m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05268
|
[1], [2] | |||
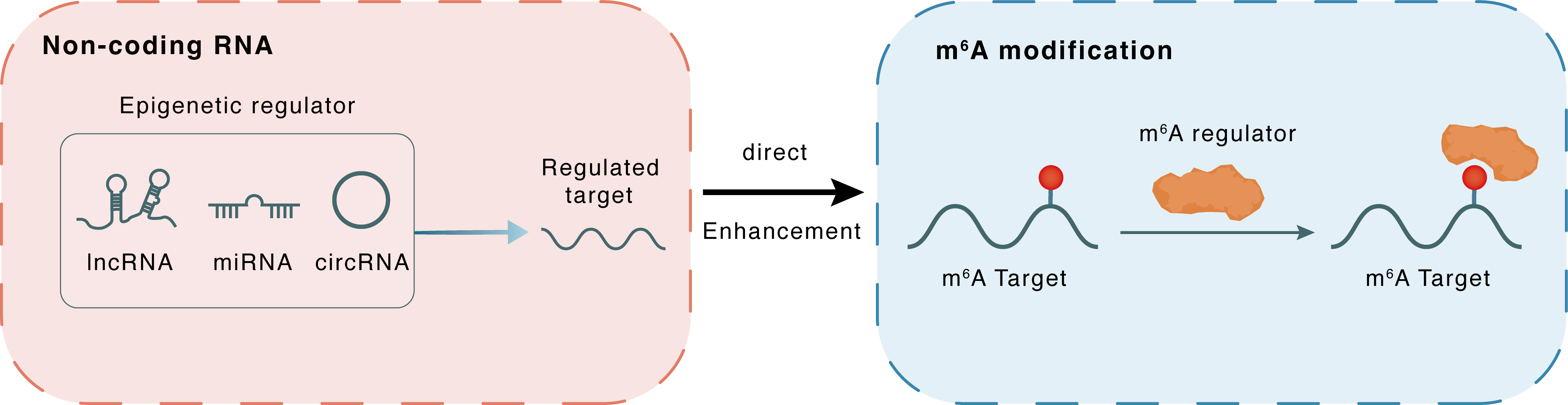 Non-coding RNA
miR-136-5p
YTHDF1
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
ARHGEF2
ARHGEF2
YTHDF1
Non-coding RNA
miR-136-5p
YTHDF1
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
ARHGEF2
ARHGEF2
YTHDF1
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | YTH domain-containing family protein 1 (YTHDF1) | READER | |||
| m6A Target | Rho guanine nucleotide exchange factor 2 (ARHGEF2) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | hsa-miR-136-5p | microRNA | View Details | ||
| Regulated Target | YTH domain-containing family protein 1 (YTHDF1) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Enhancement | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | hsa-miR-136-5p targeted YTHDF1 to restrain CRC progression and chemoresistance.YTHDF1 promotes cell growth in CRC cell lines and primary organoids and lung and liver metastasis in vivo. YTHDF1 binds to m6A sites of Rho guanine nucleotide exchange factor 2 (ARHGEF2) messenger RNA, resulting in enhanced translation of ARHGEF2. | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
In-vitro Model |
HCT 116 | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| RKO | Colon carcinoma | Homo sapiens | CVCL_0504 | ||
| SW1116 | Colon adenocarcinoma | Homo sapiens | CVCL_0544 | ||
| DLD-1 | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| LoVo | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | ||
| LS180 | Colon adenocarcinoma | Homo sapiens | CVCL_0397 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| 2B91: Colorectal cancer | 25 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Retifanlimab | Approved | [3] | ||
| Synonyms |
INCMGA0012; Retifanlimab
Click to Show/Hide
|
|||
| External Link | ||||
| Aflibercept | Approved | [4] | ||
| Synonyms |
Ziv-Aflibercept; Zaltrap (TN); VEGF Trap; VEGF Trap-Eye
Click to Show/Hide
|
|||
| External Link | ||||
| Regorafenib | Approved | [5] | ||
| Synonyms |
755037-03-7; BAY 73-4506; Regorafenibum; Stivarga; 4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide; BAY73-4506; Regorafenib (BAY 73-4506); UNII-24T2A1DOYB; 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide; BAY-73-4506; 24T2A1DOYB; CHEMBL1946170; CHEBI:68647; Stivarga (TN); BAY73-4506 hydrochloride; Regorafenib [USAN:INN]
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [6] | ||
| Synonyms |
Bevacizumab (ophthalmic slow-release tissue tablet)
Click to Show/Hide
|
|||
| External Link | ||||
| SYM-004 | Phase 3 | [6] | ||
| Synonyms |
Chimeric IgG1 antibody 1024 (cancer), Symphogen; Chimeric IgG1 antibody 992 (cancer), Symphogen; Chimeric IgG1 antibodies992 + 1024 (cancer), Symphogen
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab + Erlotinib | Phase 3 | [7] | ||
| External Link | ||||
| CPI-613 | Phase 3 | [6] | ||
| Synonyms |
95809-78-2; 6,8-bis(benzylthio)octanoic acid; CPI 613; MLS006010202; SCHEMBL1062218; 6,8-Bis(benzylsulfanyl)octanoic acid; Octanoic acid, 6,8-bis((phenylmethyl)thio)-; Octanoic acid, 6,8-bis[(phenylMethyl)thio]-; 6,8-Bis[(phenylmethyl)thio]octanoic acid; CPI613; CHEMBL3186849; QCR-193; AOB1058; MolPort-023-219-128; HMS3656L06; C22H28O2S2; BCP04663; EX-A2043; s2776; AKOS025142095; BCP9000552; DB12109; RL06062; CS-0961; NCGC00344764-01; SMR004701300; AS-16613; BC261916; AK174899; HY-15453; BCP0726000030; KB-293127; AB0035874
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [4] | ||
| External Link | ||||
| AlloStim | Phase 2/3 | [8] | ||
| Synonyms |
AlloStim (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Sibrotuzumab | Phase 2 | [9] | ||
| External Link | ||||
| CV301 | Phase 2 | [10] | ||
| External Link | ||||
| Efatutazone | Phase 2 | [11] | ||
| Synonyms |
Inolitazone; 223132-37-4; 5-[[4-[[6-(4-amino-3,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-yl]methoxy]phenyl]methyl]-2,4-Thiazolidinedione; Efatutazone [INN]; RS5444; CS-7017; SCHEMBL3246054; CHEMBL3545280; JCYNMRJCUYVDBC-UHFFFAOYSA-N; Efatutazone;CS-7017;RS5444; BCP07478; AKOS030526729; DB11894; CS-0778; KB-77905; DA-07988; HY-14792; QC-10456; 4CA-1384; FT-0737589; 5-[4-[6-(4-amino-3 ,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-ylmethoxy]benzyl]thiazolidine-2,4-dione
Click to Show/Hide
|
|||
| External Link | ||||
| LOR-2040 | Phase 2 | [12] | ||
| External Link | ||||
| RG7221 | Phase 2 | [13] | ||
| External Link | ||||
| PEG-SN38 | Phase 2 | [14] | ||
| Synonyms |
EZN-2208
Click to Show/Hide
|
|||
| External Link | ||||
| MEGF0444A | Phase 2 | [15] | ||
| External Link | ||||
| Encapsulated cell therapy | Phase 1/2 | [16] | ||
| External Link | ||||
| AB928 | Phase 1/2 | [17] | ||
| External Link | ||||
| MGD007 | Phase 1 | [13] | ||
| External Link | ||||
| BNC-101 | Phase 1 | [18] | ||
| External Link | ||||
| Navicixizumab | Phase 1 | [6] | ||
| External Link | ||||
| RG7160 | Discontinued in Phase 2 | [19] | ||
| External Link | ||||
| Nimesulide | Terminated | [20] | ||
| Synonyms |
51803-78-2; N-(4-Nitro-2-phenoxyphenyl)methanesulfonamide; Mesulid; Flogovital; Sulidene; Nimed; R-805; 4-NITRO-2-PHENOXYMETHANESULFONANILIDE; Nisulid; Nimesulidum [INN-Latin]; Nimesulida [INN-Spanish]; R 805; UNII-V4TKW1454M; 4-Nitro-2-phenoxy-methanesulfonanilide; 4'-Nitro-2'-phenoxymethanesulfonanilide; Methanesulfonamide, N-(4-nitro-2-phenoxyphenyl)-; EINECS 257-431-4; 4'-Nitro-2'-phenoxymethansulfonanilid; BRN 2421175; CHEMBL56367; MLS000069680; V4TKW1454M; Methanesulfonanilide, 4'-nitro-2'-phenoxy-; CHEBI:44445; Dulanermin
Click to Show/Hide
|
|||
| External Link | ||||
| Saracatinib | Phase 2 | [21] | ||
| External Link | ||||
| G3139 + Irinotecan | Investigative | [22] | ||
| External Link | ||||
References