m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05336
|
[1] | |||
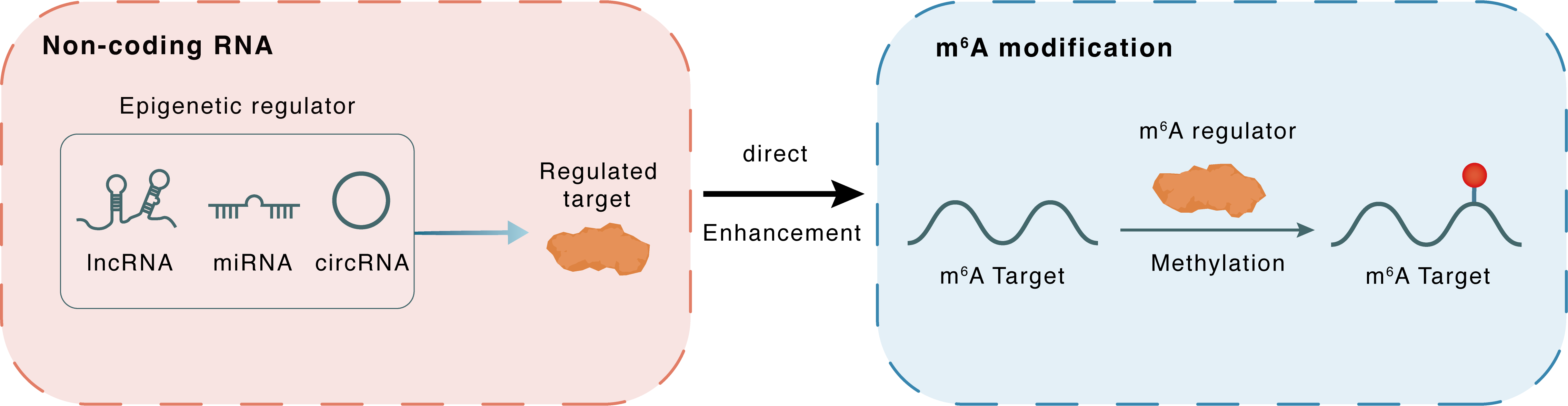 Non-coding RNA
SLERT
METTL3
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
BDNF
BDNF
METTL3
Methylation
Non-coding RNA
SLERT
METTL3
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
BDNF
BDNF
METTL3
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Neurotrophic factor BDNF precursor form (BDNF) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | SnoRNA-ended lncRNA Enhances pre-Ribosomal RNA Transcription (SLERT) | LncRNA | View Details | ||
| Regulated Target | Methyltransferase-like protein 3 (METTL3) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Enhancement | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through recruiting m6A regulator | ||||
| Crosstalk Summary | SLERT directly binds to METTL3, increasing the m6A levels of Neurotrophic factor BDNF precursor form (BDNF) mRNA; then, the m6A sites were read by IGF2BP1, enhancing BDNF mRNA stability, | ||||
| Responsed Disease | Endometrial cancer | ICD-11: 2C76 | |||
| In-vivo Model | The mice were randomly divided into four groups (n=5 per group), labeled as OE-vector (caudal vein injection of 2×106 AN3CA cells), OE-SLERT (caudal vein injection of 2×106 SLERT-overexpressing AN3CA cells), OE-SLERT+ASO-BDNF (caudal vein injection of 2×106 SLERT-overexpressing AN3CA cells transfected with ASO-BDNF), and OE-SLERT+k252a (caudal vein injection of 2×106 SLERT-overexpressing AN3CA cells and intraperitoneal injection of 25μg/kg k252a). | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Neurotrophic factor BDNF precursor form (BDNF) | 1 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| PYM-50028 | Phase 2 | [2] | ||
| Synonyms |
Cogane; Smilagenin; P-58; P-63
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| 2C76: Endometrial cancer | 18 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Dostarlimab | Approved | [3] | ||
| External Link | ||||
| Lurbinectedin | Phase 3 | [4] | ||
| Synonyms |
UNII-2CN60TN6ZS; 497871-47-3; 2CN60TN6ZS; Lurbinectedin [INN]; SCHEMBL16152477; DTXSID30198065; DB12674; CS-6323; HY-16293; J3.652.626B
Click to Show/Hide
|
|||
| External Link | ||||
| Mirvetuximab soravtansine | Approved | [4] | ||
| Synonyms |
ZOHXWSHGANNQGO-QRVRWUFNSA-N; DB12489
Click to Show/Hide
|
|||
| External Link | ||||
| AL3818 | Phase 1/2 | [4] | ||
| Synonyms |
Anlotinib; 1058156-90-3; UNII-GKF8S4C432; GKF8S4C432; AL-3818; SCHEMBL2063386; GTPL9601; KSMZEXLVHXZPEF-UHFFFAOYSA-N; MolPort-044-567-604; ZINC117924202; AKOS030526233; DB11885; CS-5396; AL 3818; HY-19716; 1-[[4-[(4-fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxyquinolin-7-yl]oxymethyl]cyclopropan-1-amine; 1-((4-(4-fluoro-2-methyl-1h-indol-5-yloxy)-6-methoxyquinolin-7-yloxy)methyl)cyclopropan-amine; Cyclopropanamine, 1-(((4-((4-fluoro-2-methyl-1H-indol-5-yl)oxy)-6-methoxy-7-quinolinyl)oxy)methyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| TKI258 | Phase 3 | [5] | ||
| Synonyms |
Dovitinib; 405169-16-6; CHIR-258; TKI-258; Chir 258; 4-Amino-5-fluoro-3-[5-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]quinolin-2(1H)-one; CHIR258; Dovitinib (TKI-258, CHIR-258); UNII-I35H55G906; CHEMBL522892; 804551-71-1; I35H55G906; TKI 258; 1027263-12-2; (3Z)-4-Amino-5-fluoro-3-[5-(4-methyl-1-piperazinyl)-1,3-dihydro-2H-benzimidazol-2-ylidene]-2(3H)-quinolinone; C21H21FN6O; 4-Amino-5-fluoro-3-(5-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl)quinolin-2(1H)-one
Click to Show/Hide
|
|||
| External Link | ||||
| DKN-01 | Phase 2 | [4] | ||
| External Link | ||||
| FP-1039 | Phase 2 | [6] | ||
| Synonyms |
FPT-039; HGS-1036; Modified growth factor receptor (cancer), FivePrime Therapeutics; FGFR-1c antagonist (protein fusion/iv,cancer), FivePrime Therapeutics; FGFR-1c antagonist (protein fusion/iv, cancer), FivePrime Therapeutics/ Human Genome Sciences
Click to Show/Hide
|
|||
| External Link | ||||
| XBIO-101 | Phase 2 | [4] | ||
| Synonyms |
sodium cridanimod; cridanimod; 38609-97-1; 9-Oxo-10(9H)-acridineacetic acid; 10-Carboxymethyl-9-acridanone; N-(Carboxymethyl)acridone; (9-oxoacridin-10(9H)-yl)acetic acid; 10(9H)-ACRIDINEACETIC ACID, 9-OXO-; Cridanimod [INN]; 2-(9-oxoacridin-10(9H)-yl)acetic acid; (9-Oxo-9H-acridin-10-yl)-acetic acid; UNII-X91E9EME19; 10-Carboxymeth-9-acridanone; Cycloferon; BRN 0227508; 9-Oxo-10-acridanacetic acid; X91E9EME19; C15H11NO3; Cridanimod (INN); 2-(9-oxoacridin-10-yl)acetic acid; 10(9H)-Acridineaceticacid, 9-oxo-; (9-Oxo-10(9H)-acrid
Click to Show/Hide
|
|||
| External Link | ||||
| PF-04691502 | Phase 2 | [7] | ||
| Synonyms |
1013101-36-4; PF 04691502; UNII-4W39NS61KI; 4W39NS61KI; 2-amino-8-((1r,4r)-4-(2-hydroxyethoxy)cyclohexyl)-6-(6-methoxypyridin-3-yl)-4-methylpyrido[2,3-d]pyrimidin-7(8H)-one; CHEMBL1234354; PF04691502; 2-Amino-8-[trans-4-(2-Hydroxyethoxy)cyclohexyl]-6-(6-Methoxypyridin-3-Yl)-4-Methylpyrido[2,3-D]pyrimidin-7(8h)-One; 2-Amino-8-[4-(2-hydroxyethoxy)cyclohexyl]-6-(6-methoxypyridin-3-yl)-4-methylpyrido[2,3-d]pyrimidin-7-one
Click to Show/Hide
|
|||
| External Link | ||||
| Folate binding protein vaccine | Phase 2 | [8] | ||
| External Link | ||||
| Virexxa | Phase 2 | [9] | ||
| External Link | ||||
| ONC201 | Phase 2 | [4] | ||
| Synonyms |
Onc-201; TIC10; 1616632-77-9; UNII-9U35A31JAI; 9U35A31JAI; TIC10(ONC-201); 7-Benzyl-4-(2-methylbenzyl)-1,2,6,7,8,9-hexahydroimidazo[1,2-A]pyrido[3,4-E]pyrimidin-5(4H)-one; TIC 10 active isomer; ONC201(TIC10 isomer); TIC 10; GTPL9978; SCHEMBL16227974; EX-A669; ONC 201; AOB2892; MolPort-039-137-731; HY-15615A; 3388AH; s7963; ZINC169620396; AKOS025404904; NSC 350625; CS-3564; AS-16735; AK174891; KB-335104; FT-0700231; J-690224; 1342897-86-2; 2,4,6,7,8,9-Hexahydro-4-((2-methylphenyl)methyl)-7-phenylmethyl)imidazo)(1,2-a)pyrido(3,4-e)pyrimid
Click to Show/Hide
|
|||
| External Link | ||||
| Folate binding protein-E39 | Phase 1/2 | [10] | ||
| External Link | ||||
| GALE-301 | Phase 2 | [11] | ||
| External Link | ||||
| COTI-2 | Phase 1 | [4] | ||
| Synonyms |
UNII-2BTA1O65BR; 2BTA1O65BR; 1039455-84-9; ZINC114475331; CS-8156; HY-19896; 1-Piperazinecarbothioic acid, 4-(2-pyridinyl)-, 2-(6,7-dihydro-8(5H)-quinolinylidene)hydrazide
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-meso-CAR vector transduced T cells | Phase 1 | [12] | ||
| External Link | ||||
| GSK1059615 | Discontinued in Phase 1 | [13] | ||
| Synonyms |
KS-00001CUQ; CTK8F0346; HMS3654N17; BCP02498; AKOS026750445; NCGC00346509-01; FT-0669060
Click to Show/Hide
|
|||
| External Link | ||||
| RU-46556 | Terminated | [14] | ||
| External Link | ||||
References