m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05294
|
[1] | |||
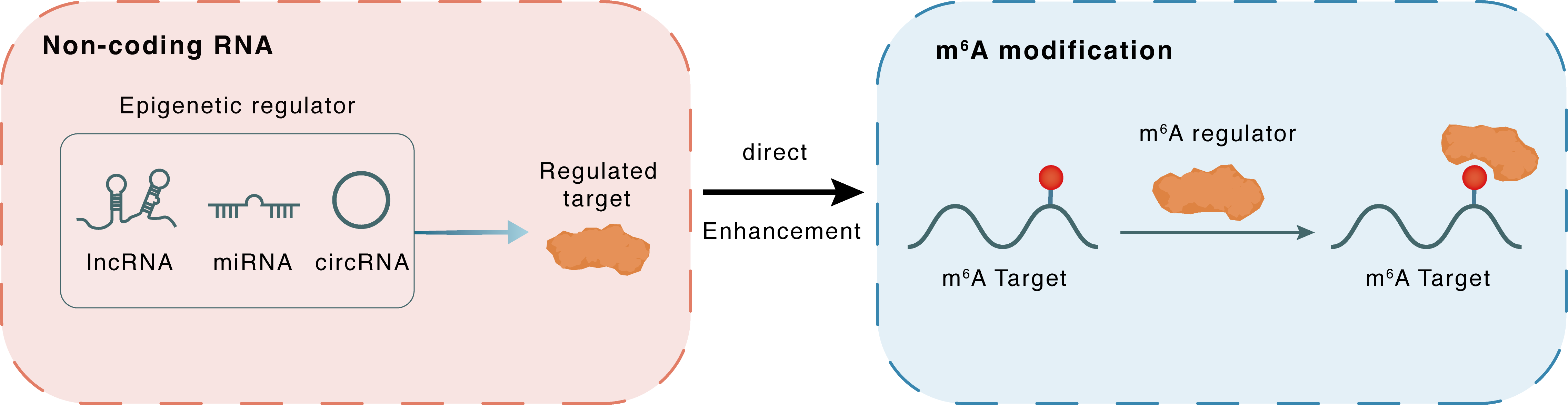 Non-coding RNA
PCAT6
IGF2BP2
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
IGF1R
IGF1R
IGF2BP2
Non-coding RNA
PCAT6
IGF2BP2
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
IGF1R
IGF1R
IGF2BP2
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) | READER | |||
| m6A Target | Insulin-like growth factor 1 receptor (IGF1R) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | Prostate cancer associated transcript 6 (PCAT6) | LncRNA | View Details | ||
| Regulated Target | Insulin like growth factor 2 mRNA binding protein 2 (IGF2BP2) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Enhancement | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through recruiting m6A regulator | ||||
| Crosstalk Summary | PCAT6 upregulated Insulin-like growth factor 1 receptor (IGF1R) expression by enhancing IGF1R mRNA stability through the PCAT6/IGF2BP2/IGF1R RNA-protein three-dimensional complex. Importantly, PCAT6 inhibition by ASO in vivo showed therapeutic potential against bone metastasis in PCa. Finally, the clinical correlation of METTL3, IGF2BP2, IGF1R, and PCAT6 was further demonstrated in PCa tissues and cells. | ||||
| Responsed Disease | Prostate cancer | ICD-11: 2C82 | |||
| Cell Process | RNA stability | ||||
| In-vivo Model | For the animal model of BM, eight BALB/c-nu mice (male, 4-6 weeks old) in the indicated groups were injected with PC-3 cells (1 × 106) in 100 μl PBS into the left cardiac ventricle and further analyzed and measured by In Vivo Imaging System (IVIS, Caliper Life Sciences), X-ray, hematoxylin and eosin (H&E), and IHC staining. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Insulin-like growth factor 1 receptor (IGF1R) | 38 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Teprotumumab | Approved | [2] | ||
| Synonyms |
RV001
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| Mecasermin | Approved | [3] | ||
| Synonyms |
Increlex (TN)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Somatomedin-1 | Approved | [4] | ||
| Synonyms |
Igef (TN)
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| OSI-906 | Phase 3 | [5] | ||
| Synonyms |
Linsitinib; 867160-71-2; Linsitinib(OSI-906); OSI906; OSI 906; OSI-906AA; OSI-906 (Linsitinib); UNII-15A52GPT8T; Kinome_3532; ASP-7487; 3-[8-amino-1-(2-phenylquinolin-7-yl)imidazo[1,5-a]pyrazin-3-yl]-1-methylcyclobutan-1-ol; 15A52GPT8T; CHEMBL1091644; MMV676605; cis-3-[8-Amino-1-(2-phenyl-7-quinolinyl)imidazo[1,5-a]pyrazin-3-yl]-1-methylcyclobutanol; C26H23N5O; cis-3-(8-amino-1-(2-phenyl-7-quinolinyl)imidazo(1,5-a)pyrazin-3-yl)-1-methylcyclobutanol; Linsitinib [USAN:INN]; OSI906/Linsitinib/
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 13 nM | |||
| External Link | ||||
| Rinfabate | Phase 2/3 | [6] | ||
| Synonyms |
RhIGFBP-3; Rinfabate, Insmed; RhIGF-BP3, Insmed; Insulin-like growth factor binding protein-3, Insmed
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| AMG 479 | Phase 2 | [7] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| VPI-2690B | Phase 2 | [8] | ||
| MOA | Antagonist | |||
| External Link | ||||
| AXL-1717 | Phase 2 | [9] | ||
| Synonyms |
BVT-51004; IGF-1 inhibitors, Axelar/Biovitrum; IGF-1 inhibitors, Karolinska/Biovitrum; Insulin-like growth factor 1 inhibitors, Axelar/Biovitrum
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| R1507 | Phase 2 | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Cixutumumab | Phase 2 | [11] | ||
| Synonyms |
LY3012217
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| TT-100 | Phase 2 | [12] | ||
| Synonyms |
TT-100, TriAct; Dual IGF-1/EGFR inhibitor (non-small-cell lung cancer), TriAct
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MM-141 | Phase 2 | [13] | ||
| MOA | Modulator | |||
| External Link | ||||
| Cyclolignan picropodophyllin | Phase 1 | [14] | ||
| Synonyms |
PPP; IGF-1R inhibitor (cancer), Karolinska
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RG-7010 | Phase 1 | [15] | ||
| Synonyms |
R-7010; PEGylated IGF1 (amyotrophic lateral sclerosis), Roche
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| HF-0299 | Phase 1 | [16] | ||
| MOA | Modulator | |||
| External Link | ||||
| BIIB 022 | Phase 1 | [17] | ||
| MOA | Antagonist | |||
| External Link | ||||
| FPI-1434 | Phase 1 | [18] | ||
| External Link | ||||
| AEW-541 | Phase 1 | [19] | ||
| Synonyms |
AECDBHGVIIRMOI-UHFFFAOYSA-N; NVP-AEW541; 475489-16-8; 475488-34-7; AEW541; NVP-AEW 541; UNII-97QB5037VR; AEW 541; AVP-AEW541; 7-((1s,3s)-3-(azetidin-1-ylmethyl)cyclobutyl)-5-(3-(benzyloxy)phenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine; CHEMBL1614712; 97QB5037VR; 7-[TRANS-3-(1-AZETIDINYLMETHYL)CYCLOBUTYL]-5-[3-(PHENYLMETHOXY)PHENYL]-7H-PYRROLO[2,3-D]PYRIMIDIN-4-AMINE; C27H29N5O; 7H-PYRROLO[2,3-D]PYRIMIDIN-4-AMINE, 7-[CIS-3-(1-AZETIDINYLMETHYL)CYCLOBUTYL]-5-[3-(PHENYLMETHOXY)PHENYL]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 60 nM | |||
| External Link | ||||
| SAR446159 | Phase 1 | [20] | ||
| External Link | ||||
| AVE-1642 | Discontinued in Phase 2 | [21] | ||
| Synonyms |
EM-164; Anti-IGF-1 receptor antibody (cancer), Aventis; Anti-IGF-1 receptor antibody (cancer), ImmunoGen; Anti-insulin-like growth factor-1 receptor antibody, Aventis; Anti-insulin-like growth factor-1 receptor antibody, ImmunoGen; Anti-IGF-1 receptor antibody (cancer), sanofi-aventis; Anti-insulin-like growthfactor-1 receptor antibody, sanofi-aventis
Click to Show/Hide
|
|||
| External Link | ||||
| KW-2450 | Discontinued in Phase 1/2 | [22] | ||
| MOA | Modulator | |||
| External Link | ||||
| Figitumumab | Discontinued in Phase 1 | [23] | ||
| Synonyms |
AC1OCENC; (2R)-3-[(4S,6R,7R,10S)-4-[(E,2R)-4-[(2S,2'S,4R,4aS,6R,8aR)-4-hydroxy-2-[(1S,3S)-1-hydroxy-3-[(6S,9R,10S)-9-methyl-5,11-dioxaspiro[5.5]undecan-10-yl]butyl]-3-methylidenespiro[4a,7,8,8a-tetrahydro-4H-pyrano[3,2-b]pyran-6,5'-oxolane]-2'-yl]but-3-en-2-yl]-7-hydroxy-2-methyl-5,11-dioxaspiro[5.5]undec-1-en-10-yl]-2-hydroxy-2-methylpropanoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BMS-695735 | Preclinical | [24] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 34 nM | |||
| External Link | ||||
| EGFR/IGFR tandem adnectin | Preclinical | [25] | ||
| Synonyms |
BMS-964210
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NVP-ADW742 | Investigative | [26] | ||
| Synonyms |
475488-23-4; ADW-742; 475489-15-7; UNII-MXS2N5862L; ADW742; MXS2N5862L; CHEMBL399021; 5-(3-(Benzyloxy)phenyl)-7-((1r,3r)-3-(pyrrolidin-1-ylmethyl)-cyclobutyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine; 5-(3-(Benzyloxy)phenyl)-7-(cis-3-(pyrrolidin-1-ylmethyl)cyclobutyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine; C28H31N5O; 5-(3-Benzyloxyphenyl)-7-[trans-3-[(pyrrolidin-1-yl)methyl]cyclobutyl]-7H-pyrrolo[2,3-d]pyrimidin-4-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 323 nM | |||
| External Link | ||||
| JB-1 | Investigative | [26] | ||
| Synonyms |
JB1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-((naphthalen-2-ylamino)methyl)benzene-1,2-diol | Investigative | [27] | ||
| Synonyms |
CHEMBL1240677; BDBM50326006
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4500 nM | |||
| External Link | ||||
| 4-((1H-indazol-6-ylamino)methyl)benzene-1,2-diol | Investigative | [27] | ||
| Synonyms |
CHEMBL1240676; BDBM50326004
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1500 nM | |||
| External Link | ||||
| AMP-PNP | Investigative | [28] | ||
| Synonyms |
Phosphoaminophosphonic acid-adenylate ester; gamma-Imino-ATP; ADENYLYL IMIDODIPHOSPHATE; AMPPNP; Adenyl imidodiphosphate; 25612-73-1; adenyl-5'-yl imidodiphosphate; CHEBI:47785; App(NH)p; O(5')-(1,2-dihydroxy-2-phosphonoaminodiphosphoryl)adenosine; 5'-O-(hydroxy{[hydroxy(phosphonoamino)phosphoryl]oxy}phosphoryl)adenosine; [[[[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]amino]phosphonic acid; p(NH)Ppf; beta,gamma-Imido-ATP; beta,gamma-Imidoadenosine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BMS-536924 | Investigative | [29] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| Fucose | Investigative | [30] | ||
| Synonyms |
L-galactomethylose; 6-Desoxygalactose; SCHEMBL13092958; AKOS030212707
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Alpha-D-Mannose | Investigative | [30] | ||
| Synonyms |
alpha-D-Mannopyranose; alpha-Mannose; alpha-D-Man; UNII-W3F28J9G0W; CHEBI:28729; 7296-15-3; W3F28J9G0W; 3h-mannose; Manalpha1,; 1rdl; 1rin; 29696-75-1; Epitope ID:130701; AC1Q59RC; AC1L4HD7; SCHEMBL76882; CHEMBL365590; WQZGKKKJIJFFOK-PQMKYFCFSA-N; ZINC3860903; FT-0773891; C00936; WURCS=2.0/1,1,0/[a1122h-1a_1-5]/1/
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ATL-1101 | Preclinical | [31] | ||
| External Link | ||||
| AG 1024 | Investigative | [31] | ||
| Synonyms |
tyrphostin AG 1024; AG-1024; AG1024
Click to Show/Hide
|
|||
| External Link | ||||
| PQ401 | Investigative | [31] | ||
| Synonyms |
PQ 401; IGF-1R inhibitor II; PQ-401
Click to Show/Hide
|
|||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| GSK1511931 | Investigative | [31] | ||
| Synonyms |
GSK1511931A; GSK-1511931; compound 14 [PMID: 19081716]
Click to Show/Hide
|
|||
| Activity | IC50 = 250 nM | |||
| External Link | ||||
| AZD3463 | Investigative | [31] | ||
| Synonyms |
AZD-3463; CS-1382; HY-15609; KB-154896
Click to Show/Hide
|
|||
| External Link | ||||
| GSK-1838705A | Investigative | [31] | ||
| Synonyms |
GSK 1838705A; GSK1838705A
Click to Show/Hide
|
|||
| Activity | IC50 = 2 nM | |||
| External Link | ||||
| 2C82: Prostate cancer | 1 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| CC-94676 | Phase 1 | [32] | ||
| External Link | ||||
References