m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05226
|
[1] | |||
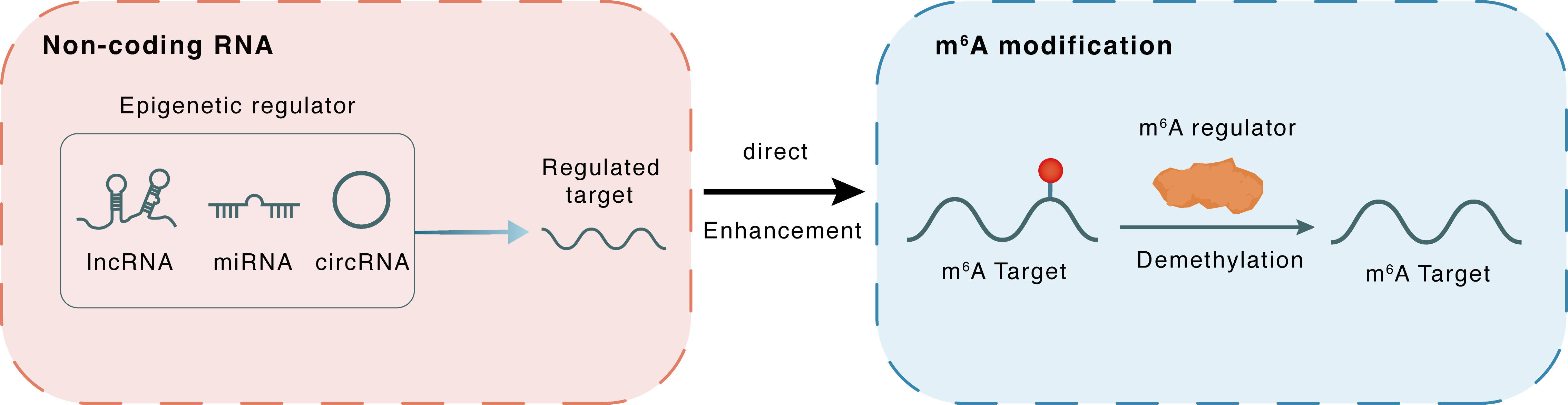 Non-coding RNA
CCRR
FTO
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
ATP2A2
ATP2A2
FTO
Demethylation
Non-coding RNA
CCRR
FTO
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
ATP2A2
ATP2A2
FTO
Demethylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | |||
| m6A Target | Sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2a/ATP2A2) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | Cardiac conduction regulatory RNA (CCRR) | LncRNA | View Details | ||
| Regulated Target | FTO alpha-ketoglutarate dependent dioxygenase (FTO) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Enhancement | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | CCRR is a protective lncRNA that acts by maintaining the function of FTO, thereby reducing the m6A RNA methylation level of Sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2a/ATP2A2), ultimately preserving calcium homeostasis for myocardial contractile function in MI. | ||||
| Responsed Disease | Acute myocardial infarction | ICD-11: BA41 | |||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2a/ATP2A2) | 2 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Mydicar | Phase 2 | [2] | ||
| Synonyms |
Mydicar (TN)
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Gallopamil | Phase 2 | [3] | ||
| Synonyms |
16662-47-8; methoxyverapamil; 5-((3,4-Dimethoxyphenethyl)(methyl)amino)-2-isopropyl-2-(3,4,5-trimethoxyphenyl)pentanenitrile; Galopamilo; Galopamilo [INN-Spanish]; Gallopamillum [INN-Latin]; Gallopamil [INN:BAN]; D600; D 600; C28H40N2O5; CHEBI:34772; (+/-)-Methoxyverapamil hydrochloride; 5-[(3,4-Dimethoxyphenethyl)methylamino]-2-isopropyl-2-(3,4,5-trimethoxyphenyl)valeronitrile; Benzeneacetonitrile, alpha-(3-((2-(3,4-dimethoxyphenyl)ethyl)methylamino)propyl)-3,4,5-trimethoxy-alpha-(1-methylethyl)-; GSK 796406
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BA41: Acute myocardial infarction | 34 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Reteplase | Approved | [4] | ||
| Synonyms |
Retevase; Rapilysin (TN); Retavase (TN); Retevase (TN); Reteplase (USAN/INN)
Click to Show/Hide
|
|||
| External Link | ||||
| M-enoxaparin | Approved | [5] | ||
| Synonyms |
M-enoxaparin (angina)
Click to Show/Hide
|
|||
| External Link | ||||
| Anistreplase | Approved | [6] | ||
| External Link | ||||
| Ridogrel | Approved | [7] | ||
| Synonyms |
Ibidel; R-68070; Ridogrel (USAN/INN); 5-[(E)-[pyridin-3-yl-[3-(trifluoromethyl)phenyl]methylidene]amino]oxypentanoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Tisokinase | Approved | [5] | ||
| Synonyms |
Plasvata (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Tirofiban | Approved | [8] | ||
| Synonyms |
AGG; Aggrastat; Agrastat; Tirofibanum; Aggrastat (TN); Agrastat (TN); L-700462; MK-383; Tirofiban (INN); Tirofiban[BAN:INN]; Tirofiban [INN:BAN]; L-700,462; N-(BUTYLSULFONYL)-O-[4-(4-PIPERIDINYL)BUTYL]-L-TYROSINE; N-(Butylsulfonyl)-O-(4-(4-piperidyl)butyl)-L-tyrosine; N-(butylsulfonyl)-O-(4-piperidin-4-ylbutyl)-L-tyrosine; N-(butylsulfonyl)-o-[4-(piperidin-4-yl)butyl]-l-tyrosine; (2S)-2-(butylsulfonylamino)-3-[4-(4-piperidin-4-ylbutoxy)phenyl]propanoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Prasugrel | Approved | [9] | ||
| Synonyms |
Effient (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Trapidil | Phase 4 | [10] | ||
| Synonyms |
Trapymin; Rocornal; 15421-84-8; Avantrin; Trapymine; N,N-diethyl-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine; AR 12008; Trapidilum [INN-Latin]; UNII-EYG5Y6355E; EINECS 239-434-2; BRN 0186842; 7-Diethylamino-5-methyl-s-triazolo(1,5-a)pyrimidine; MLS000567667; EYG5Y6355E; N,N-Diethyl-5-methyl-(1,2,4)triazolo(1,5-a)pyrimidine-7-amine; (1,2,4)Triazolo(1,5-a)pyrimidin-7-amine, N,N-diethyl-5-methyl-; 5-Methyl-7-diethylamino-s-triazolo-(1,5-a)-pyrimidine; NCGC00016715-01; AR-12008; SMR000154170; SU10991
Click to Show/Hide
|
|||
| External Link | ||||
| Entresto | Phase 3 | [11] | ||
| Synonyms |
Sacubitril valsartan sodium hydrate; UNII-WB8FT61183; Sacubitril mixture with valsartan; WB8FT61183; Entresto (TN); Valsartan mixture with AHU-377; MolPort-042-624-138; C96H120N12Na6O21; 9052AF; AKOS026670199; ACN-036829; 3-(1-Biphenyl-4-ylmethyl-3-ethoxycarbonyl-1-butylcarbamoyl)propionate-3'-methyl-2'-(pentanoyl(2'-(tetrazol-5-ylate)biphenyl-4'-ylmethyl)amino)butyrate; AK198922; AC-29037; Sacubitril valsartan sodium hydrate (JAN); FT-0700025; D10226; L-Valine, N-(1-oxopentyl)-N-((2'-(2H-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl)-, compd. wi
Click to Show/Hide
|
|||
| External Link | ||||
| MultiStem | Phase 2 | [11] | ||
| Synonyms |
MultiStem (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Losmapimod | Phase 3 | [12] | ||
| Synonyms |
585543-15-3; GSK-AHAB; GW856553; GW856553X; UNII-F2DQF16BXE; F2DQF16BXE; GW-856553; 6-[5-(cyclopropylcarbamoyl)-3-fluoro-2-methylphenyl]-N-(2,2-dimethylpropyl)pyridine-3-carboxamide; Losmapimod [USAN:INN]; Losmapimod (GW856553X); GSKAHAB; GW 856553X; SB 856553; Losmapimod (USAN/INN); SCHEMBL1070401; GTPL7835; CHEMBL1088752; EX-A486; CHEBI:131167; KKYABQBFGDZVNQ-UHFFFAOYSA-N; MolPort-009-194-138; losmapimod pound> HMS3653G19; BCP09909; AOB87105; ZINC35793138; s7215; BDBM50418610; 2523AH; AKOS015994587
Click to Show/Hide
|
|||
| External Link | ||||
| Bendavia | Phase 2 | [13] | ||
| Synonyms |
Elamipretide
Click to Show/Hide
|
|||
| External Link | ||||
| Dalcetrapib | Phase 3 | [11] | ||
| Synonyms |
JTT 705; JTT-705; R-1658; RG-1658; RO-4607381; S-[2-[[1-(2-ethylbutyl)cyclohexanecarbonyl]amino]phenyl] 2-methylpropanethioate
Click to Show/Hide
|
|||
| External Link | ||||
| REG1 | Phase 3 | [14] | ||
| External Link | ||||
| RGN-352 | Phase 2 | [11] | ||
| Synonyms |
Thymosin Beta 4 (injectable)
Click to Show/Hide
|
|||
| External Link | ||||
| CSL 112 | Phase 2 | [15] | ||
| External Link | ||||
| MEDI6012 | Phase 1 | [16] | ||
| Synonyms |
RhLCAT; ACP-501
Click to Show/Hide
|
|||
| External Link | ||||
| TF0023 | Phase 2 | [11] | ||
| External Link | ||||
| E5555 | Phase 2 | [17] | ||
| Synonyms |
Atopaxar
Click to Show/Hide
|
|||
| External Link | ||||
| VT-111a | Phase 2 | [18] | ||
| External Link | ||||
| RO-4905417 | Phase 2 | [19] | ||
| External Link | ||||
| Aliskiren/valsartan | Phase 2 | [20] | ||
| Synonyms |
Valturna (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| M118 | Phase 2 | [21] | ||
| Synonyms |
Adomiparin
Click to Show/Hide
|
|||
| External Link | ||||
| Ischemic tolerant allogeneic mesenchymal stem cell therapy | Phase 2 | [22] | ||
| External Link | ||||
| CER-001 | Phase 2 | [23] | ||
| External Link | ||||
| KAI-9803 | Phase 1/2 | [24] | ||
| Synonyms |
DELCASERTIB; UNII-5G7N7E908H; 5G7N7E908H; BMS-875944; Delcasertib [USAN:INN]; 949100-39-4; HY-106262; CS-0025460; L-Arginine, L-cysteinyl-L-tyrosylglycyl-L-arginyl-L-lysyl-L-lysyl-L-arginyl-L-arginyl-L- glutaminyl-L-arginyl-L-arginyl-, (1->1')-disulfide with L-cysteinyl-L-seryl-L-phenylalanyl-L- asparaginyl-L-seryl-L-tyrosyl-L-alpha-glutamyl-L-leucylglycyl-L-seryl-L-leucine; L-Cysteinyl-L-tyrosylglycyl-L-arginyl-L-lysyl-L-lysyl-L-arginyl-L-arginyl-L-glutaminyl-L- arginyl-L-arginyl-L-arginine (1->1')-d
Click to Show/Hide
|
|||
| External Link | ||||
| MDCO-216 | Phase 1 | [25] | ||
| External Link | ||||
| PF-06282999 | Phase 1 | [26] | ||
| External Link | ||||
| REC-01 | Phase 1 | [11] | ||
| External Link | ||||
| Mesenchymal stem cell therapy, ischemic-tolerant stem cells | Phase 1 | [11] | ||
| External Link | ||||
| Darexaban maleate | Discontinued in Phase 3 | [27] | ||
| Synonyms |
UNII-03RTP2436R; 365462-24-4; 03RTP2436R; YM150; Darexaban maleate (JAN); SCHEMBL5406349; CHEMBL1922345; D09817; N-[2-[[4-(Hexahydro-4-methyl-1H-1,4-diazepin-1-yl)benzoyl]amino]-3-hydroxyphenyl]-4-methoxybenzamide (2Z)-2-Butenedioate
Click to Show/Hide
|
|||
| External Link | ||||
| Streptokinase | Phase 4 | [28] | ||
| External Link | ||||
| RG1512 | Discontinued in Phase 2 | [29] | ||
| Synonyms |
P-selectin huMAb
Click to Show/Hide
|
|||
| External Link | ||||
| LA-8045 | Investigative | [30] | ||
| Synonyms |
LA-816
Click to Show/Hide
|
|||
| External Link | ||||
References