; Digimerck; Digipural; Digisidin; Digitoksim; Digitoksin; Digitophyllin; Digitossina; Digitoxina; Digitoxine; Digitoxinum; Digitoxoside; Digitoxosidum; Digitrin; Ditaven; Glucodigin; Lanatoxin; Lanostabil; Monodigitoxoside; Myodigin; Natigal; Pandigal; Panlanat; Purodigin; Purpurid; Tardigal; Unidigin; Crystalline digitalin; Digitaline cristallisee; Digitaline nativelle; Digitalinum verum; Digitossina [DCIT]; Digitoxigenin tridigitoxoside; LT00244784; Crystodigin (TN); De-Tone; Digitalin, crystalline; Digitaline (TN); Digitoxigenin-tridigitoxosid; Digitoxigenin-tridigitoxosid [German]; Digitoxina [INN-Spanish]; Digitoxine [INN-French]; Digitoxinum [INN-Latin]; Mono-digitoxid; Mono-digitoxid [German]; Mono-glycocard; Purodigin, crystalline; Tri-digitoxoside; Tri-digitoxoside [German]; Digitoxin [INN:BAN:JAN]; Digitoxin (JP15/USP/INN); Inhibits Na+/K+ ATPase; 5.beta.-Card-20(22)-enolide, 3.beta.,14-dihydroxy-, 3-[tris-(digitoxoside)]
Click to Show/Hide
|
|||
| External Link | ||||
| Celacade | Approved | [3] | ||
| Synonyms |
Celacade (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Deslanoside | Approved | [4] | ||
| Synonyms |
Ceglunat; Desace; Deslanosido; Deslanosidum; Glucodigoxin; Lekozid; Sediranido; Deacetyllanatoside C; DesacetylLanatoside; Desacetyldigilanide C; Desacetyllanatoside C; Deslanatoside C; Deslanosidum C; Lanatosid C; Cedilanid-D; Desacetyl-Lanatoside C; Deslanosido [INN-Spanish]; Deslanosidum [INN-Latin]; Cedilanid-d (TN); Deslanoside (JP15/USP/INN); Deslanoside [USAN:BAN:INN:JAN]; (3beta,5beta,12beta)-3-{[beta-D-glucopyranosyl-(1->4)-2,6-dideoxy-beta-D-ribo-hexopyranosyl-(1->4)-2,6-dideoxy-beta-D-ribo-hexopyranosyl-(1->4)-2,6-dideoxy-beta-D-ribo-hexopyranosyl]oxy}-12,14-dihydroxycard-20(22)-enolide; 3-[(O-beta-D-glucopyranosyl-(1->4)-O-2,6-dideoxy-beta-D-ribo-hexopyranosyl-(1->4)-O-2,6-dideoxy-beta-D-ribo-hexopyranosyl-(1->4)-O-2,6-dideoxy-beta-D-ribo-hexopyranosyl)oxy]-12,14-dihydroxy-3beta,5beta,12beta-card-20(22)-enolide; 3beta-(O-beta-D-Glucopyranosyl-(1-4)-O-beta-D-digitoxosyl-(1-4)-O-beta-D-digitoxosyl-(1-4)-beta-D-digitoxosyloxy-12beta,14-dihydroxy-5beta,14beta-card-20(22)-enolid; 3beta-(O-beta-D-Glucopyranosyl-(1-4)-O-beta-D-digitoxosyl-(1-4)-O-beta-D-digitoxosyl-(1-4)-beta-D-digitoxosyloxy=12beta.14=dihydroxy-5beta,14beta-card-20(22)-enolid; 3beta-{[beta-D-glucopyranosyl-(1->4)-2,6-dideoxy-beta-D-ribo-hexopyranosyl-(1->4)-2,6-dideoxy-beta-D-ribo-hexopyranosyl-(1->4)-2,6-dideoxy-beta-D-ribo-hexopyranosyl]oxy}-12beta,14-dihydroxy-5beta-card-20(22)-enolide
Click to Show/Hide
|
|||
| External Link | ||||
| Digoxin | Approved | [5] | ||
| Synonyms |
20830-75-5; 12beta-Hydroxydigitoxin; Digoxine; Lanoxin; Lanoxicaps; Digossina; Digoxina; Digoxinum; Digosin; Lanicor; Digacin; Dilanacin; CHEBI:4551; MLS000069819; Lanacordin; Cardiogoxin; Eudigox; Davoxin; SMR000059217; Rougoxin; Mapluxin; Lenoxin; Lanacrist; Dynamos; Vanoxin; Neo-Lanicor; Lanoxin PG; Digoxin Pediatric; Digoxin Nativelle; SK-Digoxin; UNII-73K4184T59; Homolle's digitalin; Hemigoxine Nativelle; MFCD00003674; Digitek (TN); Lanoxicaps (TN); Lanoxin (TN); Digoxin (JP15/USP); (3beta,5beta,12beta)-3-{[2,6-dideoxy-beta-D-ribo-hexopyranosyl-(1->4)-2,6-dideoxy-beta-D-ribo-hexopyranosyl-(1->4)-2,6-dideoxy-beta-D-ribo-hexopyranosyl]oxy}-12,14-dihydroxycard-20(22)-enolide; 4-[(1S,2S,5S,7R,10R,11S,14R,15S,16R)-5-{[(2R,4S,5S,6R)-5-{[(2S,4S,5S,6R)-5-{[(2S,4S,5S,6R)-4,5-dihydroxy-6-methyloxan-2-yl]oxy}-4-hydroxy-6-methyloxan-2-yl]oxy}-4-hydroxy-6-methyloxan-2-yl]oxy}-11,16-dihydroxy-2,15-dimethyltetracyclo[8700^{2,7}; [3H]digoxin
Click to Show/Hide
|
|||
| External Link | ||||
| Recombinant human neuregulin-1 beta | Phase 3 | [6] | ||
| External Link | ||||
| Satavaptan | Phase 3 | [7] | ||
| External Link | ||||
| Neucardin | Phase 2 | [8] | ||
| Synonyms |
Human neuregulin-1 recombinant peptide fragment (injectable, heart failure/cardiomyopathy), Zensun; RhNRG-1 (injectable, heartfailure/cardiomyopathy), Zensun
Click to Show/Hide
|
|||
| External Link | ||||
| Subcutaneous furosemide | Application submitted | [8] | ||
| External Link | ||||
| C-Cure | Phase 2/3 | [9] | ||
| Synonyms |
Autologous stem cell/cardiopoietic cell therapy (C-Cath, chronic heart failure), Mayo/Cardio3
Click to Show/Hide
|
|||
| External Link | ||||
| RT-400 | Phase 2 | [8] | ||
| Synonyms |
JNJ-39588146
Click to Show/Hide
|
|||
| External Link | ||||
| Cinaciguat | Phase 2 | [10] | ||
| Synonyms |
329773-35-5; BAY 58-2667; BAY582667; UNII-59K0Y58UAD; BAY-58-2667; 59K0Y58UAD; 4-({(4-carboxybutyl)[2-(2-{[4-(2-phenylethyl)benzyl]oxy}phenyl)ethyl]amino}methyl)benzoic acid; Cinaciguat [INN:JAN]; cinaciguatum; Z90; Cinaciguat (JAN/INN); SCHEMBL249267; GTPL5168; CHEMBL1236936; QCR-279; Cinaciguat (BAY 58-2667); MolPort-039-139-611; CHEBI:142433; ZINC3934935; BCP07942; 3550AH; AKOS026750293; AN-1845; CS-1169; HY-14181; KB-40005; AB0095741; D07577; W-5794
Click to Show/Hide
|
|||
| External Link | ||||
| Neladenoson bialanate | Phase 2 | [8] | ||
| Synonyms |
UNII-IV690462VZ; IV690462VZ; 1239309-58-0; Neladenoson dalanate; Neladenoson dalanate [INN]; Neladenoson bialanate [INN]; SCHEMBL7904070; AKOS032946515; DB13138; L-Alanine, L-alanyl-, 2-(4-(2-(((2-(4-chlorophenyl)-4-thiazolyl)methyl)thio)-3,5-dicyano-6-(1-pyrrolidinyl)-4-pyridinyl)phenoxy)ethyl ester; 2-(4-(2-(((2-(4-Chlorophenyl)-1,3-thiazol-4-yl)methyl)sulfanyl)-3,5-dicyano-6-(pyrrolidin-1-yl)pyridin-4-yl)phenoxy)ethyl L-alanyl-L-alaninate
Click to Show/Hide
|
|||
| External Link | ||||
| CLR325 | Phase 2 | [8] | ||
| External Link | ||||
| Mesenchymal stem cell therapy | Phase 2 | [8] | ||
| External Link | ||||
| CLP-1001 | Phase 2 | [11] | ||
| Synonyms |
Cross-linked polyelectrolyte polymer (renal disease), Sorbent Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-986231 | Phase 2 | [12] | ||
| Synonyms |
N-Hydroxy-5-methylfuran-2-sulfonamide; Cimlanod; Cimlanod [USAN]; UNII-2US4FK1EPV; 2US4FK1EPV; SCHEMBL15878610; LIXKIXWSKOENAB-UHFFFAOYSA-N; ZINC145617689; 2-Furansulfonamide, N-hydroxy-5-methyl-; 1620330-72-4
Click to Show/Hide
|
|||
| External Link | ||||
| CXL-1020 | Phase 2 | [13] | ||
| External Link | ||||
| SLV 306 | Phase 2 | [14] | ||
| Synonyms |
Daglutril; SLV-306; SLV306; UNII-KKV299446X; 182821-27-8; KKV299446X; ((3S)-3-{1-((2R)-2-Ethoxycarbonyl-4-phenylbutyl)cyclopentanecarboxamido}-2-oxo-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl)acetic acid; Daglutril [INN]; 2-[(3S)-3-[[1-[(2R)-2-ethoxycarbonyl-4-phenylbutyl]cyclopentanecarbonyl]amino]-2-oxo-4,5-dihydro-3H-1-benzazepin-1-yl]acetic acid; 1H-1-Benzazepine-1-acetic acid, 3-(((1-(2-(ethoxycarbonyl)-4-phenylbutyl)cyclopentyl)carbonyl)amino)-2,3,4,5-tetrahydro-2-oxo-, (S-(R*,S*))-
Click to Show/Hide
|
|||
| External Link | ||||
| Tonapofylline | Phase 2 | [15] | ||
| Synonyms |
Bg 9928; 340021-17-2; BG-9928; UNII-83VNU4U44T; BG9928; CHEMBL386974; CHEMBL414157; 83VNU4U44T; 3-[4-(2,6-dioxo-1,3-dipropyl-7H-purin-8-yl)-1-bicyclo[222]octanyl]propanoic acid; bicyclo[222]octane-1-propanoic acid, 4-(2,3,6,9-tetrahydro-2,6-dioxo-1,3-dipropyl-1h-purin-8-yl)-; 3-[4-(2,6-dioxo-1,3-dipropyl-2,3,6,7-tetrahydro-1H-purin-8-yl)-bicyclo[222]oct-1-yl]-propionic acid; Bicyclo(222)octane-1-propanoic acid, 4-(2,3,6,7-tetrahydro-2,6-dioxo-1,3-dipropyl-1H-purin-8-yl)-; Tonapofylline [USAN:INN]
Click to Show/Hide
|
|||
| External Link | ||||
| mRNA-0184 | Phase 1 | [16] | ||
| External Link | ||||
| LY3461767 | Phase 1 | [17] | ||
| External Link | ||||
| JTT-861 | Phase 1 | [18] | ||
| External Link | ||||
| ANX-042 | Phase 1 | [19] | ||
| Synonyms |
BNP sliced variant (heart failure), Anexon; B-type natriuretic peptide spliced variant (heart failure), Anexon
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-986224 | Phase 1 | [8] | ||
| External Link | ||||
| NOX-F37 | Preclinical | [15] | ||
| External Link | ||||
References