m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05221
|
[1] | |||
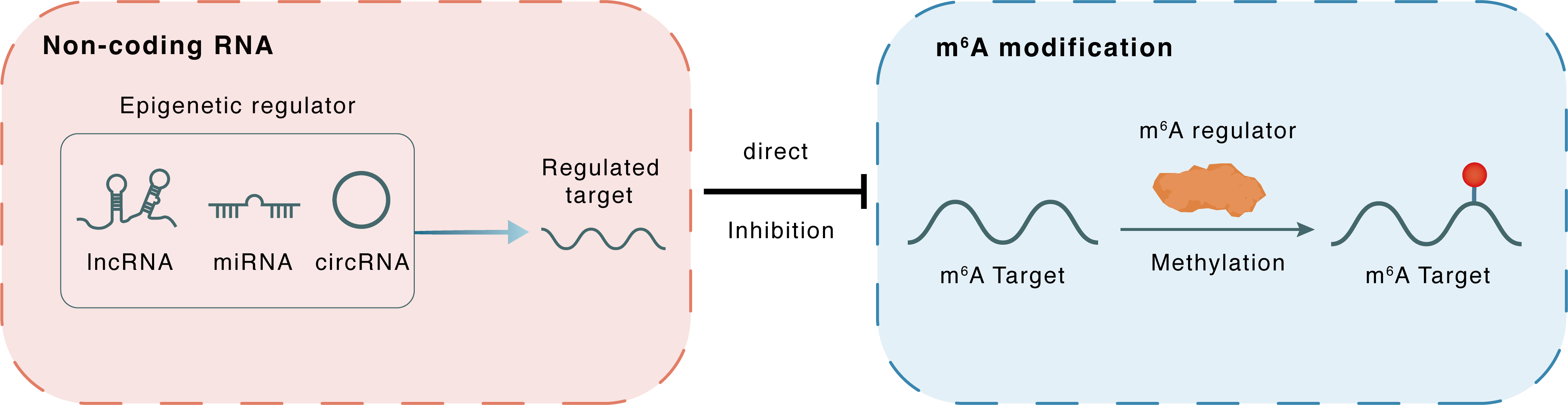 Non-coding RNA
Circ_0066715
WTAP
lncRNA miRNA circRNA
Direct
Inhibition
m6A modification
ETS1
ETS1
WTAP
Methylation
Non-coding RNA
Circ_0066715
WTAP
lncRNA miRNA circRNA
Direct
Inhibition
m6A modification
ETS1
ETS1
WTAP
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Wilms tumor 1-associating protein (WTAP) | WRITER | |||
| m6A Target | Protein C-ets-1 (ETS1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | hsa_circ_0066715 (Circ_CBLB) | circRNA | View Details | ||
| Regulated Target | Pre-mRNA-splicing regulator WTAP (WTAP) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Inhibition | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | WTAP may be involved in the methylation process of Protein C-ets-1 (ETS1) in RA. ETS1 m6A methylation levels were altered upon WTAP intervention. The overexpression or interference of Circ_CBLB decreased or increased WTAP expression. | ||||
| Responsed Disease | Rheumatoid arthritis | ICD-11: FA20 | |||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| FA20: Rheumatoid arthritis | 434 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Sarilumab | Approved | [2] | ||
| Synonyms |
Kevzara
Click to Show/Hide
|
|||
| External Link | ||||
| Sulindac | Approved | [3] | ||
| Synonyms |
Aclin; Aflodac; Arthrobid; Arthrocine; Chibret; Clinoril; Copal; Copals; Kenalin; Klinoril; Mobilin; Sulindaco; Sulindacum; Sulindal; Alphapharm Brand of Sulindac; Apo Sulin; Apotex Brand of Sulindac; Cahill May Roberts Brand of Sulindac; Chemia Brand of Sulindac; Copal resin; Copal rosin varnish; Gum copal; KendrickBrand of Sulindac; Merck Brand of Sulindac; Novo Sundac; Novopharm Brand of Sulindac; Nu Pharm Brand of Sulindac; Nu Sulindac; Resin copal; Sulindac sulfoxide; MK 231; MK231; S 8139; Apo-Sulin; Clinoril (TN); MK-231; Merck Sharp & Dohme Brand of Sulindac; Novo-Sundac; Nu-Pharm Brand of Sulindac; Nu-Sulindac; Sulindaco [INN-Spanish]; Sulindacum [INN-Latin]; Sulindac (JAN/USP/INN); Sulindac [USAN:BAN:INN:JAN]; Z-5-Fluoro-2-methyl-1-[p-(methlsulfinyl)benzylidene]indene-3-acetic acid; Cis-5-Fluoro-2-methyl-1-((p-methylsulfinyl)benzylidene)indene-3-acetic acid; {5-fluoro-2-methyl-1-[4-(methylsulfinyl)benzylidene]-1h-inden-3-yl}acetic acid; Cis-5-Fluoro-2-methyl-1-((4-(methylsulfinyl)phenyl)methylene)-1H-indene-3-acetic acid; [(1E)-5-fluoro-2-methyl-1-{[4-(methylsulfinyl)phenyl]methylidene}-1H-inden-3-yl]acetic acid; {(1E)-5-fluoro-2-methyl-1-[4-(methylsulfinyl)benzylidene]-1H-inden-3-yl}acetic acid; {(1Z)-5-fluoro-2-methyl-1-[4-(methylsulfinyl)benzylidene]-1H-inden-3-yl}acetic acid; (E)-(1)-5-Fluoro-2-methyl-1-((4-(methylsulphinyl)phenyl)methylene)-1H-indene-3-acetic acid; (Z)-5-Fluoro-2-methyl-1-((p-(methylsulfinyl)phenyl)methylene)-1H-indene-3-acetic acid; (Z)-5-Fluoro-2-methyl-1-[[4-(methyl-sulfinyl)phenyl]methylene]-1H-indene-3-acetic acid; (Z)-5-Fluoro-2-methyl-1-[p-(methylsulfinyl)benzylidene]indene-3-acetic acid; 2-[(3E)-6-fluoro-2-methyl-3-[(4-methylsulfinylphenyl)methylidene]inden-1-yl]acetic acid; 2-[(3Z)-6-fluoro-2-methyl-3-[(4-methylsulfinylphenyl)methylidene]inden-1-yl]acetic acid; 2-[6-fluoro-2-methyl-3-[(4-methylsulfinylphenyl)methylidene]inden-1-yl]acetic acid; 5-Fluoro-2-methyl-1-((4-(methylsulphinyl)phenyl)methylene)-1H-indene-3-acetic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Ofatumumab | Phase 3 | [4] | ||
| Synonyms |
Arzerra (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Baricitinib | Approved | [5] | ||
| Synonyms |
Baricitinib (LY3009104, INCB028050); Baricitinib [USAN:INN]; C16H17N7O2S; INCB 028050; INCB-028050; INCB028050; ISP4442I3Y; J-503551; LY-3009104; LY3009104; Olumiant (TN); UNII-ISP4442I3Y; olumiant
Click to Show/Hide
|
|||
| External Link | ||||
| Felbinac | Approved | [6] | ||
| Synonyms |
Felbinac); Felbinac, Takeda Chemical Industries Ltd; Seltouch, Takeda
Click to Show/Hide
|
|||
| External Link | ||||
| Salsalate | Approved | [6] | ||
| Synonyms |
Diacesal; Diplosal; Disalcid; Disalicylsaeure; Disalyl; Nobacid; Salflex; Salical; Salina; Saloxium; Salsalato; Salsalatum;Salysal; Sasapirin; Sasapyrin; Sasapyrine; Sasapyrinum; Disalicylic acid; Sal Ester Sal; Salicylic Acid Salicylate; Salicyloylsalicylic acid; Salicylsalicylic acid; Disalcid (TN); O-Salicylcylsalicylsaeure; O-Salicyloylsalicylic Acid; O-Salicylsalicylic acid; Salflex (TN); Salicylic acid, bimolecular ester; Salicylic acid, salicylate; Salsalato [INN-Spanish]; Salsalatum [INN-Latin]; Salsitab (TN); Sasapyrine (JAN); Mono-Gesic (TN); Salsalate (USP/INN); Salsalate [USAN:INN:BAN]; Benzoic acid, 2-hydroxy-, 2-carboxyphenyl ester; 2-((2-Hydroxybenzoyl)oxy)benzoic acid; 2-(2-hydroxybenzoyl)oxybenzoic acid; 2-Carboxyphenyl salicylate; 2-Salicyloyloxybenzoic Acid; 2-{[(2-hydroxyphenyl)carbonyl]oxy}benzoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Fostamatinib | Phase 3 | [7] | ||
| Synonyms |
901119-35-5; R788; Tavalisse; UNII-SQ8A3S5101; R-788 Free acid; R 788; R-788; R-935788 Free acid; SQ8A3S5101; R7935788; Fostamatinib [USAN:INN]
Click to Show/Hide
|
|||
| External Link | ||||
| Belumosudil | Approved | [8] | ||
| External Link | ||||
| Canakinumab | Approved | [9] | ||
| Synonyms |
Ilaris (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Methotrexate | Phase 3 | [10] | ||
| Synonyms |
Rheumatrex; Amethopterin; Metatrexan; Hdmtx; Abitrexate; Mexate; Methylaminopterinum; Methotrexatum; Antifolan; Metotrexato; Methylaminopterin; MTX; (S)-2-(4-(((2,4-Diaminopteridin-6-yl)methyl)(methyl)amino)benzamido)pentanedioic acid; Methotrexat; Amethopterine; Maxtrex; Rasuvo; L-Amethopterin; A-Methopterin; A-Methpterin; Amethopterin L-; Folex-Pfs; Methotrexat-Ebewe; N-Bismethylpteroylglutamic acid; Methotrexate, L-; Metotressato [DCIT]; Methotextrate; Mexate-Aq; [3H]methotrexate
Click to Show/Hide
|
|||
| External Link | ||||
| IPI-145 | Phase 3 | [11] | ||
| Synonyms |
Duvelisib; 1201438-56-3; INK-1197; UNII-610V23S0JI;
Click to Show/Hide
|
|||
| External Link | ||||
| Indomethacin | Approved | [6] | ||
| Synonyms |
Aconip; Amuno; Arthrexin; Artracin; Artrinovo; Artrivia; Bonidin; Bonidon; Catlep; Confortid; Dolcidium; Dolovin; Durametacin; Elmetacin; Hicin; IMN; Idomethine; Imbrilon; Inacid; Indacin; Indameth; Indmethacine; Indocid; Indocin; Indomecol; Indomed; Indomee; Indometacin; Indometacina; Indometacine; Indometacinum; Indometacyna; Indomethacine; Indomethacinum; Indomethancin; Indomethazine; Indomethegan; Indomethine; Indometicina; Indomo; Indomod; Indoptic; Indoptol; Indorektal; Indoxen; Inflazon; Infrocin; Lausit; Liometacen; Metacen; Metartril; Methazine; Metindol; Mezolin; Miametan; Mikametan; Mobilan; Novomethacin; Reumacide; Sadoreum; Tannex; Vonum; Bonidon Gel; DESMETHYL INDOMETHACIN; Dolcidium PL;Flexin continus; Indocid Pda; Indocid Sr; Indocin Sr; Indolar SR; Indometacyna [Polish]; Indometicina [Spanish]; Inteban sp; Rhemacin LA; Rheumacin LA; I 7378; IN1454; Indomet 140; Aconip (TN); Apo-Indomethacin; Chibro-amuno; Chrono-indicid; Chrono-indocid; Indo-Lemmon; Indo-Spray; Indo-phlogont; Indo-rectolmin; Indo-tablinen; Indocid (TN); Indocid (pharmaceutical); Indocin (TN); Indocin I.V; Indometacina [INN-Spanish]; Indometacine [INN-French]; Indometacinum [INN-Latin]; Indomethacin (USP); Indomethacin [USAN:BAN]; Novo-Methacin; Indochron E-R (TN); Indocin I.V.; Indocin-SR (TN); Indometacin (JP15/INN); Indomethacin & MAP-30; Indomethacin, Indochron E-R, Indocin-SR, Indocid, Indocin, Indomethacin
Click to Show/Hide
|
|||
| External Link | ||||
| Inosine pranobex | Approved | [6] | ||
| Synonyms |
Imunovir (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Secukinumab | Phase 2 | [12] | ||
| External Link | ||||
| Infliximab | Approved | [13] | ||
| Synonyms |
Remicade (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Citrofen | Approved | [6] | ||
| Synonyms |
Citrofen (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Meclofenamate Sodium | Approved | [6] | ||
| External Link | ||||
| Oxaprozin | Approved | [6] | ||
| Synonyms |
Actirin; Alvo; Danoprox; Daypro; Dayrun; Deflam; Oxaprozina; Oxaprozine; Oxaprozinum; Voir; Xopane; Apotex brand of oxaprozin; CSC brand of oxaprozin; Pfizer brand of oxaprozin; Rhoxalpharma brand of oxaprozin; TRB brand of oxaprozin; Lyl)propenoic acid; NCI310839; O 9637; WY 21743; Apo-Oxaprozin; Daypro (TN); Duraprox (TN); Oxaprozina [INN-Spanish]; Oxaprozine [INN-French]; Oxaprozinum [INN-Latin]; Rhoxal-oxaprozin; WY-21743; WY-21,743; Oxaprozin (JP15/USAN/INN); Oxaprozin [USAN:BAN:INN:JAN]; Beta-(4,5-Diphenyloxazol-2-yl)propionic acid; 3-(4, 5-Diphenyl-2-oxazo; 3-(4,5-Diphenyl-1,3-oxazol-2-yl)propanoic acid; 3-(4,5-Diphenyl-2-oxazolyl)propenoic acid; 4, 5-Diphenyl-2-oxazolepropionic acid; 4,5-Diphenyl-2-oxazolepropanoic acid; 4,5-Diphenyl-2-oxazolepropionic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Zinc salts | Approved | [6] | ||
| External Link | ||||
| Adalimumab | Approved | [14] | ||
| Synonyms |
Humira; Adalimumab (genetical recombination); Humira (TN); Adalimumab (USAN/INN); Adalimumab (genetical recombination) (JAN)
Click to Show/Hide
|
|||
| External Link | ||||
| Orthokine | Approved | [6] | ||
| Synonyms |
Orthokine (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Dexamethasone | Approved | [15] | ||
| Synonyms |
Adexone; Anaflogistico; Aphtasolon; Aphthasolone; Auxiron; Azium; Calonat; Corson; Corsone; Cortisumman; DXM; Decacort; Decacortin; Decaderm; Decadron; Decagel; Decaject; Decalix; Decameth; Decasone; Decaspray; Dectancyl; Dekacort; Deltafluorene; Dergramin; Deronil; Desadrene; Desametasone; Desamethasone; Desameton; Deseronil; Dexacort; Dexacortal; Dexacortin; Dexadeltone; Dexafarma; Dexair; Dexalona; Dexaltin; Dexametasona; Dexameth; Dexamethansone; Dexamethasonum; Dexamethazone; Dexamonozon; Dexapolcort; Dexaprol; Dexason; Dexasone; Dexinolon; Dexinoral; Dexone; Dexonium; Dexpak; Dextelan; Dezone; Dinormon; Dxms; Fluormethylprednisolone; Fluormone; Fluorocort; Fortecortin; Gammacorten; Hexadecadrol; Hexadrol; IontoDex; Loverine; Luxazone; Maxidex; Mediamethasone; Methylfluorprednisolone; Mexidex; Millicorten; Mymethasone; Oradexon; Policort; Posurdex; Prodex; Spoloven; Superprednol; Turbinaire; Visumetazone; Alcon Brand of Dexamethasone; Bisu DS; Desametasone [DCIT]; Dexa Mamallet; Dexamethasone Base; Dexamethasone Intensol; Dexamethasone alcohol; ECR Brand of Dexamethasone; Foy Brand of Dexamethasone; Hexadrol Elixir; Hexadrol Tablets; ICN Brand of Dexamethasone; Lokalison F; Merck Brand of Dexamethasone; Pet Derm III; Prednisolon F; Prednisolone F; Sunia Sol D; Dexone 4; MK 125; Merz Brand 1 of Dexamethasone; Merz Brand 2 of Dexamethasone; Aeroseb-D; Aeroseb-Dex; Azium (Veterinary); Decadron (TN); Decadron Tablets, Elixir; Decadron, Dexamethasone; Decadron-LA; Dex-ide; Dexa-Cortidelt;Dexa-Cortisyl; Dexa-Mamallet; Dexa-Scheroson; Dexa-sine; Dexacen-4; Dexametasona [INN-Spanish]; Dexamethasonum [INN-Latin]; Dexone 0.5; Dexone 0.75; Dexone 1.5; Hl-dex; Isopto-Dex; OTO-104; Ocu-trol;Pet-Derm Iii; SK-Dexamethasone; Decaject L.A.; Dexamethasone [INN:BAN:JAN]; Decaject-L.A.; Dexamethasone (JP15/USP/INN); Delta1-9alpha-Fluoro-16alpha-methylcortisol; Delta(sup 1)-9-alpha-Fluoro-16-alpha-methylcortisol; (3H)-Dexamethasone; 1-Dehydro-16.alpha.-methyl-9.alpha.-fluorohydrocortisone; 1-Dehydro-16alpha-methyl-9alpha-fluorohydrocortisone; 16-alpha-Methyl-9-alpha-fluoro-1-dehydrocortisol; 16-alpha-Methyl-9-alpha-fluoro-delta(sup 1)-hydrocortisone; 16-alpha-Methyl-9-alpha-fluoro-delta1-hydrocortisone; 16-alpha-Methyl-9-alpha-fluoroprednisolone; 16.alpha.-Methyl-9.alpha.-fluoro-1-dehydrocortisol; 16.alpha.-Methyl-9.alpha.-fluoroprednisolone; 16alpha-Methyl-9alpha-fluoro-1-dehydrocortisol; 16alpha-Methyl-9alpha-fluoro-delta(sup 1)-hydrocortisone; 16alpha-Methyl-9alpha-fluoroprednisolone; 9-alpha-Fluoro-16-alpha-methylprednisolone; 9.alpha.-Fluoro-16.alpha.-methylprednisolone; 9A-FLUORO-16BETA-METHYLPREDNISOLONE; 9alpha-Fluoro-16alpha-methylprednisolone
Click to Show/Hide
|
|||
| External Link | ||||
| SK-1306X | Approved | [6] | ||
| Synonyms |
Cararthron; Joins
Click to Show/Hide
|
|||
| External Link | ||||
| Tenoxicam | Approved | [16] | ||
| Synonyms |
Artriunic; Liman; Mobiflex; Reutenox; Tenoxicamum; Tilcotil; Apotex brand of tenoxicam; Novag brand of tenoxicam; Novopharm brand of tenoxicam; Roche brand of tenoxicam; Solvay brand of tenoxicam; Apo-Tenoxicam; Mobiflex (TN); Novo-Tenoxicam; Ro 12-0068; Ro-120068; Tenoxicamum [INN-Latin]; Tilcotil (TN); Ro 12-0068/000; Ro-12-0068; Tenoxicam (JAN/USAN/INN); Tenoxicam [USAN:BAN:INN:JAN]; (3E)-3-[hydroxy-(pyridin-2-ylamino)methylidene]-2-methyl-1,1-dioxothieno[2,3-e]thiazin-4-one; (3Z)-3-[hydroxy-(pyridin-2-ylamino)methylidene]-2-methyl-1,1-dioxothieno[2,3-e]thiazin-4-one; 2H-Thieno(2,3-e)-1,2-thiazine-3-carboxamide, 4-hydroxy-2-methyl-N-2-pyridinyl-, 1,1-dioxide; 3-[hydroxy-(pyridin-2-ylamino)methylidene]-2-methyl-1,1-dioxothieno[2,3-e]thiazin-4-one; 4-HYDROXY-2-METHYL-N-2-PYRIDINYL-2H-THIENO[2,3-E]-1,2-THIAZINE-3-CARBOXAMIDE 1,1-DIOXIDE; 4-Hydroxy-2-methyl-N-2-pyridinyl-2H-thieno(2,3-e)-1,2-thiazine-3-carboxamide1,1-dioxide; 4-Hydroxy-2-methyl-N-2-pyridyl-2H-thieno(2,3-e)-1,2-thiazine-3-carboxamide 1,1-dioxide; 4-hydroxy-2-methyl-N-(pyridin-2-yl)-2H-thieno[2,3-e][1,2]thiazine-3-carboxamide 1,1-dioxide
Click to Show/Hide
|
|||
| External Link | ||||
| Etanercept | Approved | [17] | ||
| Synonyms |
Etanercept (sciatica); Etanercept (sciatica), BioAssets Development; Etanercept (sciatica), Cephalon
Click to Show/Hide
|
|||
| External Link | ||||
| Meloxicam | Approved | [18] | ||
| Synonyms |
Meloxicam (SoluMatrix/arthritis); Meloxicam nanoformulation capsules (arthritis), iCeutica; Meloxicam (SoluMatrix/arthritis), iCeutica
Click to Show/Hide
|
|||
| External Link | ||||
| Gold sodium thiomalate | Approved | [6] | ||
| Synonyms |
Sodium aurothiomalate
Click to Show/Hide
|
|||
| External Link | ||||
| Vamorolone | Approved | [19] | ||
| Synonyms |
vamorolone; 13209-41-1; UNII-8XP29XMB43; VBP-15; 8XP29XMB43; CHEMBL2348780; 17,21-Dihydroxy-16alpha-methylpregna-1,4,9(11)-triene-3,20-dione; A-Methyl-9,11-dehydro Prednisolone; (8S,10S,13S,14S,16R,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13,16-trimethyl-7,8,12,14,15,16-hexahydro-6H-cyclopenta[a]phenanthren-3-one; Vamorolone [USAN]; VBP-15 free alcohol; VBP 15; EINECS 236-177-8; AC1MHYKY; Vamorolone (USAN/INN); SCHEMBL143459; GTPL9247; A)-17,21-dihydroxy-16-methylpregna-1,4,9(11)-triene-3,20-dione; ZINC33650317
Click to Show/Hide
|
|||
| External Link | ||||
| Abatacept | Approved | [20] | ||
| Synonyms |
Orencia (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Tofacitinib | Approved | [21] | ||
| Synonyms |
Tasocitinib; 477600-75-2; 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrile; CP-690550; CP 690550; racemic-tofacitinib; 1259404-17-5; tofacitinibum; CP-690,550; UNII-87LA6FU830; 3-((3R,4R)-rel-4-Methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrile; 3-{(3R,4R)-4-methyl-3-[methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]piperidin-1-yl}-3-oxopropanenitrile; Tofacitinib (CP-690550,Tasocitinib); CHEMBL221959; CHEBI:71200; Xeljanz (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| OM-89 | Approved | [6] | ||
| Synonyms |
Subreum; UroVaxom; UroVaxon; OM-8980
Click to Show/Hide
|
|||
| External Link | ||||
| Rimexolone | Approved | [22] | ||
| Synonyms |
Rimexolone (sustained release intra-articular steroid suspension, Plexis, arthritis)
Click to Show/Hide
|
|||
| External Link | ||||
| Certolizumab | Approved | [23] | ||
| Synonyms |
CDP-870; Cimzia; PHA-738144; Simziya
Click to Show/Hide
|
|||
| External Link | ||||
| Alclofenac | Approved | [6] | ||
| Synonyms |
22131-79-9; Alclophenac; Prinalgin; Medifenac; Reufenac; Allopydin; Mervan; Alclofenacum; Alclofenaco; Zumaril; Neoston; Neosten; 2-(4-(Allyloxy)-3-chlorophenyl)acetic acid; [4-(Allyloxy)-3-chlorophenyl]acetic acid; Desinflam; Marvan Forte; 3-Chloro-4-(2-propenyloxy)benzeneacetic acid; (4-Allyloxy-3-chlorphenyl)essigsaeure; MY 101; Epinal; Benzeneacetic acid, 3-chloro-4-(2-propenyloxy)-; Alclofenacum [INN-Latin]; Alclofenaco [INN-Spanish]; UNII-M9CP5H21N8; W 7320; CHEBI:31183; EINECS 244-795-4
Click to Show/Hide
|
|||
| External Link | ||||
| Careram | Approved | [24] | ||
| Synonyms |
Iguratimod
Click to Show/Hide
|
|||
| External Link | ||||
| Flurbiprofen | Approved | [25] | ||
| Synonyms |
Adfeed; Adofeed; Anmetarin; Ansaid; Anside; Antadys; Cebutid; FLP; Flubiprofen; Flugalin; Flurbiprofene; Flurbiprofeno; Flurbiprofenum; Fluriproben; Flurofen; Froben; Ocufen; Ocuflur; Stayban; Yakuban; Zepolas; FLURBIPROFEN SODIUM; Froben Sr; BTS 18322; F0371; FP 70; IN1332; U 27182; Ansaid (TN); Apo-Flurbiprofen; BTS-18322; Flurbiprofene [INN-French]; Flurbiprofeno [INN-Spanish]; Flurbiprofenum [INN-Latin]; Froben (TN); MKS-11; Novo-Flurprofen; Nu-Flurbiprofen; U 27,182; U-27182; L-790,330; Flurbiprofen (JP15/USP/INN); Flurbiprofen [USAN:INN:BAN:JAN]; [+/-]-2-Fluoro-alpha-methyl-4-biphenylacetic acid; (+-)-2-(2-Fluoro-4-biphenylyl)propionic acid; (+-)-2-Fluoro-alpha-methyl-4-biphenylacetic acid; (+/-)-2-Fluoro-alpha-methyl-4-biphenylacetic acid; (+/-)-2-Fluoro-alpha-methyl[1,1′ -biphenyl]-4-acetic Acid; (1)-2-Fluoro-alpha-methyl(1,1'-biphenyl)-4-acetic acid; 2-(2-Fluorobiphenyl-4-yl)propionic Acid; 2-(2-fluoro-[1,1'-biphenyl-4-yl])propanoic acid; 2-(2-fluorobiphenyl-4-yl)propanoic acid; 2-(3-fluoro-4-phenylphenyl)propanoic acid; 2-Fluoro-alpha-methyl-(1,1'-biphenyl)-4-acetic acid; 2-Fluoro-alpha-methyl-4-biphenylacetic acid; 3-Fluoro-4-phenylhydratropic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Nafcillin | Approved | [26] | ||
| Synonyms |
Nafcilina; Nafcilline; Nafcillinum; Nallpen; Naphcillin; Naphthamidopenicillin; Unipen; NAFCILLIN SODIUM; Nafcillin sodium for injection; Nallpen In Plastic Container; Nafcilin-1; Nafcilina [INN-Spanish]; Nafcillin & VRC3375; Nafcillin (INN); Nafcillin [INN:BAN]; Nafcilline [INN-French]; Nafcillinum [INN-Latin]; Nafcillin, Monosodium Salt, Anhydrous; (2-Ethoxy-1-naphthyl)penicillin; (2-ethoxy-1-naphthalenyl)penicillin; (2S,5R,6R)-6-({[2-(ethyloxy)naphthalen-1-yl]carbonyl}amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,5R,6R)-6-[(2-ethoxynaphthalene-1-carbonyl)amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; (2S,5R,6R)-6-{[(2-ethoxynaphthalen-1-yl)carbonyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; 6-(2-ethoxy-1-naphthamido)penicillanic acid; 6-[(2-Ethoxy-naphthalene-1-carbonyl)-amino]-3,3-dimethyl-7-oxo-4-thia-1-aza-bicyclo[3.2.0]heptane-2-carboxylic acid anion; 6beta-(2-ethoxynaphthalene-1-carboxamido)-2,2-dimethylpenam-3alpha-carboxylic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Etofenamate | Approved | [6] | ||
| Synonyms |
30544-47-9; Rheumon; Bayrogel; Rheumon gel; UNII-KZF0XM66JC; Etofenamatum [INN-Latin]; Etofenamato [INN-Spanish]; Bay d 1107; C18H18F3NO4; TVX 485; WHR 5020; EINECS 250-231-8; KZF0XM66JC; BRN 2953263; 2-(2-Hydroxyethoxy)ethyl 2-((3-(trifluoromethyl)phenyl)amino)benzoate; 2-(2-hydroxyethoxy)ethyl 2-[3-(trifluoromethyl)anilino]benzoate; WHR-5020; NCGC00016804-01; 2-(2-Hydroxyaethoxy)aethylester der flutenaminsaeure [German]; CAS-30544-47-9; 2-(2-hydroxyethoxy)ethyl 2-{[3-(trifluoromethyl)phenyl]amino}benzoate
Click to Show/Hide
|
|||
| External Link | ||||
| Etoricoxib | Approved | [27] | ||
| Synonyms |
Algix; Arcoxia; Etoricoxibe; Nucoxia; Tauxib; L791456; MK 0663; MK 663; Algix (TN); Arcoxia (TN); L-791456; MK-0663; MK-663; Merck Sharp & Dohme brand of etoricoxib; Tauxib (TN); Etoricoxib (USAN/INN); Etoricoxib [USAN:INN:BAN]; 5-Chloro-6'-methyl-3-(p-(methylsulfonyl)phenyl)-2,3'-bipyridine; 5-chloro-2-(6-methylpyridin-3-yl)-3-(4-methylsulfonylphenyl)pyridine; 5-chloro-6'-methyl-3-[4-(methylsulfonyl)phenyl]-2,3'-bipyridine; 5CH
Click to Show/Hide
|
|||
| External Link | ||||
| Golimumab | Approved | [28] | ||
| Synonyms |
Simponi (TN); Golimumab (USAN/INN)
Click to Show/Hide
|
|||
| External Link | ||||
| Rilonacept | Approved | [29] | ||
| Synonyms |
Rilonacept (USAN/INN)
Click to Show/Hide
|
|||
| External Link | ||||
| Tolmetin | Approved | [30] | ||
| Synonyms |
Tolectin; Tolmetina; Tolmetine; Tolmetino; Tolmetinum; Tolmetina [DCIT]; MCN 2559; McN-2559; Tolectin (TN); Tolmetin Sodium, Dihydrate; Tolmetine [INN-French]; Tolmetino [INN-Spanish]; Tolmetinum [INN-Latin]; Tolmetin (USAN/INN); Tolmetin [USAN:BAN:INN]; Acido 1-metil-5-(p-tolnil)-pirrol-2-acetico; Acido 1-metil-5-(p-tolnil)-pirrol-2-acetico [Spanish]; 1-Methyl-5-(4-methylbenzoyl)-1H-pyrrole-2-acetic acid; 1-Methyl-5-(4-methylbenzoyl)-pyrrole-2-acetic acid; 1-Methyl-5-p-toluoylpyrrole-2-acetic acid; 2-[1-methyl-5-(4-methylbenzoyl)pyrrol-2-yl]acetic acid; 5-(p-Toluoyl)-1-methylpyrrole-2-acetic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Denosumab | Phase 2 | [31] | ||
| Synonyms |
Denosumab (USAN); Denosumab (genetical recombination); Prolia (TN); Denosumab (genetical recombination) (JAN)
Click to Show/Hide
|
|||
| External Link | ||||
| Leflunomide | Approved | [32] | ||
| Synonyms |
Arava; Leflunomid; Leflunomida; Leflunomidum; Lefunamide; Aventis Behring Brand of Leflunomide; Aventis Brand of Leflunomide; Aventis Pharma Brand of Leflunomide; Hoechst Brand of Leflunomide; HWA 486; L 5025; SU 101; SU101; Arava (TN); Arava, Leflunomide; HWA-486; Leflunomida [INN-Spanish]; Leflunomide [USAN:INN]; Leflunomidum [INN-Latin]; Lefunomide [Inn-Spanish]; RS-34821; SU 101 (pharmaceutical); SU-101; AP-501/42475599; Leflunomide (JAN/USAN/INN); N-(4-trifluoromethyphenyl)-5-methylisoxazole-4-carboxamide; N-(4'-Trifluoromethylphenyl)-5-methylisoxazole-4-carboxamide; Alpha,alpha,alpha-Trifluoro-5-methyl-4-isoxazolecarboxy-p-toluidide; 4-Isoxazolecarboxamide, 5-methyl-N-(4-(trifluoromethyl)phenyl; 4-isoxazolecarboxamide,5-methyl-N-(4-(trifluoromethyl)phenyl); 5-Methyl-N-(4-(trifluoromethyl)phenyl)-4-isoxazolecarboxamide; 5-Methylisoxazole-4-(4-trifluoromethyl)carboxanilide; 5-Methylisoxazole-4-(4-trifluoromethylcarboxanilide); 5-Methylisoxazole-4-carboxylic acid (4-trifluoromethyl)anilide; 5-methyl-N-[4-(trifluoromethyl)phenyl]-1,2-oxazole-4-carboxamide; 5-methyl-N-[4-(trifluoromethyl)phenyl]-4-isoxazolecarboxamide; 5-methyl-N-[4-(trifluoromethyl)phenyl]isoxazole-4-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| Penicillamine | Approved | [33] | ||
| Synonyms |
D-Penicillamine; 52-67-5; Cuprimine; D-(-)-Penicillamine; 3-Mercapto-D-valine; Depen; Cuprenil; D-Penamine; (-)-Penicillamine; (2S)-2-Amino-3-methyl-3-sulfanylbutanoic acid; D-Mercaptovaline; Mercaptovaline; Perdolat; Penicillamin; Pendramine; Kuprenil; Depamine; Mercaptyl; Trolovol; Metalcaptase; Artamine; Cupripen; (S)-3,3-Dimethylcysteine; D-Valine, 3-mercapto-; Penicillaminum; Penicilamina; Sufirtan; beta-Thiovaline; Dimethylcysteine; D-beta,beta-Dimethylcysteine; D-3-Mercaptovaline; beta,beta-Dimethylcysteine; Penicillamina; Penicilllamine; Sufortan; Copper penicillaminate; D Penicillamine; Penicillamina [DCIT]; Reduced penicillamine; D 3 Mercaptovaline; TBB068824; Beta,beta Dimethylcysteine; Cuprimine (TN); D-Penicilamine; D-Penicyllamine; Depen (TN); P-1280; Penicilamina [INN-Spanish]; Penicillaminate, Copper; Penicillaminum [INN-Latin]; Reduced D-penicillamine; D,3-Mercaptovaline; D-beta-Mercaptovaline; Distamine (*Hydrochloride*); Metalcaptase (*Hydrochloride*); Penicillamine (JAN/USP/INN); Penicillamine [USAN:INN:BAN:JAN]; Alpha-Amino-beta-methyl-beta-mercaptobutyric acid; D-(-)-2-Amino-3-mercapto-3-methylbutanoic acid; (2S)-2-amino-3-methyl-3-sulfanyl-butanoic acid; (D)-PENICILLAMINE; (S)-Penicillamin; (S)-Penicillamine; 2-Amino-3-mercapto-3-methylbutanoic acid; 3,3-Dimethyl-D-cysteine; 3-Mercaptovaline; 3-sulfanyl-D-valine
Click to Show/Hide
|
|||
| External Link | ||||
| Dimethyl Sulfoxide | Approved | [6] | ||
| Synonyms |
Rimso-50
Click to Show/Hide
|
|||
| External Link | ||||
| Tocilizumab | Approved | [34] | ||
| Synonyms |
Actemra; Actemra (TN); Tocilizumab (USAN/INN); Tocilizumab (genetical recombination); Tocilizumab (genetical recombination) (JAN); humanized IgG1 monoclonal antibody
Click to Show/Hide
|
|||
| External Link | ||||
| Benorilate | Approved | [6] | ||
| Synonyms |
5003-48-5; BENORYLATE; 4-acetamidophenyl 2-acetoxybenzoate; Benoral; Salipran; Benortan; Fenasprate; Fenasparate; Aspirin acetaminophen ester; Benorilato; Benorilato [Spanish]; p-Acetamidophenyl acetylsalicylate; Benorilatum [INN-Latin]; Benorilato [INN-Spanish]; UNII-W1QX9DV96G; p-N-Acetylaminophenylacetylsalicylate; TO 125; WIN 11450; 4'-(Acetamido)phenyl-2-acetoxybenzoate; 2-Acetoxy-4'-(acetamino)phenylbenzoate; CCRIS 1739; Benzoic acid, 2-(acetyloxy)-, 4-(acetylamino)phenyl ester; 4-Acetamidophenyl salicylate acet
Click to Show/Hide
|
|||
| External Link | ||||
| Halopredone acetate | Approved | [6] | ||
| Synonyms |
Haloart (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Teriflunomide | Approved | [35] | ||
| External Link | ||||
| Upadacitinib | Approved | [36] | ||
| Synonyms |
ABT-494
Click to Show/Hide
|
|||
| External Link | ||||
| Siponimod | Phase 2 | [19] | ||
| Synonyms |
BAF312
Click to Show/Hide
|
|||
| External Link | ||||
| Oxyphenbutazone | Approved | [6] | ||
| Synonyms |
Tandearil
Click to Show/Hide
|
|||
| External Link | ||||
| FENBUFEN | Approved | [6] | ||
| Synonyms |
36330-85-5; Lederfen; Cinopal; Napanol; Bufemid; 4-(4-Biphenylyl)-4-oxobutyric acid; 3-(4-Phenylbenzoyl)propionic acid; 3-(4-Biphenylylcarbonyl)propionic acid; 4-(biphenyl-4-yl)-4-oxobutanoic acid; gamma-Oxo(1,1'-biphenyl)-4-butanoic acid; 3-(4-Biphenylcarbonyl)propionic acid; Cinopol; Fenbufenum [INN-Latin]; Fenbufene [INN-French]; 4-([1,1'-biphenyl]-4-yl)-4-oxobutanoic acid; CL-82204; CL 82204; 4-biphenyl-4-yl-4-oxobutanoic acid; Butyric acid 4-(4-biphenyl)-4-oxo-; UNII-9815R1WR9B; 4-oxo-4-(4-phenylphenyl)butanoic a
Click to Show/Hide
|
|||
| External Link | ||||
| Dexchlorpheniramine Maleate | Approved | [6] | ||
| Synonyms |
Polaramine
Click to Show/Hide
|
|||
| External Link | ||||
| Enbrel | Approved | [37] | ||
| External Link | ||||
| Fenoprofen | Approved | [38] | ||
| Synonyms |
Fenoprofene; Fenoprofeno; Fenoprofenum; Feprona; Nalfon; Nalgesic; Feneprofen calcium salt dihydrate; Fenoprofen calcium; Fenoprofen calcium hydrate; Lilly 53838; Fenoprofen Dihydrate, Calcium Salt; Fenoprofene [INN-French]; Fenoprofeno [INN-Spanish]; Fenoprofenum [INN-Latin]; Fenopron (TN); Fenoprofen (USAN/INN); Fenoprofen [USAN:BAN:INN]; Alpha-(m-phenoxyphenyl)propionic acid; Alpha-Methyl-3-phenoxybenzeneacetic acid; (+-)-2-(3-Phenoxyphenyl)propionic acid; (+-)-m-Phenoxyhydratropic acid; 2-(3-Phenoxyphenyl)propanoic acid; 2-(3-Phenoxyphenyl)propionic acid; 2-(m-phenoxyphenyl)propionic acid; 2-[3-(phenyloxy)phenyl]propanoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Celecoxib | Approved | [39] | ||
| Synonyms |
CEL; Celebra; Celebrex; Celecox; Celecoxi; Celocoxib; Eurocox; Medicoxib; Onsenal; Solexa; Xilebao; Celecoxib [Old RN]; Celecoxib [USAN]; Pfizer brand of celecoxib; SC 58635; SC58635; YM 177; YM177; AI-525; CEP-33222; Celebra (TN); Celebrex (TN); SC-58635; TPI-336; YM-177; Celebrex, Celebra, Celecoxib; Celecoxib (SC-58635); Celecoxib (JAN/USAN/INN); SC-58553, SC-58635; P-(5-p-Tolyl-3-(trifluoromethyl)pyrazol-1-yl)benzenesulfonamide; Benzenesulfonamide,4-(5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl); 4-(5-(4-Methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)benzenesulfonamide; 4-[5-(4-METHYLPHENYL)-3-(TRIFLUOROMETHYL)-1H-PYRAZOL-1-YL]BENZENESULFONAMIDE; 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1Hpyrazol-1-yl] benzenesulfonamide; 4-[5-(4-methylphenyl)-3-(trifluoromethyl)pyrazol-1-yl]benzenesulfonamide
Click to Show/Hide
|
|||
| External Link | ||||
| Sodium Succinate | Approved | [6] | ||
| Synonyms |
Disodium succinate; 150-90-3; Disodium butanedioate; Butanedioic acid, disodium salt; Succinic acid disodium salt; Soduxin; Jantaran sodny [Czech]; UNII-V8ZGC8ISR3; Succinic acid, disodium salt; FEMA No. 3277; CCRIS 3700; Sodium succinate dibasic; EINECS 205-778-7; V8ZGC8ISR3; Butanedioic acid, sodium salt (1:2); Succinic acid disodium salt anhydrous; Butanedioic acid disodium salt; sodium succinate (anhydrous); Jantaran sodny; Butanedioic acid disodium hexahydrate; Succinic acid, disodium salt, 99%, anhydrous
Click to Show/Hide
|
|||
| External Link | ||||
| Sulfasalazine | Approved | [40] | ||
| Synonyms |
599-79-1; Salicylazosulfapyridine; Salazosulfapyridine; Azulfidine; Asulfidine; Salazopyridin; Sulcolon; Azopyrin; Accucol; Colo-Pleon; Salazopiridazin; Salisulf; Reupirin; Benzosulfa; Azopyrine; Salazosulfapyridin; Sulfasalazina; w-t Sasp oral; Sulfasalazinum; Sulfasalazin; Azulfidine EN; Sulfazalazine; Azulfidine EN-tabs; Salazosulfapiridina; Sas-500; Salazosulfapyridinum; Azosulfidin; SASP; Salazo-sulfapyridinum; 5-(p-(2-Pyridylsulfamyl)phenylazo)salicylic acid; Sulfasalizine
Click to Show/Hide
|
|||
| External Link | ||||
| Anakinra | Approved | [41] | ||
| Synonyms |
Kineret; Kineret (TN); Anakinra (USAN/INN)
Click to Show/Hide
|
|||
| External Link | ||||
| Cyproheptadine | Approved | [6] | ||
| Synonyms |
Ciproheptadina; Ciprovit; Cypoheptadine; Cyproheptadiene; Cyproheptadinum; Dihexazin; Dronactin; Eiproheptadine; Periactin; Periactine; Periactinol; Viternum; Cyproheptadine Hcl; MK 141; Ciproheptadina [INN-Spanish]; Ciprovit (TN); Cyproheptadine (INN); Cyproheptadine [INN:BAN]; Cyproheptadinum [INN-Latin]; Dibenzosuberonone/Cyproheptadine; Periactin (TN); 1-Methyl-4-(5-dibenzo(a,e)cycloheptatrienylidene)piperidine; 1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)piperidine; 4-(5-Dibenzo(a,d)cyclohepten-5-ylidine)-1-methylpiperidine; 4-(5H-Dibenzo(a,d)cyclohepten-5-ylidene)-1-methylpiperidene; 4-(5H-Dibenzo(a,d)cyclohepten-5-ylidene)-1-methylpiperidine; 4-(5H-Dibenzo[a,d]cyclohepten-5-ylidene)-1-methylpiperidine; 4-(5H-dibenzo[a,d][7]annulen-5-ylidene)-1-methylpiperidine; 5-(1-Methylpiperidylidene-4)-5H-dibenzo(a,d)cyclopheptene
Click to Show/Hide
|
|||
| External Link | ||||
| Niflumic Acid | Approved | [42] | ||
| Synonyms |
Actol; Donalgin; Flunir; Forenol; Landruma; NFL; Niflactol; Niflam; Niflugel; Niflumate; Nifluril; Acide niflumique; Acide niflumique [French]; Acido niflumico; Acido niflumico [Italian]; Acidum niflumicum; Nifluminic acid; UPSA Conseil Brand of Niflumic Acid; Upsamedica Brand of Niflumic Acid; N 0630; SC 1332; UP 83; UPSA Brand 1 of Niflumic Acid; UPSA Brand 2 of Niflumic Acid; Acid, Niflumic; Acide niflumique [INN-French]; Acido niflumico [INN-Spanish]; Acidum niflumicum [INN-Latin]; Niflugel (TN); Niflumic acid (INN); Niflumic acid [INN:DCF]; Aza-2 dimethyl-2',3' (tetrazolyl-5)-6 diphenylamino; Aza-2 dimethyl-2',3' (tetrazolyl-5)-6 diphenylamino [French]; 2-(3-(Trifluoromethyl)-phenyl)aminonicotinic acid; 2-(3-(Trifluoromethyl)anilino)nicotinic acid; 2-(3-Trifluoromethyl-phenylamino)-nicotinic acid; 2-(3-Trifluoromethylanilino)nicotinic Acid; 2-(3-[Trifluoromethyl]anilino)nicotinic acid; 2-(A,A,A-Trifluoro-m-toluidino)nicotinic acid; 2-(alpha,alpha,alpha-Trifluoro-m-toluidino)nicotinic acid; 2-[(3-TRIFLUOROMETHYL)PHENYL]AMINO-3-PYRIDINE-CARBOXYLIC ACID; 2-[(3-Trifluoromethyl)phenyl]amino]-3-pyridinecarboxylic Acid; 2-[(3-Trifluoromethylphenyl)amino]nicotinic Acid; 2-[3-(Trifluoromethyl)anilino]nicotinic acid; 2-[3-(trifluoromethyl)anilino]pyridine-3-carboxylic acid; 2-[alpha,alpha,alpha-trifluoro-m-toluidino]-nicotinic acid; 2-{[3-(TRIFLUOROMETHYL)PHENYL]AMINO}NICOTINIC ACID; 2-{[3-(trifluoromethyl)phenyl]amino}pyridine-3-carboxylic acid; 39690A
Click to Show/Hide
|
|||
| External Link | ||||
| Nipocalimab | Phase 2 | [43] | ||
| External Link | ||||
| Nipocalimab? | Phase 3 | [43] | ||
| External Link | ||||
| BI 695501 | Phase 3 | [44] | ||
| Synonyms |
BI-695501)
Click to Show/Hide
|
|||
| External Link | ||||
| PF-06410293 | Phase 3 | [19] | ||
| External Link | ||||
| IB-MECA | Phase 3 | [19] | ||
| Synonyms |
152918-18-8; piclidenoson; CF-101; 3-IB-Meca; N(6)-Ibamu; CF 101; Cf101; N(6)-(3-iodobenzyl)-5'-N-methylcarboxamidoadenosine; UNII-30679UMI0N; N(6)-(3-Iodobenzyl)adenosine-5'-N-methyluronamide; 1-Deoxy-1-(6-(((3-iodophenyl)methyl)amino)-9H-purin-9-yl)-N-methyl-beta-D-ribofuranuronamide; CHEMBL119709; CHEBI:73286; 30679UMI0N; RPR-113090; 3-iodobenzyl-5'-N-methylcarboxamidoadenosine; N(6)-(3-iodo-benzyl)adenosine-5'-N-methyluronamide
Click to Show/Hide
|
|||
| External Link | ||||
| SA-237 | Phase 3 | [45] | ||
| Synonyms |
Second generation IL-6 antagonist (mAb, inflammatory disease), Chugai
Click to Show/Hide
|
|||
| External Link | ||||
| Flobufen | Phase 3 | [46] | ||
| Synonyms |
VUFB-16066; VUFB-17203
Click to Show/Hide
|
|||
| External Link | ||||
| BI-695500 | Application submitted | [19] | ||
| Synonyms |
Rituximab biosimilar
Click to Show/Hide
|
|||
| External Link | ||||
| GS-5745 | Phase 3 | [19] | ||
| External Link | ||||
| CG-100649 | Phase 3 | [47] | ||
| Synonyms |
Polmacoxib; 301692-76-2; UNII-IJ34D6YPAO; CG100649; IJ34D6YPAO; 4-(3-(3-fluorophenyl)-5,5-dimethyl-4-oxo-4,5-dihydrofuran-2-yl)benzenesulfonamide; 4-[3-(3-Fluorophenyl)-5,5-Dimethyl-4-Oxidanylidene-Furan-2-Yl]benzenesulfonamide; 4-(3-(3-Fluorophenyl)-5,5-dimethyl-4-oxo-4,5-dihydrofuran-2-yl)-benzenesulfonamide; Polmacoxib [USAN:INN]; Acelex (S. Korea); CG 100649; Polmacoxib (USAN/INN); SCHEMBL3233093; CHEMBL166863; GTPL8316; EX-A601; IJWPAFMIFNSIGD-UHFFFAOYSA-N; ZINC589683; BCP15550; AKOS025149767; SB17177; DB12399
Click to Show/Hide
|
|||
| External Link | ||||
| Resveratrol | Phase 3 | [19] | ||
| Synonyms |
Resvida; KUC104385N; R 5010; SRT 501; Cis-resveratrol; PREVENTION 8 (RESVERATROL); RM-1812; SRT-501; Trans-resveratrol; CU-01000001503-3; KSC-10-164; Resveratrol-3-sulfate; Trans-3,4',5-trihydroxystilbene; Trans-3,4′,5-Trihydroxystilbene; Trans-1,2-(3,4',5-Trihydroxydiphenyl)ethylene; (E)-5-(2-(4-hydroxyphenyl)ethenyl)-1,3-benzenediol
Click to Show/Hide
|
|||
| External Link | ||||
| GP-2017 | Phase 3 | [19] | ||
| External Link | ||||
| RAVAX | Phase 3 | [48] | ||
| External Link | ||||
| Cartistem | Phase 3 | [49] | ||
| Synonyms |
Stem cell therapy (cartilage disease/rheumatoid arthritis), Medipost
Click to Show/Hide
|
|||
| External Link | ||||
| IR-201 | Phase 3 | [50] | ||
| Synonyms |
Seryl-glutaminyl-isoleucyl-valyl-asparaginyl-aspartyl-phenylalanyl-glutaminyl-lysyl-glycyl-aspartyl-isoleucyl-alanyl-glutamyl-glycyl-tyrosyl-serine
Click to Show/Hide
|
|||
| External Link | ||||
| Esonarimod | Phase 3 | [51] | ||
| Synonyms |
Sonatimod; KE-298; KE-749; KE-758; NE-298; (R)-KE-298; (S)-KE-298
Click to Show/Hide
|
|||
| External Link | ||||
| CIPEMASTAT | Phase 3 | [52] | ||
| Synonyms |
Trocade; Ro 32-3555; 190648-49-8; UNII-02HQ4TYQ60; 02HQ4TYQ60; GFUITADOEPNRML-SJORKVTESA-N; Cipemastat [USAN:INN]; Ro 32-3555/000; Trocade (TN); Cipemastat (USAN/INN); GTPL6466; CHEMBL115653; SCHEMBL7088217; CTK8F1312; BDBM30344; MolPort-023-276-601; ZINC600699; (alphaR,betaR)-beta-(Cyclopentylmethyl)-gamma-oxo-alpha-((3,4,4-piperidinebutyrohydroxamic acid; AKOS024457333; HY-19677; Ro-32-03555; Ro-323555000; RT-015421; RO32-3555; CS-0016192; D03517; J-012306
Click to Show/Hide
|
|||
| External Link | ||||
| Methylprednisolone | Approved | [6] | ||
| External Link | ||||
| STI-002 | Phase 3 | [19] | ||
| External Link | ||||
| ABT-874 | Discontinued in Phase 2 | [23] | ||
| External Link | ||||
| MK-8808 | Phase 1 | [53] | ||
| External Link | ||||
| Sivelestat | Application submitted | [19] | ||
| Synonyms |
127373-66-4; UNII-DWI62G0P59; 2-[[2-[[4-(2,2-dimethylpropanoyloxy)phenyl]sulfonylamino]benzoyl]amino]acetic acid; Elastase Inhibitor IV; C20H22N2O7S; N-(2-(4-(2,2-Dimethylpropionyloxy)phenylsulfonylamino)benzoyl)aminoacetic acid; Sivelestat (ONO-5046); LY544349; CHEMBL76688; LY 544349; DWI62G0P59; Glycine, N-(2-(((4-(2,2-dimethyl-1-oxopropoxy)phenyl)sulfonyl)amino)benzoyl)-; N-(o-(p-Pivaloyloxybenzene)sulfonylaminobenzoyl)glycine
Click to Show/Hide
|
|||
| External Link | ||||
| ABP 798 | Phase 3 | [19] | ||
| External Link | ||||
| TG-C | Phase 3 | [54] | ||
| Synonyms |
3-Thiaglutaryl-Coa; S-[[(2-Hydroxy-2-oxoethyl)thio]acetyl]coenzyme A; (3R,5S,9R)-1-[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)tetrahydrofuran-2-yl]-3,5,9-trihydroxy-8,8-dimethyl-10,14,19-trioxo-2,4,6-trioxa-18,21-dithia-11,15-diaza-3,5-diphosphatricosan-23-oic acid 3,5-dioxide; TGC
Click to Show/Hide
|
|||
| External Link | ||||
| PF-06438179 | Phase 3 | [19] | ||
| External Link | ||||
| Baricitinib | Approved | [6] | ||
| External Link | ||||
| Cilengitide | Phase 3 | [55] | ||
| Synonyms |
188968-51-6; EMD-121974; Cilengitide [USAN:INN]; UNII-4EDF46E4GI; EMD121974; EMD-12192; EMD 121974; 4EDF46E4GI; CHEMBL429876; 2-[(2S,5R,8S,11S)-5-benzyl-11-[3-(diaminomethylideneamino)propyl]-7-methyl-3,6,9,12,15-pentaoxo-8-propan-2-yl-1,4,7,10,13-pentazacyclopentadec-2-yl]acetic acid; Cyclo(L-arginylglycyl-L-aspartyl-D-phenylalanyl-N-methyl-L-valyl); Cyclo(L-arginylglycyl-L-alpha-aspartyl-D-phenylalanyl-N-methyl-L-valyl); Cilengitide (TFA salt)
Click to Show/Hide
|
|||
| External Link | ||||
| CEP-41750 | Phase 3 | [56] | ||
| External Link | ||||
| Sarilumab | Approved | [6] | ||
| External Link | ||||
| Andolast | Phase 3 | [57] | ||
| Synonyms |
Dizolast; CR-2039; Calcium-activated potassium channel opener (inhaled powder, asthma), Rottapharm
Click to Show/Hide
|
|||
| External Link | ||||
| Vanadate | Phase 3 | [19] | ||
| Synonyms |
Ammonium metavanadate; 7803-55-6; Ammonium vanadate(V); UNII-FL85PX638G; FL85PX638G; Ammonium metavanadate, ACS reagent; Ammoniummetavanadate; Ammonium metavanadate, 99.5%, for analysis; Ammonium metavanadate, 99.996%, (trace metal basis); Ammonium meta-Vanadate; Vanadate (VO31-), ammonium (1:1); AC1LANGP; ammonium vanadiumoylolate; H4NO3V; EC 232-261-3; azanium oxido(dioxo)vanadium; KS-00000WUM; UNTBPXHCXVWYOI-UHFFFAOYSA-O; MolPort-044-723-987; MolPort-003-925-733; MFCD00011430; AKOS030228605; AMMONIUM VANADATE (META), NH4VO3
Click to Show/Hide
|
|||
| External Link | ||||
| CPL-7075 | Phase 3 | [19] | ||
| Synonyms |
Ajulemic acid; Lenabasum; IP-751; CT-3; 137945-48-3; IP 751; UNII-OGN7X90BT8; CPL7075; JBT-101; OGN7X90BT8; Resunab; (6aR,10aR)-1-hydroxy-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,10,10a-tetrahydrobenzo[c]chromene-9-carboxylic acid; (6ar,10ar)-3-(1,1-Dimethylheptyl)-1-Hydroxy-6,6-Dimethyl-6a,7,10,10a-Tetrahydro-6h-Benzo[c]chromene-9-Carboxylic Acid; DMH-THC-11-OIC; AB-III-56; CT 3; CPL 7075; Lenabasum [USAN]; HU-239; AC1MJ0SY; SCHEMBL26441; CHEMBL456341; GTPL9772; IP751; Mixed CB agonist/sodim channel blocker (pain), Cervelo
Click to Show/Hide
|
|||
| External Link | ||||
| Spermidine | Phase 3 | [19] | ||
| Synonyms |
Spermidin; UNII-U87FK77H25; BRN 1698591; AI3-26636; EINECS 204-689-0; CHEMBL19612; CHEBI:16610; ATHGHQPFGPMSJY-UHFFFAOYSA-N; U87FK77H25; MFCD00008229; Spermidine hydrochloride; NSC528399; 1pot; Aminopropylbutandiamine; N-(4-Aminobutyl)-1,3-diaminopropane; Spectrum_000005; Tocris-0959; ACMC-20ajn3; AC1L1AQB; Spectrum2_000874; Spectrum3_000977; Spectrum4_001101; Spectrum5_001561; Lopac-S-2501; Biomol-NT_000212; bmse000116; bmse000951; bmse000955; Spermidine 0.1 M solution; Lopac0_001047; SCHEMBL15618; BSPBio_002613; KBioGR_001542; KBioSS_000345; 4-04-00-01300 (Beilstein Handbook Reference); DivK1c_001007; SPBio_000947; Spermidine, > =99% (GC); Spermidine, analytical standard; BPBio1_001276; GTPL2390; DTXSID4036645; CTK3J1693; KBio1_001007; KBio2_000345; KBio2_002913; KBio2_005481; KBio3_001833; MolPort-001-761-230; NINDS_001007; HY-B1776; ZINC1532612; BDBM50009353; PA(34); N-(3-Aminopropyl)-4-aminobutylamine; AKOS006222987; CCG-205124; DB03566; MCULE-8096530192; RTR-003757; SDCCGMLS-0066822.P001; IDI1_001007; NCGC00015937-01; NCGC00015937-02; NCGC00015937-03; NCGC00015937-04; NCGC00015937-05; NCGC00024903-01; NCGC00024903-02; NCGC00024903-03; AJ-26792; AN-22947; LS-45643; M923; NCI60_004294; SC-69371; DB-026892; TR-003757; CS-0013804; FT-0629162; ST24048721; ST45025991; C00315; 124S209; SR0
Click to Show/Hide
|
|||
| External Link | ||||
| Varespladib methyl | Phase 3 | [58] | ||
| Synonyms |
A-002; LY-333013; S-3013; Varespladib methyl (oral formulation, coronary artery disease); PLA2 inhibitors (inflammation), Lilly/Shionogi; Varespladib (oral formulation, coronary artery disease), Anthera; Varespladib methyl (oral formulation, coronary artery disease), Anthera
Click to Show/Hide
|
|||
| External Link | ||||
| GP2013 | Phase 3 | [19] | ||
| External Link | ||||
| ABP 501 | Phase 3 | [59] | ||
| External Link | ||||
| ASP-015K | Phase 3 | [19] | ||
| Synonyms |
Peficitinib; ASP015K; UNII-HPH1166CKX; 944118-01-8; HPH1166CKX; 4-[[(1R,3S)-5-hydroxy-2-adamantyl]amino]-1H-pyrrolo[2,3-b]pyridine-5-carboxamide; 4-[[(1S,3R)-5-oxidanyl-2-adamantyl]amino]-1H-pyrrolo[2,3-b]pyridine-5-carboxamide; Peficitinib [USAN:INN]; ASP 015K; JNJ-54781532; 9T6; Peficitinib (USAN/INN); SCHEMBL1154421; SCHEMBL9990248; SCHEMBL4447032; GTPL8315; SCHEMBL9990240; SCHEMBL1154418; CHEMBL3137308; SCHEMBL17645135; BCP18465; BDBM50124208; SB16834; DB11708; SC-17960; D10653; Peficitinib pound A
Click to Show/Hide
|
|||
| External Link | ||||
| Anakinra | Approved | [60] | ||
| External Link | ||||
| CHS-0214 | Phase 3 | [19] | ||
| External Link | ||||
| Atacicept | Phase 2 | [61] | ||
| External Link | ||||
| Tenidap | Phase 3 | [62] | ||
| External Link | ||||
| Sirukumab | Phase 3 | [63] | ||
| External Link | ||||
| Olokizumab | Phase 3 | [19] | ||
| External Link | ||||
| LY3090106 | Phase 1 | [64] | ||
| Synonyms |
Tibulizumab
Click to Show/Hide
|
|||
| External Link | ||||
| VX-509 | Phase 2/3 | [65] | ||
| Synonyms |
Decernotinib; Adelatinib; 944842-54-0; UNII-MZK2GP0RHK; Decernotinib(VX-509); VX509; MZK2GP0RHK; VRT-831509; VX 509; (2r)-2-Methyl-2-[[2-(1h-Pyrrolo[2,3-B]pyridin-3-Yl)pyrimidin-4-Yl]amino]-N-[2,2,2-Tris(Fluoranyl)ethyl]butanamide; (R)-2-((2-(1H-Pyrrolo[2,3-b]pyridin-3-yl)pyrimidin-4-yl)amino)-2-methyl-N-(2,2,2-trifluoroethyl)butanamide; Decernotinib [USAN:INN]; VRT 831509; Decernotinib,VX-509; Decernotinib (USAN/INN); Decernotinib (VX-509); VX-509 (Decernotinib); SCHEMBL2630387; GTPL8309; CHEMBL3039513; KS-00001CYK
Click to Show/Hide
|
|||
| External Link | ||||
| Olokizumab | Phase 3 | [66] | ||
| External Link | ||||
| CreaVax-RA | Phase 2a | [67] | ||
| Synonyms |
Autologous DC therapy (injectable, rheumatoid arthritis), CreaGene; Autologous dendritic cell therapy (injectable, rheumatoid arthritis), CreaGene
Click to Show/Hide
|
|||
| External Link | ||||
| RhuDex | Phase 2a | [61] | ||
| External Link | ||||
| VX-702 | Phase 2a | [68] | ||
| Synonyms |
ST51054128; I14-1965; EC-000.2363; 6-[carbamoyl-(2,6-difluorophenyl)amino]-2-(2,4-difluorophenyl)pyridine-3-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| Cevidoplenib | Phase 2 | [69] | ||
| Synonyms |
(S)-cyclopropyl(5-(4-(4-((4-hydroxyisoxazolidin-2-yl)methyl)-3-methyl-1H-pyrazol-1-yl)pyrimidin-2-ylamino)-1-methyl-1H-indol-3-yl)methanone; 1703788-21-9; 3N3H8BX897; AKOS040748110; BDBM196772; Cevidoplenib; Cevidoplenib [INN]; Cevidoplenib [WHO-DD]; CHEMBL3921923; CS-0039259; cyclopropyl-[5-[[4-[4-[[(4S)-4-hydroxy-1,2-oxazolidin-2-yl]methyl]-3-methylpyrazol-1-yl]pyrimidin-2-yl]amino]-1-methylindol-3-yl]methanone; EX-A5914; HY-109082; Methanone, cyclopropyl(5-((4-(4-(((4S)-4-hydroxy-2-isoxazolidinyl)methyl)-3-methyl-1H-pyrazol-1-yl)-2-pyrimidinyl)amino)-1-methyl-1H-indol-3-yl)-; MS-28778; SCHEMBL16653204; UNII-3N3H8BX897; US9212178, 1; YCZUBLQESBVOSH-IBGZPJMESA-N
Click to Show/Hide
|
|||
| External Link | ||||
| Zunsemetinib | Phase 2 | [70] | ||
| Synonyms |
(-)-3-Chloro-4-[(3,5-difluoro-2-pyridinyl)methoxy]-2'-[2-(1-hydroxy-1-methylethyl)-4-pyrimidinyl]-5',6-dimethyl[1(2H),4'-bipyridin]-2-one; (1(2H),4'-Bipyridin)-2-one, 3-chloro-4-((3,5-difluoro-2-pyridinyl)methoxy)-2'-(2-(1-hydroxy-1-methylethyl)-4-pyrimidinyl)-5',6-dimethyl-, (-)-; (2'S)-3-Chloro-4-[(3,5-difluoro-2-pyridinyl)methoxy]-2'-[2-(1-hydroxy-1-methylethyl)-4-pyrimidinyl]-5',6-dimethyl[1(2H),4'-bipyridin]-2-one; (P)-(3-chloro-4-((3,5-difluoropyridin-2-yl)methoxy)-2'-(2-(2-hydroxypropan-2-yl)pyrimidin-4-yl)-5',6-dimethyl-2H-[1,4'-bipyridin]-2-one); (p)-3-Chloro-4-((3,5-difluoropyridin-2-yl)methoxy)-2'-(2-(2-hydroxypropan-2-yl)pyrimidin-4-yl)-5',6-dimethyl-2H-(1,4'-bipyridin)-2-one; (R)-Zunsemetinib; [1(2H),4'-Bipyridin]-2-one, 3-chloro-4-[(3,5-difluoro-2-pyridinyl)methoxy]-2'-[2-(1-hydroxy-1-methylethyl)-4-pyrimidinyl]-5',6-dimethyl-, (2'S)-; 1639791-42-6; 1640282-42-3; 1640282-44-5; 3-Chloro-4-((3,5-difluoropyridin-2-yl)methoxy)-2'-(2-(2-hydroxypropan-2-yl)pyrimidin-4-yl)-5',6-dimethyl-2H-[1,4'-bipyridin]-2-one; 3-chloro-4-[(3,5-difluoropyridin-2-yl)methoxy]-1-[2-[2-(2-hydroxypropan-2-yl)pyrimidin-4-yl]-5-methylpyridin-4-yl]-6-methylpyridin-2-one; 3-chloro-4-[(3,5-difluoropyridin-2-yl)methoxy]-1-{2-[2-(2-hydroxypropan-2-yl)pyrimidin-4-yl]-5-methylpyridin-4-yl}-6-methylpyridin-2-one; AKOS040756965; Ati 450; ATI 450 [WHO-DD]; ATI450; ATI-450; AX2VWG0ZCR; BDBM175242; CDD450; CDD-450; CHEMBL3704901; CS-0204147; CS-0374185; EX-A6292; GTPL11681; HY-139553; HY-139553A; MS-29543; SCHEMBL16279876; UNII-AX2VWG0ZCR; US9115089, 49; WHO 11983; zunsemetinib; Zunsemetinib [INN]; Zunsemetinib [USAN:INN]; Zunsemetinib [USAN]; Zunsemetinib M-atropisomer
Click to Show/Hide
|
|||
| External Link | ||||
| Dazodalibep | Phase 2 | [71] | ||
| Synonyms |
VIB4920
Click to Show/Hide
|
|||
| External Link | ||||
| Natrunix | Phase 2 | [72] | ||
| External Link | ||||
| A223 | Phase 2 | [73] | ||
| External Link | ||||
| GS-5718 | Phase 2 | [74] | ||
| Synonyms |
1-BOC-3-IODO-1H-PYRAZOLO[3,4-B]PYRIDINE; 1H-PYRAZOLO[3,4-B]PYRIDINE-1-CARBOXYLIC ACID, 3-IODO-, 1,1-DIMETHYLETHYLESTER; 1H-Pyrazolo[3,4-b]pyridine-1-carboxylicacid,3-iodo-,1,1-diMethylethylester; 920036-34-6; A860172; AKOS022178093; AMY21637; CS-0048281; FT-0702852; GS-5718; MFCD13183727; OKKLWSNHFSFXEM-UHFFFAOYSA-N; PB26872; SCHEMBL3606964; tert-Butyl 3-iodo-1H-pyrazolo[3,4-b]pyridine-1-carboxylate; tert-butyl 3-iodopyrazolo[3,4-b]pyridine-1-carboxylate; tert-Butyl3-iodo-1H-pyrazolo[3,4-b]pyridine-1-carboxylate
Click to Show/Hide
|
|||
| External Link | ||||
| Branebrutinib | Phase 2 | [75] | ||
| Synonyms |
(S)-4-(3-(2-BUTYNOYLAMINO)PIPERIDIN-1-YL)-5-FLUORO-2,3-DIMETHYL-1H-INDOLE-7-CARBOXAMIDE; (S)-4-(3-(but-2-ynamido)piperidin-1-yl)-5-fluoro-2,3-dimethyl-1H-indole-7-carboxamide; 1912445-55-6; 1H-Indole-7-carboxamide, 5-fluoro-2,3-dimethyl-4-((3S)-3-((1-oxo-2-butyn-1-yl)amino)-1-piperidinyl)-; 4-((3S)-3-(2-BUTYNOYLAMINO)-1-PIPERIDINYL)-5-FLUORO-2,3-DIMETHYL-1HINDOLE-7-CARBOXAMIDE; 4-((3S)-3-(2-Butynoylamino)-1-piperidinyl)-5-fluoro-2,3-dimethyl-1H-indole-7-carboxamide; 4-((3S)-3-(But-2-ynamido)piperidin-1-yl)-5-fluoro-2,3-dimethyl-1H-indole-7-carboxamide; 4-[(3S)-3-(but-2-ynamido)piperidin-1-yl]-5-fluoro-2,3-dimethyl-1H-indole-7-carboxamide; 4-[(3S)-3-(but-2-ynoylamino)piperidin-1-yl]-5-fluoro-2,3-dimethyl-1H-indole-7-carboxamide; 7LBRZUYSHU; AC-31535; AKOS037649047; BCP29496; BDBM164638; BDBM166759; BMS986195; BMS986195; BMS986195; BMS-986195; Branebrutinib; Branebrutinib (BMS-986195); Branebrutinib (USAN); BRANEBRUTINIB [INN]; Branebrutinib [USAN]; BRANEBRUTINIB [WHO-DD]; BS-16393; C20H23FN4O2; CHEMBL4297674; CS-0043577; D11478; DB15347; EN300-2007801; EX-A2720; Example 223 [US20160115126A1]; GTPL9869; HY-112161; MFCD31631584; NSC807627; NSC-807627; Q50825082; s8832; SCHEMBL17699728; UNII-7LBRZUYSHU; US9688629, 123; US9688629, 223; VJPPLCNBDLZIFG-ZDUSSCGKSA-N; WHO 11026
Click to Show/Hide
|
|||
| External Link | ||||
| EQ121 | Phase 2 | [76] | ||
| External Link | ||||
| Vobarilizumab | Phase 2 | [77] | ||
| Synonyms |
ALX-0061
Click to Show/Hide
|
|||
| External Link | ||||
| IRL201805 | Phase 2 | [78] | ||
| External Link | ||||
| KPL-404 | Phase 2 | [79] | ||
| External Link | ||||
| ABBV-154 | Phase 2 | [80] | ||
| External Link | ||||
| FK-3311 | Phase 2 | [81] | ||
| Synonyms |
116686-15-8; FK 3311; FK3311; COX-2 Inhibitor V, FK3311; N-[4-acetyl-2-(2,4-difluorophenoxy)phenyl]methanesulfonamide; N-(4-Acetyl-2-(2,4-difluorophenoxy)phenyl)methanesulfonamide; 4'-Acetyl-2'-(2,4-difluorophenoxy)methanesulfonanilide; Methanesulfonamide, N-[4-acetyl-2-(2,4-difluorophenoxy)phenyl]-; C15H13F2NO4S; Methanesulfonamide, N-(4-acetyl-2-(2,4-difluorophenoxy)phenyl)-; DIIYLGZNZGPXRR-UHFFFAOYSA-N; AC1L4U0O; AC1Q6W4K; SCHEMBL441676; ZINC3880; DIIYLGZNZGPXRR-UHFFFAOYSA-; CTK8E9207; EX-A545; DTXSID90151474
Click to Show/Hide
|
|||
| External Link | ||||
| TAK-783 | Phase 2 | [82] | ||
| Synonyms |
Rheumatoid arthritis therapy (oral), Takeda Pharmaceutical
Click to Show/Hide
|
|||
| External Link | ||||
| Piclamilast | Phase 2 | [83] | ||
| Synonyms |
144035-83-6; Cpodpmb; RP 73401; 3-(Cyclopentyloxy)-N-(3,5-dichloropyridin-4-yl)-4-methoxybenzamide; UNII-WM58D7C3ZT; RP 73-401; RP-73401; RP-73-401; RPR 73401; 3-(Cyclopentyloxy)-N-(3,5-dichloro-4-pyridinyl)-4-methoxybenzamide; WM58D7C3ZT; Benzamide, 3-(cyclopentyloxy)-N-(3,5-dichloro-4-pyridinyl)-4-methoxy-; CHEMBL42126; CHEBI:47619; RRRUXBQSQLKHEL-UHFFFAOYSA-N; RPR-73401; 3-(Cyclopentyloxy)-N-(3,5-dichloro-4-pyridyl)-p-anisamide; 3-(Cyclopentyloxy)-N-(3,5-dichloro-4-pyridyl)-4-methoxybenzamide; PIL
Click to Show/Hide
|
|||
| External Link | ||||
| ADL-5859 | Phase 2 | [84] | ||
| Synonyms |
PF-04856880
Click to Show/Hide
|
|||
| External Link | ||||
| SBI-087 | Phase 2 | [85] | ||
| Synonyms |
PF-05230895
Click to Show/Hide
|
|||
| External Link | ||||
| SB-705498 | Phase 2 | [86] | ||
| Synonyms |
501951-42-4; SB705498; SB 705498; UNII-T74V9O0Y2W; (R)-1-(2-bromophenyl)-3-(1-(5-(trifluoromethyl)pyridin-2-yl)pyrrolidin-3-yl)urea; T74V9O0Y2W; 1-(2-bromophenyl)-3-[(3R)-1-[5-(trifluoromethyl)pyridin-2-yl]pyrrolidin-3-yl]urea; N-(2-Bromophenyl)-N'-[(3R)-1-[5-(trifluoromethyl)-2-pyridinyl]-3-pyrrolidinyl]urea; 1-(2-Bromophenyl)-3-[(3R)-1-[5-(trifluoromethyl)-2-pyridyl]pyrrolidin-3-yl]urea; JYILLRHXRVTRSH-GFCCVEGCSA-N; MLS006011113; SCHEMBL1350298; GTPL4311; CHEMBL207433; BMCL163287 Compound 15; BDBM20504
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-817399 | Phase 2 | [87] | ||
| External Link | ||||
| PRALNACASAN | Phase 2 | [88] | ||
| Synonyms |
VX-740; UNII-N986NI319S; 192755-52-5; N986NI319S; HMR3480/VX-740; Pralnacasan [USAN:INN]; HMR 3480; VX 470; Pralnacasan (USAN/INN); AC1L4A1A; SCHEMBL142187; GTPL6467; CHEMBL437526; DTXSID60172873; HMR3480; HMR-3480; BDBM50189360; AKOS030230853; DB04875; D08978; (4S,7S)-N-[(2R,3S)-2-ethoxy-5-oxooxolan-3-yl]-7-(isoquinoline-1-carbonylamino)-6,10-dioxo-2,3,4,7,8,9-hexahydro-1H-pyridazino[1,2-a]diazepine-4-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| F-8-IL-10 fusion protein | Phase 2 | [19] | ||
| Synonyms |
Dekavil; F-8-IL-10; F-8-IL-10 fusion protein (rheumatoid arthritis/endometriosis); F-8-IL-10 fusion protein (rheumatoid arthritis/endometriosis), Philogen
Click to Show/Hide
|
|||
| External Link | ||||
| GLPG-0634 | Phase 2 | [89] | ||
| Synonyms |
Small molecule, JAK1/JAK2 inhibitor (rheumatoid arthritis), Galapagos/GSK
Click to Show/Hide
|
|||
| External Link | ||||
| CFZ533 | Phase 2 | [90] | ||
| External Link | ||||
| PD-360324 | Phase 2 | [91] | ||
| Synonyms |
PD-0360324; MCSF mAb (RA), Pfizer; Macrophage colony stimulating factor monoclonal antibody (rheumatoid arthritis), Pfizer
Click to Show/Hide
|
|||
| External Link | ||||
| MGCD-290 | Phase 2 | [92] | ||
| Synonyms |
MG-1; MG-3290; Histone deacetylase inhibitors (cardiovascular diseases), MethylGene; Histone deacetylase inhibitors (diabetes), MethylGene; Histone deacetylase inhibitors (fungal infection), MethylGene; Histone deacetylase inhibitors (inflammation), MethylGene; HDAC inhibitors (non-oncology), MethylGene; Histone deacetylase inhibitors (non-oncology), MethylGene
Click to Show/Hide
|
|||
| External Link | ||||
| Prednisone/ dipyridamole | Phase 2 | [93] | ||
| Synonyms |
Synavive (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| QAL964 | Phase 2 | [94] | ||
| External Link | ||||
| M2951 | Phase 2 | [19] | ||
| Synonyms |
evobrutinib; UNII-ZA45457L1K; ZA45457L1K; 1415823-73-2; 1-(4-(((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)amino)methyl)piperidin-1-yl)prop-2-en-1-one; 1-[4-[[[6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl]amino]methyl]piperidin-1-yl]prop-2-en-1-one; Evobrutinib [INN]; GTPL9752; SCHEMBL14165673; QUIWHXQETADMGN-UHFFFAOYSA-N; MSC2364447C; MSC-2364447C; ZINC205623965; AKOS032954004; CS-6303; MSC 2364447; HY-101215; A250 [WO2012170976]; N-[(1-acryloylpiperidin-4-yl)methyl]-5-(4-phenoxyphenyl)pyrimidine-4,6-diamine; 1-(4-((6-Amin
Click to Show/Hide
|
|||
| External Link | ||||
| AK-106-001616 | Phase 2 | [95] | ||
| External Link | ||||
| ABT-122 | Phase 2 | [96] | ||
| External Link | ||||
| ETIPREDNOL DICLOACETATE | Phase 2 | [97] | ||
| Synonyms |
BNP-166; Cronaze (Ivax); Ethinase (Ivax); Etiprednol dicloacetate < USAN; Respicort (Ivax); (11beta,17alpha)-17-(2,2-Dichloroacetoxy)-11-hydroxy-3-oxoandrosta-1,4-diene-17-carboxylic acid ethyl ester
Click to Show/Hide
|
|||
| External Link | ||||
| Meninge ACYW | Phase 2 | [98] | ||
| External Link | ||||
| TAK-715 | Phase 2 | [99] | ||
| Synonyms |
P38 MAP kinase inhibitor (rheumatoid arthritis), Takeda
Click to Show/Hide
|
|||
| External Link | ||||
| ALD-518 | Phase 2 | [100] | ||
| External Link | ||||
| MPC-300-IV | Phase 2 | [19] | ||
| External Link | ||||
| GSK2982772 | Phase 1 | [19] | ||
| Synonyms |
LYPAFUINURXJSG-AWEZNQCLSA-N; 1622848-92-3; UNII-T5W3M0VO9B; T5W3M0VO9B; GSK-2982772; (S)-5-benzyl-N-(5-methyl-4-oxo-2,3,4,5-tetrahydrobenzo[b][1,4]oxazepin-3-yl)-1H-1,2,4-triazole-3-carboxamide; GTPL9554; SCHEMBL15956219; MolPort-044-830-634; s8484; AKOS030528033; compound 5 [PMID: 28151659]; ACN-041458; CS-6899; GSK 2982772; AS-35128; AC-29894; HY-101760; J3.650.802G; 5-benzyl-N-[(3S)-5-methyl-4-oxo-2,3-dihydro-1,5-benzoxazepin-3-yl]-1H-1,2,4-triazole-3-carboxamide; 3-Benzyl-N-[(3s)-5-Methyl-4-Oxo-2,3,4,5-Tetrahydr
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-LT alpha | Phase 2 | [101] | ||
| External Link | ||||
| NNC-0151-0000-0000 | Phase 2 | [102] | ||
| Synonyms |
Neutrazumab; Anti-C5aR monoclonal antibodies (rheumatoid arthritis/SLE), G2/Novo Nordisk
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-945429 | Phase 2 | [103] | ||
| External Link | ||||
| ONO-4641 | Phase 2 | [104] | ||
| Synonyms |
Sphingosine-1-phosphate agonist (tablet, mutiple sclerosis), Ono
Click to Show/Hide
|
|||
| External Link | ||||
| IMO-8400 | Phase 2 | [19] | ||
| Synonyms |
Bazlitoran; Bazlitoran [INN]; Bazlitoran [USAN]; UNII-2U46M95B5M; 2U46M95B5M
Click to Show/Hide
|
|||
| External Link | ||||
| CNT0-1959 | Phase 2 | [105] | ||
| External Link | ||||
| Apratastat | Phase 2 | [106] | ||
| Synonyms |
TMI-005; TMI-05; UNII-C6BZ5263BJ; 287405-51-0; C6BZ5263BJ; CHEMBL206815; TMI 005; Apratastat [USAN:INN]; Apratastat (USAN/INN); MLS006010301; SCHEMBL2834310; GTPL6482; TMI005; Apratastat, > MolPort-021-805-014; BCPP000041; ZINC28571311; BDBM50181008; DB13020; API0013699; compound 5h [PMID: 16426848]; SMR004701369; 4CA-0170; D08859; 3-Thiomorpholinecarboxamide,N-hydroxy-4-[[4-[(4-hydroxy-2-butyn-1-yl)oxy]phenyl]sulfonyl]-2,2-dimethyl-,(3S)-; TMI-1; Dual TACE/MMP-13 inhibitors (inflammation), Wyeth; Dual TACE/MMP-13 inhibitors (rheumatoid arthritis), Wyeth; Dual TACE/MMP-13 inhibitors, Wyeth-Ayerst
Click to Show/Hide
|
|||
| External Link | ||||
| FLUASTERONE | Phase 2 | [107] | ||
| Synonyms |
HE-2500; 16alpha-Fluoroandrost-5-en-17-one
Click to Show/Hide
|
|||
| External Link | ||||
| GS-4059 | Phase 2 | [19] | ||
| Synonyms |
tirabrutinib; 1351636-18-4; UNII-LXG44NDL2T; LXG44NDL2T; Tirabrutinib [INN]; ONO-4059(Free base); SCHEMBL14798454; MolPort-044-728-902; BDBM194087; ZINC72318699; AKOS030526437; CS-5676; HY-15771; US9199997, 9; F10085; (R)-6-amino-9-(1-(but-2-ynoyl)pyrrolidin-3-yl)-7-(4-phenoxyphenyl)-7H-purin-8(9H)-one; 6-Amino-9-((3R)-1-(2-butynoyl)-3-pyrrolidinyl)-7-(4-phenoxyphenyl)-7,9-dihydro-8H-purin-8-one; 8H-Purin-8-one,6-amino-7,9-dihydro-9-((3R)-1-(1-oxo-2-butyn-1-yl)-3-pyrrolidinyl)-7-(4-phenoxyphenyl); 6-Amino-9-[(3R)-1-(2-butyno
Click to Show/Hide
|
|||
| External Link | ||||
| VVZ-149 | Phase 2 | [108] | ||
| Synonyms |
VVZ-000149
Click to Show/Hide
|
|||
| External Link | ||||
| MRC-375 | Phase 2 | [109] | ||
| Synonyms |
Tetracycline (enteric-coated oral tablet, rheumatoid arthritis), Molecular Research Center Inc
Click to Show/Hide
|
|||
| External Link | ||||
| AWD-12-281 | Phase 2 | [110] | ||
| Synonyms |
GW-842470; AWD-12-343; AWD-12-281 (COPD), elbion/GlaxoSmithKline; AWD-12-281 (allergic rhinitis), elbion/GlaxoSmithKline; AWD-12-281 (asthma), elbion/GlaxoSmithKline; AWD-12-281 (inhaled), elbion/GlaxoSmithKline; AWD-12-281 (intranasal), elbion/GlaxoSmithKline
Click to Show/Hide
|
|||
| External Link | ||||
| APX-001 | Investigative | [111] | ||
| Synonyms |
EPI-0010; SSS-07; Anti-TNF alpha recombinant humanized monoclonal antibody (RabMab/MLG, rheumatoid arthritis), 3SBio/Apexigen; Anti-TNF alpha recombinant humanized monoclonal antibody (RabMab/MLG, rheumatoid arthritis), 3SBio/Epitomics
Click to Show/Hide
|
|||
| External Link | ||||
| SR-31747 | Phase 2 | [112] | ||
| Synonyms |
SR-317417A
Click to Show/Hide
|
|||
| External Link | ||||
| STA-5326 | Phase 2 | [113] | ||
| Synonyms |
Apilimod; STA 5326; STA5326; N-[(3-methylphenyl)methylideneamino]-6-morpholin-4-yl-2-(2-pyridin-2-ylethoxy)pyrimidin-4-amine
Click to Show/Hide
|
|||
| External Link | ||||
| BI 655064 | Phase 2 | [114] | ||
| External Link | ||||
| GSK3196165 | Phase 2 | [19] | ||
| External Link | ||||
| GSK315234 | Phase 2 | [115] | ||
| Synonyms |
Oncostatin M mAb
Click to Show/Hide
|
|||
| External Link | ||||
| GS-9876 | Phase 1 | [19] | ||
| Synonyms |
XCIGZBVOUQVIPI-UHFFFAOYSA-N; lanraplenib; UNII-A6U64OU57E; A6U64OU57E; 6-(6-aminopyrazin-2-yl)-N-[4-[4-(oxetan-3-yl)piperazin-1-yl]phenyl]imidazo[1,2-a]pyrazin-8-amine; 6-(6-Aminopyrazin-2-yl)-N-(4-(4-(oxetan-3-yl)piperazin-1-yl)phenyl)imidazo[1,2-a]pyrazin-8-amine; Lanraplenib [INN]; GTPL9764; SCHEMBL16820581; CHEMBL3986824; example 2 [US9290505]; BDBM212271; GS9876; US9290505, Ex.-2; Ex.-2, US9290505; Imidazo(1,2-a)pyrazin-8-amine, 6-(6-amino-2-pyrazinyl)-N-(4-(4-(3-oxetanyl)-1-piperazinyl)phenyl)-; 6-(6-Aminopyr
Click to Show/Hide
|
|||
| External Link | ||||
| TZI-41078 | Phase 2 | [116] | ||
| Synonyms |
3,5-Di-tert-butyl-4-hydroxybenzophenone oxime
Click to Show/Hide
|
|||
| External Link | ||||
| SAN-300 | Phase 2 | [117] | ||
| External Link | ||||
| CNTO-6785 | Phase 2 | [118] | ||
| External Link | ||||
| AMG-714 | Discontinued in Phase 1 | [23] | ||
| External Link | ||||
| LX2931 | Phase 2 | [119] | ||
| External Link | ||||
| VAY736 | Phase 2 | [19] | ||
| External Link | ||||
| KB002/003 | Phase 2 | [120] | ||
| External Link | ||||
| SSR-180575 | Phase 2 | [121] | ||
| Synonyms |
2-(7-Chloro-5-methyl-4-oxo-3-phenyl-4,5-dihydro-3H-pyridazino[4,5-b]indol-1-yl)-N,N-dimethylacetamide
Click to Show/Hide
|
|||
| External Link | ||||
| Rabeximod | Phase 2 | [122] | ||
| Synonyms |
Rob-803; B-220 analog (RA), OxyPharma; B-220 analog (rheumatoid arthritis), OxyPharma
Click to Show/Hide
|
|||
| External Link | ||||
| BIIB 023 | Phase 2 | [123] | ||
| External Link | ||||
| Baminercept | Phase 2 | [124] | ||
| Synonyms |
BG-9924; Soluble lymphotoxin beta receptor, Biogen; Soluble lymphotoxin beta receptor, Biogen Idec
Click to Show/Hide
|
|||
| External Link | ||||
| PF-06823859 | Phase 2 | [125] | ||
| External Link | ||||
| MK-8457 | Phase 2 | [126] | ||
| External Link | ||||
| CDP323 | Phase 2 | [23] | ||
| Synonyms |
Zaurategrast; 455264-31-0; (S)-3-(4-((2,7-Naphthyridin-1-yl)amino)phenyl)-2-((2-bromo-3-oxospiro[3.5]non-1-en-1-yl)amino)propanoic acid; UNII-06A0IC74I3; 06A0IC74I3; C26H25BrN4O3; Zaurategrast [INN]; SCHEMBL2976322; CTK8C0588; DTXSID90196547; MolPort-023-332-826; KS-00001DY0; ANW-64932; 6274AB; ZINC100041912; AKOS016005046; CS-0322; N-(2-Bromo-3-oxospiro[3.5]non-1-en-1-yl)-4-(2,7-naphthyridin-1-ylamino)-L-phenylalanine; NCGC00378753-01
Click to Show/Hide
|
|||
| External Link | ||||
| Tofacitinib | Approved | [127] | ||
| External Link | ||||
| BMS-986142 | Phase 2 | [19] | ||
| Synonyms |
Unii-pjx9GH268R; PJX9GH268R; CHEMBL3900554; 1643368-58-4; GTPL9857; SCHEMBL16319712; BMS986142; BDBM50194720; AKOS032954006; compound 14f [PMID: 27583770]; J3.563.199B; 73T; 6-Fluoro-5-(R)-(3-(S)-(8-Fluoro-1-Methyl-2,4-Dioxo-1,2-Dihydroquinazolin-3(4h)-Yl)-2-Methylphenyl)-2-(S)-(2-Hydroxypropan-2-Yl)-2,3,4,9-Tetrahydro-1h-Carbazole-8-Carboxamide; (7S)-3-fluoro-4-[3-(8-fluoro-1-methyl-2,4-dioxoquinazolin-3-yl)-2-methylphenyl]-7-(2-hydroxypropan-2-yl)-6,7,8,9-tetrahydro-5H-carbazole-1-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| NN8765 | Phase 2 | [128] | ||
| External Link | ||||
| CDP-6038 | Phase 2 | [100] | ||
| External Link | ||||
| DE-098 | Phase 2 | [129] | ||
| Synonyms |
ARG-098; Anti-Fas antibody, Santen; Anti-APO-1 antibody, Santen
Click to Show/Hide
|
|||
| External Link | ||||
| AMG0714 | Phase 2 | [130] | ||
| Synonyms |
HuMax-IL15
Click to Show/Hide
|
|||
| External Link | ||||
| Fosbretabulin | Phase 2 | [131] | ||
| Synonyms |
Zybrestat (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Rimacalib | Phase 2 | [132] | ||
| Synonyms |
SMP-114
Click to Show/Hide
|
|||
| External Link | ||||
| Mavrilimumab | Phase 2 | [133] | ||
| External Link | ||||
| NN8555 | Phase 2 | [134] | ||
| External Link | ||||
| GSK2586184 | Phase 2 | [135] | ||
| Synonyms |
GSK-2586184
Click to Show/Hide
|
|||
| External Link | ||||
| PTC299 | Phase 2 | [136] | ||
| Synonyms |
6-(4-Fluorophenyl)-2,3-dihydro-5-(4-pyridinyl)imidazo(2,1-b)thiazole; skf-86002; 72873-74-6; Skf 86002; F 86002; F 86002-A(2); UNII-9R6QDF1UO7; 9R6QDF1UO7; CHEMBL313417; 5-(4-Pyridyl)-6-(4-fluorophenyl)-2,3-dihydroimidazo(2,1-b)-thiazole; 4-[6-(4-fluorophenyl)-2H,3H-imidazo[2,1-b][1,3]thiazol-5-yl]pyridine; 6-(4-fluorophenyl)-5-(4-pyridyl)-2,3-dihydroimidazo[2,1-b]thiazole; 6-(4-Fluorophenyl)-2,3-dihydro-5-(4-pyridyl)imidazo[2,1-b]thiazole; Imidazo(2,1-b)thiazole,; SK&F 86002
Click to Show/Hide
|
|||
| External Link | ||||
| CCX-354 | Phase 2 | [19] | ||
| Synonyms |
CCX-354-C; GSK2941266
Click to Show/Hide
|
|||
| External Link | ||||
| [L/D]-aminopterin | Phase 2 | [137] | ||
| External Link | ||||
| ALX-0061 | Phase 2 | [19] | ||
| Synonyms |
Nanobody therapeutic (autoimmune/inflammatory disease), Ablynx; Anti-IL-6R nanobody therapeutic (autoimmune/inflammatory disease), Ablynx
Click to Show/Hide
|
|||
| External Link | ||||
| Ozoralizumab | Phase 2 | [138] | ||
| Synonyms |
ATN-103
Click to Show/Hide
|
|||
| External Link | ||||
| JNJ-38518168 | Phase 2 | [139] | ||
| External Link | ||||
| Clazakizumab | Phase 2 | [19] | ||
| External Link | ||||
| UCB-35440 | Phase 2 | [140] | ||
| Synonyms |
5-[4-(N-Carbamoyl-N-hydroxyamino)-1-butynyl]-2-[2-[4-[1(R)-(4-chlorophenyl)-1-phenylmethyl]piperazin-1-yl]ethoxy]benzamide
Click to Show/Hide
|
|||
| External Link | ||||
| Otilimab | Phase 3 | [141] | ||
| External Link | ||||
| Pegsunercept | Phase 2 | [142] | ||
| Synonyms |
STNF-R1
Click to Show/Hide
|
|||
| External Link | ||||
| AD-452 | Phase 2 | [143] | ||
| Synonyms |
RS(+)-mefloquine; (+)-erythro-mefloquine
Click to Show/Hide
|
|||
| External Link | ||||
| SSR-240612 | Phase 2 | [144] | ||
| Synonyms |
N2-[3(R)-(1,3-Benzodioxol-5-yl)-3-(6-methoxynaphthalen-2-ylsulfonamido)propionyl]-4-[2(R),6(S)-dimethylpiperidin-1-ylmethyl]-N1-isopropyl-N1-methyl-D-phenylalaninamide hydrochloride
Click to Show/Hide
|
|||
| External Link | ||||
| TOK-8801 | Phase 2 | [145] | ||
| Synonyms |
105963-46-0; Imidazo[2,1-b]thiazole-2-carboxamide, 5,6-dihydro-3,6,6-trimethyl-N-(2-phenylethyl)-; N-(2-Phenylethyl)-3,6,6-trimethyl-5,6-dihydroimidazo(2,1-b)thiazole-2-carboxamide; Imidazo[2,1-b]thiazole-2-carboxamide,5,6-dihydro-3,6,6-trimethyl-N-(2-phenylethyl)-; Tok 8801; N-(2-Phenylethyl)-3,6,6-trimethyl-5,6-dihydroimidazo[2,1-b]thiazole-2-carboxamide; Imidazo(2,1-b)thiazole-2-carboxamide, 5,6-dihydro-3,6,6-trimethyl-N-(2-phenylethyl)-; AC1L3UYM; ACMC-20m9c6; SCHEMBL9774378; CTK4A4247; AKOS030604802
Click to Show/Hide
|
|||
| External Link | ||||
| Sirukumab | Phase 3 | [146] | ||
| External Link | ||||
| PG-760564 | Phase 2 | [147] | ||
| Synonyms |
Rheumatoid arthritis therapy, Procter & Gamble
Click to Show/Hide
|
|||
| External Link | ||||
| Peresolimab | Phase 2 | [148] | ||
| External Link | ||||
| CH-4051 | Phase 2 | [149] | ||
| External Link | ||||
| VX-745 | Phase 2 | [150] | ||
| Synonyms |
5-(2,6-Dichlorophenyl)-2-((2,4-difluorophenyl)thio)-6H-pyrimido[1,6-b]pyridazin-6-one; 209410-46-8; Neflamapimod; VX 745; VX745; VRT-031745; UNII-TYL52QM320; TYL52QM320; CHEBI:90528; 5-(2,6-dichlorophenyl)-2-(2,4-difluorophenylthio)-6H-pyrimido[1,6-b]pyridazin-6-one; 5-(2,6-dichlorophenyl)-2-(2,4-difluorophenyl)sulfanylpyrimido[1,6-b]pyridazin-6-one; Neflamapimod (USAN); Neflamapimod [USAN]; AK-44905; C19H9Cl2F2N3OS; 5-(2,6-Dichlorophenyl)-2-[(2,4-Difluorophenyl)sulfanyl]-6h-Pyrimido[1,6-B]pyridazin-6-One; VX745, VX-745; 5-(2,6-Dichlorophenyl)-2-((2,4-difluorophenyl)thio)-6H-pyrimido(1,6-b)pyridazin-6-one; Vertex 745 (VX745)
Click to Show/Hide
|
|||
| External Link | ||||
| PF-04171327 | Phase 2 | [151] | ||
| Synonyms |
Fosdagrocorat; UNII-HPI19004QS; 1044535-58-1; HPI19004QS; 2-Phenanthrenecarboxamide, 4b,5,6,7,8,8a,9,10-octahydro-N-(2-methyl-3-pyridinyl)-4b-(phenylmethyl)-7-(phosphonooxy)-7-(trifluoromethyl)-, (4bS,7R,8aR)-;2-Phenanthrenecarboxamide, 4b,5,6,7,8,8a,9,10-octahydro-N-(2-methyl-3-pyridinyl)-4b-(phenylmethyl)-7-(phosphonooxy)-7-(trifluoromethyl)-, (4bS,7R,8aR)-; Fosdagrocorat [USAN:INN]; PF 04171327; Fosdagrocorat (USAN/INN); SCHEMBL1707427; GTPL9649; US8901310, Example 2; CHEMBL3137316; BDBM140010; SB17317
Click to Show/Hide
|
|||
| External Link | ||||
| MANOALIDE | Phase 2 | [152] | ||
| Synonyms |
UNII-E1DK0157K9; 75088-80-1; CHEMBL463914; CHEBI:66666; E1DK0157K9; 2(5H)-Furanone, 4-(3,6-dihydro-6-hydroxy-5-(4-methyl-6-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-hexenyl)-2H-pyran-2-yl)-5-hydroxy-; 2(5H)-Furanone, 4-((2R,6R)-3,6-dihydro-6-hydroxy-5-((3E)-4-methyl-6-(2,6,6-trimethyl-1-cyclohexen-1-yl)-3-hexenyl)-2H-pyran-2-yl)-5-hydroxy-, (5R)-; AC1O5NJS; SCHEMBL20551728; MolPort-020-000-975
Click to Show/Hide
|
|||
| External Link | ||||
| Cenplacel-L | Phase 2 | [153] | ||
| Synonyms |
PDA-001
Click to Show/Hide
|
|||
| External Link | ||||
| BT-016 | Phase 2 | [154] | ||
| Synonyms |
Tregalizumab
Click to Show/Hide
|
|||
| External Link | ||||
| Xmab 5871 | Phase 1/2 | [155] | ||
| Synonyms |
XmAb 5871 (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Vidofludimus | Phase 2 | [156] | ||
| Synonyms |
717824-30-1; 4SC-101; UNII-8Y1PJ3VG81; SC12267; Vidofludimus(4SC-101; SC12267); CHEMBL197194; 8Y1PJ3VG81; 2-[[2-fluoro-4-(3-methoxyphenyl)phenyl]carbamoyl]cyclopentene-1-carboxylic Acid; 2-{[2-fluoro-4-(3-methoxyphenyl)phenyl]carbamoyl}cyclopent-1-ene-1-carboxylic acid; 2-[[2-fluoranyl-4-(3-methoxyphenyl)phenyl]carbamoyl]cyclopentene-1-carboxylic acid; Vidofludimus [INN]; SC 12267; SCHEMBL247888; GTPL9860; KS-00000TTT; BDBM16111; EX-A546; DTXSID50431325; MolPort-039-193-851; BCP14555; AOB87354; s7262; ZINC14960644
Click to Show/Hide
|
|||
| External Link | ||||
| K-832 | Phase 2 | [23] | ||
| Synonyms |
10465-81-3; 1,1'-(Azodicarbonyl)-dipiperidine; AdDP; 1,1-(Azodicarbonyl)dipiperidine; 1,1'-(Azodicarbonyl)dipiperidine; diazene-1,2-diylbis(piperidin-1-ylmethanone); NSC356027; NSC 356027; (NE)-N-(piperidine-1-carbonylimino)piperidine-1-carboxamide; azodicarboxylic acid dipiperidine; (E)-diazene-1,2-diylbis(piperidin-1-ylmethanone); Azodicarboxylic acid dipiperidide; J-503690; SR-4077; (E)-diazene-1,2-diylbis(piperidin-1-yl methanone); SR 4077; PubChem19605; piperidyl (piperidylcarbonyl)diazenyl ketone; AC1Q5JWC; AC1NWBQ9
Click to Show/Hide
|
|||
| External Link | ||||
| PRTX-100 | Phase 1/2 | [157] | ||
| Synonyms |
Staphylococcal protein A
Click to Show/Hide
|
|||
| External Link | ||||
| Cx-611 | Phase 1/2 | [158] | ||
| Synonyms |
Allogeneic adipose-derived stem cell therapy (intravenous, rheumatoid arthritis), Cellerix; Allogeneic adipose-derived stem cell therapy (intravenous, rheumatoid arthritis), TiGenix
Click to Show/Hide
|
|||
| External Link | ||||
| BIO-300 | Phase 1/2 | [159] | ||
| Synonyms |
Radioprotective therapy, Humanetics
Click to Show/Hide
|
|||
| External Link | ||||
| Chondrogen | Phase 1/2 | [160] | ||
| Synonyms |
Chondrogen (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| ART621 | Phase 1/2 | [161] | ||
| External Link | ||||
| Indomethacin | Approved | [162] | ||
| External Link | ||||
| HM71224 | Phase 1 | [163] | ||
| Synonyms |
Poseltinib; 1353552-97-2; UNII-D01E4B1U35; D01E4B1U35; LY3337641; N-(3-((2-((4-(4-methylpiperazin-1-yl)phenyl)amino)furo[3,2-d]pyrimidin-4-yl)oxy)phenyl)acrylamide; Poseltinib [INN]; GTPL9862; CHEMBL4163691; SCHEMBL14915064; BDBM50369724; BS-15248; HM-71224; LY333764; Example 228 [WO2011162515A2]; HY-109010; CS-0030508; Q27275916; 2-Propenamide, N-(3-((2-((4-(4-methyl-1-piperazinyl)phenyl)amino)furo(3,2-d)pyrimidin-4-yl)oxy)phenyl)-; N-(3-((2-(4-(4-Methylpiperazin-1-yl)anilino)furo(3,2-d)pyrimidin-4-yl)oxy)phenyl)prop-2-enamide; N-[3-[2-[4-(4-methylpiperazin-1-yl)anilino]furo[3,2-d]pyrimidin-4-yl]oxyphenyl]prop-2-enamide
Click to Show/Hide
|
|||
| External Link | ||||
| LABP-104 | Phase 1 | [164] | ||
| Synonyms |
NIM-1324
Click to Show/Hide
|
|||
| External Link | ||||
| ORTD-1 | Phase 1 | [165] | ||
| Synonyms |
.theta.-Defensin 1; 1-[3-[benzyl-tetrakis(3-guanidinopropyl)-[(1R)-1-hydroxyethyl]-isobutyl-isopropyl-[(1S)-1-methylpropyl]-octadecaoxo-[?]yl]propyl]guanidine; 251442-64-5; BDBM50236198; CHEMBL4073105; Cyclic-(GVCRCICTRGFCRCLCRR); DTXSID60179822; oRTD-1; RTD-1; theta-Defensin 1; theta-Defensin RTD-1
Click to Show/Hide
|
|||
| External Link | ||||
| JNJ-67484703 | Phase 1 | [166] | ||
| Synonyms |
JNJ-4703
Click to Show/Hide
|
|||
| External Link | ||||
| IA-14069 | Phase 1 | [167] | ||
| External Link | ||||
| BX-U001 | Phase 1 | [168] | ||
| External Link | ||||
| KPE-06001 | Phase 1 | [169] | ||
| Synonyms |
Asthma/arthritis therapy, Kemin
Click to Show/Hide
|
|||
| External Link | ||||
| PF-05230905 | Phase 1 | [170] | ||
| Synonyms |
ATN-192
Click to Show/Hide
|
|||
| External Link | ||||
| RG6125 | Phase 1 | [19] | ||
| External Link | ||||
| TAK-020 | Phase 1 | [19] | ||
| External Link | ||||
| AXS-06 | Phase 1 | [171] | ||
| External Link | ||||
| Aminoguanidine | Discontinued in Phase 2 | [172] | ||
| Synonyms |
Pimagedine; Hydrazinecarboximidamide; Guanyl hydrazine; Monoaminoguanidine; 2-aminoguanidine; 79-17-4; Imino semicarbazide; Aminate base; 2-azanylguanidine; Pimagedine [INN]; GUANIDINE, AMINO-; 1-aminoguanidine; UNII-SCQ4EZQ113; Hydrazinecarboximidamide(9CI); CCRIS 3511; EINECS 201-183-1; Aminoguanidine, Hemisulfate; SCQ4EZQ113; CHEMBL225304; CHEBI:40618; HAMNKKUPIHEESI-UHFFFAOYSA-N; guanylhydrazine; GER-11; AGU; amino guanidine; 1-amino-guanidine; Aminoguanidine (AG); Tocris-0787; Carbonohydrazonic diamide; INCB3284
Click to Show/Hide
|
|||
| External Link | ||||
| OPL-CCL2-LPM | Phase 1 | [173] | ||
| Synonyms |
CCR2 targeting agent (arthritis/nephritis/cardiovascular/pulmonaryl/CNS); Leukocyte population modulator (arthritis/nephritis/cardiovascular/pulmonary/CNS), Osprey
Click to Show/Hide
|
|||
| External Link | ||||
| AZD9567 | Phase 1 | [19] | ||
| Synonyms |
ZQFNDBISEYQVRR-LOSJGSFVSA-N; AZD-9567; GTPL9812; SCHEMBL17643955; AZD 9567; example 1 [WO2016046260A1]; compound 15 [PMID: 29424542]; B9W; 2,2-difluoro-N-[(1R,2S)-3-methyl-1-[1-(1-methyl-6-oxopyridin-3-yl)indazol-5-yl]oxy-1-phenylbutan-2-yl]propanamide; 2,2-bis(fluoranyl)-~{N}-[(1~{R},2~{S})-3-methyl-1-[1-(1-methyl-6-oxidanylidene-pyridin-3-yl)indazol-5-yl]oxy-1-phenyl-butan-2-yl]propanamide
Click to Show/Hide
|
|||
| External Link | ||||
| AVE1701 | Phase 1 | [23] | ||
| External Link | ||||
| AVP-13358 | Phase 1 | [174] | ||
| Synonyms |
IgE inhibitors, AVANIR; Allergy/asthma therapeutic, AVANIR
Click to Show/Hide
|
|||
| External Link | ||||
| Cadherin-11 | Phase 1 | [175] | ||
| Synonyms |
SDP051
Click to Show/Hide
|
|||
| External Link | ||||
| NCX-1015 | Phase 1 | [176] | ||
| Synonyms |
Nitroprednisolone; NCX-1004; NCX-1016; NO-prednisolone
Click to Show/Hide
|
|||
| External Link | ||||
| ONS-3010 | Phase 1 | [19] | ||
| External Link | ||||
| BF-389 | Phase 1 | [177] | ||
| Synonyms |
Biofor 389; UNII-MGJ3XBS5KD; MGJ3XBS5KD; BF 389; 127245-22-1; 4-(3,5-Di-tert-butyl-4-hydroxybenzylidene)-2-methyl-5,6-dihydro-2H-1,2-oxazin-3(4H)-one; Dihydro-4-(3,5-di-tert-butyl-4-hydroxybenzylidene)-2-methyl-2H-1,2-oxazin-3(4H)-one; 2H-1,2-Oxazin-3(4H)-one, dihydro-4-((3,5-bis(1,1-dimethylethyl)-4-hydroxyphenyl)methylene)-2-methyl-; 2H-1,2-Oxazin-3(4H)-one, 4-((3,5-bis(1,1-dimethylethyl)-4-hydroxyphenyl)methylene)dihydro-2-methyl-; Biofor-389; AC1O5RBB; SCHEMBL8291651; LS-100029
Click to Show/Hide
|
|||
| External Link | ||||
| HMR-1031 | Phase 1 | [178] | ||
| Synonyms |
MDL-819767; 3(S)-[2(S)-[4,4-Dimethyl-3-[4-[3-(2-methylphenyl)ureido]benzyl]-2,5-dioxoimidazolidin-1-yl]-4-methylpentanoylamino]-3-phenylpropionic acid
Click to Show/Hide
|
|||
| External Link | ||||
| VI7734 | Phase 1 | [125] | ||
| External Link | ||||
| BMS-986104 | Phase 1 | [19] | ||
| Synonyms |
Unii-KJ9D084FO4; KJ9D084FO4; 1622180-31-7; CHEMBL3806158; SCHEMBL15953811; BPMMYKAHRIEVDH-VOQZNFBZSA-N; J3.582.853B; ((1R,3 S)-1-amino-3-((R)-6-hexyl-5,6,7,8-tetrahydronaphthalen-2-yl)cyclopentyl)methanol; ((1R,3S)-1-amino-3-((R)-6-hexyl-5,6,7,8-tetrahydronaphthalen-2-yl)cyclopentyl)methanol; Cyclopentanemethanol, 1-amino-3-((6R)-6-hexyl-5,6,7,8-tetrahydro-2-naphthalenyl)-, (1R,3S)-
Click to Show/Hide
|
|||
| External Link | ||||
| AMAP-102 | Phase 1 | [179] | ||
| Synonyms |
5-HT antagonist (rheumatism), AnaMar
Click to Show/Hide
|
|||
| External Link | ||||
| MEDI5117 | Phase 1 | [180] | ||
| External Link | ||||
| Sc Veltuzumab | Phase 1 | [181] | ||
| External Link | ||||
| MORAb-022 | Phase 1 | [19] | ||
| Synonyms |
Antibody (inflammation/autoimmune disease), Morphotek
Click to Show/Hide
|
|||
| External Link | ||||
| ARG301 | Phase 1 | [182] | ||
| External Link | ||||
| SCH-900117 | Phase 1 | [183] | ||
| External Link | ||||
| DWP-422 | Phase 1 | [184] | ||
| External Link | ||||
| Gerilimzumab | Phase 1 | [19] | ||
| External Link | ||||
| VEL-0230 | Phase 1 | [185] | ||
| Synonyms |
NC-2300; Osteoporosis therapy (oral), Velcura/Nippon Chemiphar
Click to Show/Hide
|
|||
| External Link | ||||
| Synthetic neutrophil inhibitor peptide | Phase 1 | [186] | ||
| Synonyms |
SNIP; Nalpha-Acetyl-L-arginyl-L-glutamyl-glycyl-L-seryl-L-tyrosyl-L-phenylalanyl-L-phenylalanyl-glycyl-L-aspartyl-L-asparaginyl-L-alaninamide
Click to Show/Hide
|
|||
| External Link | ||||
| RWJ-67657 | Phase 1 | [187] | ||
| Synonyms |
215303-72-3; RWJ 67657; RWJ67657; 4-[4-(4-Fluorophenyl)-1-(3-phenylpropyl)-5-(4-pyridinyl)-1H-imidazol-2-yl]-3-butyn-1-ol; CHEMBL190333; 4-(4-(4-fluorophenyl)-1-(3-phenylpropyl)-5-(pyridin-4-yl)-1H-imidazol-2-yl)but-3-yn-1-ol; 4-(4-Fluorophenyl)-2-(4-hydroxy-1-butynyl)-1-(3-Phenylpropyl)-5-(4-Pyridyl)imidazole; 4-[4-(4-fluorophenyl)-1-(3-phenylpropyl)-5-(4-pyridyl)imidazol-2-yl]but-3-yn-1-ol; 4-(4-(4-Fluorophenyl)-1-(3-phenylpropyl)-5-(4-pyridinyl)-1H-imidazol-2-yl)-3-butyn-1-ol
Click to Show/Hide
|
|||
| External Link | ||||
| MEDI4920 | Phase 1 | [19] | ||
| External Link | ||||
| RPI-78M | Discontinued in Phase 1 | [188] | ||
| Synonyms |
Antiviral agent, Receptogen; Receptin (injectable), ReceptoPharm; Receptin (oral), ReceptoPharm; RPI-78M (injectable), ReceptoPharm; RPI-78M (oral), ReceptoPharm
Click to Show/Hide
|
|||
| External Link | ||||
| RX-10001 | Phase 1 | [189] | ||
| External Link | ||||
| ASP2408 | Phase 1 | [190] | ||
| Synonyms |
CTLA4-Ig fusion protein
Click to Show/Hide
|
|||
| External Link | ||||
| GSK3117391 | Phase 1 | [191] | ||
| External Link | ||||
| ABBV-257 | Phase 1 | [19] | ||
| External Link | ||||
| VGX-1027 | Phase 1 | [192] | ||
| Synonyms |
6501-72-0; GIT 27; git-27; 2-(3-phenyl-4,5-dihydroisoxazol-5-yl)acetic acid; 4,5-DIHYDRO-3-PHENYL-5-ISOXAZOLEACETIC ACID; 2-(3-phenyl-4,5-dihydro-1,2-oxazol-5-yl)acetic Acid; GIT27; SCHEMBL1404343; CHEMBL1320667; CTK5C2027; VGX-127; EX-A366; DTXSID50445023; AOB1858; MUFJHYRCIHHATF-UHFFFAOYSA-N; MolPort-022-917-316; HMS3653J11; BCP09284; VGX 1027; VGX-1027(GIT 27); MFCD08696167; s7515; 2792AH; GIT-27 (VGX-1027); AKOS022538967; AKOS000282755; CS-2737; NCGC00186000-02; NCGC00186000-01; AK175225; HY-15507; AB0279943; KB-272590
Click to Show/Hide
|
|||
| External Link | ||||
| GSK-1827771 | Phase 1 | [193] | ||
| Synonyms |
IL-1 antagonist (antibody, rheumatoid arthritis), GSK
Click to Show/Hide
|
|||
| External Link | ||||
| ASP5094 | Phase 1 | [19] | ||
| External Link | ||||
| EVT 401 | Phase 1 | [194] | ||
| External Link | ||||
| SKI-O-703 | Phase 1 | [19] | ||
| External Link | ||||
| AZ-4217 | Clinical trial | [195] | ||
| Synonyms |
AZ4217; AZ 4217
Click to Show/Hide
|
|||
| External Link | ||||
| PMID27998201-Compound-9 | Patented | [196] | ||
| External Link | ||||
| Floctafenine | Withdrawn from market | [6] | ||
| Synonyms |
23779-99-9; Idarac; Novodolan; Diralgan; Idalon; 2,3-Dihydroxypropyl 2-((8-(trifluoromethyl)quinolin-4-yl)amino)benzoate; RU 15750; R 4318; Floctafeninum [INN-Latin]; Floctafenina [INN-Spanish]; 8-Trifluoromethyl-7-deschloroglafenine; C20H17F3N2O4; EINECS 245-881-4; BRN 0457808; 2,3-Dihydroxypropyl 2-((8-(trifluoromethyl)-quinolin-4-yl)amino)benzoate; 2,3-Dihydroxypropyl N-(8-(trifluoromethyl)-4-quinolyl)anthranilate; 4-(o-(2',3'-Dihydroxypropyloxycarbonyl)phenyl)-amino-8-trifluoromethylquinoline
Click to Show/Hide
|
|||
| External Link | ||||
| Aurothioglucose | Withdrawn from market | [6] | ||
| Synonyms |
HMS3262I11; LP00685
Click to Show/Hide
|
|||
| External Link | ||||
| Amiprilose | Discontinued in Preregistration | [197] | ||
| Synonyms |
Therafectin; Amiprilose hydrochloride; KAP-690; SM-1213
Click to Show/Hide
|
|||
| External Link | ||||
| Nerelimomab | Discontinued in Phase 3 | [198] | ||
| Synonyms |
BAY-X-1351
Click to Show/Hide
|
|||
| External Link | ||||
| M-5010 | Discontinued in Phase 3 | [199] | ||
| Synonyms |
M-5011; T-3788; 2(R)-[4-(3-Methyl-2-thienyl)phenyl]propionic acid
Click to Show/Hide
|
|||
| External Link | ||||
| BR3-Fc | Discontinued in Phase 2 | [200] | ||
| External Link | ||||
| KC706 | Discontinued in Phase 2 | [201] | ||
| External Link | ||||
| EFLETIRIZINE | Discontinued in Phase 2 | [202] | ||
| Synonyms |
Efletirizine < Rec INN; Ucb-28754; 2-[2-[4-[Bis(4-fluorophenyl)methyl]piperazin-1-yl]ethoxy]acetic acid
Click to Show/Hide
|
|||
| External Link | ||||
| IX207-887 | Discontinued in Phase 2 | [203] | ||
| External Link | ||||
| AMG 108 | Discontinued in Phase 2 | [204] | ||
| External Link | ||||
| HR325 | Discontinued in Phase 2 | [205] | ||
| External Link | ||||
| Apricoxib | Discontinued in Phase 2 | [206] | ||
| Synonyms |
TG01
Click to Show/Hide
|
|||
| External Link | ||||
| TA-383 | Discontinued in Phase 2 | [207] | ||
| Synonyms |
Cis-2-(4-Chlorophenyl)-4,5-diphenyl-2-imidazoline hydrochloride; Cis-2-(4-Chlorophenyl)-4,5-diphenyl-4,5-dihydro-1H-imidazole hydrochloride
Click to Show/Hide
|
|||
| External Link | ||||
| Camobucol | Discontinued in Phase 2 | [208] | ||
| Synonyms |
AGIX-4207; AGIX-4207 IV; V-protectant (rheumatoid arthritis), AtheroGenics
Click to Show/Hide
|
|||
| External Link | ||||
| SM-8849 | Discontinued in Phase 2 | [209] | ||
| Synonyms |
113759-19-6; 4-(1-(2-fluoro-4-biphenylyl)ethyl)-2-methylaminothiazole; 4-[1-(2-fluoro-4-biphenylyl)ethyl]-2-methylaminothiazole; ZTFDMDJGJVUYQE-UHFFFAOYSA-N; AC1L3Y3S; SCHEMBL5078854; 4-(1-(2-Fluoro-4-biphenyl)ethyl)-2-methylaminothiazole; (+)-2-methylamino-4-(1-(2-fluoro-4-biphenylyl)ethyl)thiazole; 4-[1-(3-fluoro-4-phenylphenyl)ethyl]-N-methyl-1,3-thiazol-2-amine; 2-Thiazolamine, 4-(1-(2-fluoro(1,1'-biphenyl)-4-yl)ethyl)-N-methyl-
Click to Show/Hide
|
|||
| External Link | ||||
| Zolimomab aritox | Discontinued in Phase 2 | [210] | ||
| Synonyms |
XomaZyme-CD5 Plus; XomaZyme-H65; XomaZyme-lym; OrthoZyme-CD5+; Xmmly-h65-rta; XomaZyme-H65-rta
Click to Show/Hide
|
|||
| External Link | ||||
| Fontolizumab | Discontinued in Phase 2 | [23] | ||
| Synonyms |
Fontolizumab (USAN/INN)
Click to Show/Hide
|
|||
| External Link | ||||
| CP-195543 | Discontinued in Phase 2 | [211] | ||
| Synonyms |
LB4 antagonist (RA), Pfizer; LB4 antagonist (inflammation), Pfizer; Leukotriene B4 antagonist (inflammation), Pfizer; Leukotriene B4 antagonist (rheumatoid arthritis), Pfizer
Click to Show/Hide
|
|||
| External Link | ||||
| MLN3897 | Discontinued in Phase 2 | [23] | ||
| External Link | ||||
| S-5751 | Discontinued in Phase 2 | [212] | ||
| Synonyms |
AC1NSK82; GTPL1898; S5751; (Z)-7-[(1R,2R,3R,5S)-2-[(5-hydroxy1-benzothiophene-3-carbonyl)amino]-7,7-dimethyl-3-bicyclo[3.1.1]heptanyl]hept-5-enoic acid; (Z)-7-[(1S,3R,4R,5R)-4-[(5-hydroxy-1-benzothiophene-3-carbonyl)amino]-6,6-dimethyl-3-bicyclo[3.1.1]heptanyl]hept-5-enoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| INCB47986 | Discontinued in Phase 2 | [213] | ||
| External Link | ||||
| MP-435 | Discontinued in Phase 2 | [214] | ||
| Synonyms |
C5a antagonist (rheumatoid arthritis), Mitsubishi Tanabe
Click to Show/Hide
|
|||
| External Link | ||||
| CPA-926 | Discontinued in Phase 2 | [215] | ||
| Synonyms |
6-(2-Acetamido-2-deoxy-beta-D-glucopyranosyloxy)-7-hydroxy-2H-1-benzopyran-2-one
Click to Show/Hide
|
|||
| External Link | ||||
| ISIS 104838 | Discontinued in Phase 2 | [216] | ||
| External Link | ||||
| SINOMENINE | Discontinued in Phase 2 | [217] | ||
| Synonyms |
Cucoline; Kukoline; (9alpha,13alpha,14alpha)-4-Hydroxy-3,7-dimethoxy-17-methyl-7,8-didehydromorphinan-6-one
Click to Show/Hide
|
|||
| External Link | ||||
| Linetastine | Discontinued in Phase 2 | [218] | ||
| Synonyms |
Tmk688; UNII-7U248Z56LA; 110501-66-1; TMK-688; 159776-68-8; 7U248Z56LA; Linetastine [INN]; Tmk 688; Linazolast; CCRIS 6902; Linazolast (JAN); YM-257; AC1O5R98; CHEMBL314338; SCHEMBL1614484; [4-[(1E,3E)-5-[2-(4-benzhydryloxypiperidin-1-yl)ethylamino]-5-oxopenta-1,3-dienyl]-2-methoxyphenyl] ethyl carbonate; Carbonic acid, 4-(5-((2-(4-(diphenylmethoxy)-1-piperidinyl)ethyl)amino)-5-oxo-1,3-pentadienyl)-2-methoxyphenyl ethyl ester; D09850; Molecule 26
Click to Show/Hide
|
|||
| External Link | ||||
| SC-106 | Discontinued in Phase 2 | [219] | ||
| Synonyms |
AC1LAPZA; SCHEMBL6773940; IABCGGIFHPDBCJ-JTQLQIEISA-N; (3R)-3-[[2-[(3-guanidinobenzoyl)amino]acetyl]amino]pent-4-ynoic acid; (R)-3-(2-{[1-(3-Guanidino-phenyl)-methanoyl]-amino}-ethanoylamino)-pent-4-ynoic acid; (3R)-3-[[2-[[3-(diaminomethylideneamino)benzoyl]amino]acetyl]amino]pent-4-ynoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| PRO-515 | Discontinued in Phase 2 | [220] | ||
| External Link | ||||
| GW-215864 | Discontinued in Phase 2 | [221] | ||
| Synonyms |
GR-215864; GR-215864X; GW-215864X
Click to Show/Hide
|
|||
| External Link | ||||
| HSR-609 | Discontinued in Phase 2 | [222] | ||
| Synonyms |
FY-609; 3-[4-(8-Fluoro-5,11-dihydrobenz[b]oxepino[4,3-b]pyridin-11-ylidene)piperidin-1-yl]propionic acid dihydrate
Click to Show/Hide
|
|||
| External Link | ||||
| AnergiX.RA | Discontinued in Phase 2 | [223] | ||
| Synonyms |
AG-4263; RA-AnergiX; Antigen specific therapy (rheumatoid arthritis, AnergiX), Corixa/ Organon
Click to Show/Hide
|
|||
| External Link | ||||
| GLPG-0259 | Discontinued in Phase 2 | [224] | ||
| Synonyms |
G-13919, GT-146, GT-1498, GT-1704, GT-314, GT-416, GT-514, GT-562
Click to Show/Hide
|
|||
| External Link | ||||
| CBF-BS2 | Discontinued in Phase 2 | [225] | ||
| Synonyms |
KSB-302
Click to Show/Hide
|
|||
| External Link | ||||
| NN8209 | Discontinued in Phase 2 | [226] | ||
| External Link | ||||
| IDEC-151 | Discontinued in Phase 2 | [227] | ||
| Synonyms |
Clenoliximab
Click to Show/Hide
|
|||
| External Link | ||||
| PAMAPIMOD | Discontinued in Phase 2 | [228] | ||
| Synonyms |
449811-01-2; RO 4402257; UNII-8S2C9V11K4; Pamapimod (R-1503, Ro4402257); CHEMBL1090089; CHEBI:90685; 8S2C9V11K4; Ro4402257; Ro-4402257; R1503; R 1503; R-1503; 6-(2,4-Difluorophenoxy)-2-{[3-Hydroxy-1-(2-Hydroxyethyl)propyl]amino}-8-Methylpyrido[2,3-D]pyrimidin-7(8h)-One; Pamapimod [USAN:INN]; 6-(2,4-Difluorophenoxy)-2-((3-hydroxy-1-(2-hydroxyethyl)propyl)amino)-8-methylpyrido(2,3-d)pyrimidin-7(8H)-one; 6-(2,4-Difluorophenoxy)-2-[[3-hydroxy-1-(2-hydroxyethyl)propyl]amino]-8-methylpyrido[2,3-d]pyrimidin-7(8H)-one
Click to Show/Hide
|
|||
| External Link | ||||
| S-2474 | Discontinued in Phase 2 | [229] | ||
| Synonyms |
158089-95-3; CTK4C9584; DTXSID20724630; PHENOL, 2,6-BIS(1,1-DIMETHYLETHYL)-4-[(E)-(2-ETHYL-1,1-DIOXIDO-5-ISOTHIAZOLIDINYLIDENE)METHYL]-
Click to Show/Hide
|
|||
| External Link | ||||
| NPC-567 | Discontinued in Phase 2 | [230] | ||
| Synonyms |
Npc 567; Arg-3-hyp-7-phe-bradykinin; UNII-DV64B0PLEH; 109333-26-8; Bradykinin, arg-hyp(3)-phe(7)-; DV64B0PLEH; Bradykinin, arg(0)-hyp(3)-phe(7)-; CHEMBL446325; CHEBI:73294; arg(0)-hyp(3)-phe(7)-bradykinin; Bradykinin, arginyl-hydroxyprolyl(3)-phenylalanine(7)-; [D-Arg(0),Hyp(3),D-Phe(7)]bradykinin; Bradykinin, N2-D-arginyl-3-(trans-4-hydroxy-L-proline)-7-D-phenylalanine-
Click to Show/Hide
|
|||
| External Link | ||||
| T-5224 | Discontinued in Phase 2 | [231] | ||
| Synonyms |
AKOS003047196; T5224
Click to Show/Hide
|
|||
| External Link | ||||
| AD-121 | Discontinued in Phase 1/2 | [232] | ||
| Synonyms |
PW-9101; Rheumatoid arthritis therapeutic (oral controlled release, Syncrodose), Arakis/Penwest; Rheumatoid arthritis therapeutic (oral controlled release, TIMERx), Arakis/Penwest
Click to Show/Hide
|
|||
| External Link | ||||
| AME-527 | Discontinued in Phase 1/2 | [233] | ||
| External Link | ||||
| AZD-7140 | Discontinued in Phase 1 | [234] | ||
| External Link | ||||
| PF-4629991 | Discontinued in Phase 1 | [235] | ||
| Synonyms |
PF-991; S1P1 receptor agonist (autoimmune disease), Pfizer
Click to Show/Hide
|
|||
| External Link | ||||
| NN8210 | Discontinued in Phase 1 | [236] | ||
| External Link | ||||
| ARRY-614 | Discontinued in Phase 1 | [237] | ||
| Synonyms |
Pexmetinib; 945614-12-0; Pexmetinib (ARRY-614); UNII-3750D0U8B5; 3750D0U8B5; Pexmetinib [INN]; Pexmetinib;ARRY-614; Pexmetinib(ARRY-614); ARRY614; SCHEMBL379035; GTPL9917; MolPort-039-193-822; LNMRSSIMGCDUTP-UHFFFAOYSA-N; BCP28410; EX-A1421; s7799; ZINC41747181; AKOS032945154; SB16914; Urea, N-(3-(1,1-dimethylethyl)-1-(4-methylphenyl)-1H-pyrazol-5-yl)-N'-((5-fluoro-2-((1-(2-hydroxyethyl)-1H-indazol-5-yl)oxy)phenyl)methyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| Oncolysin CD6 | Discontinued in Phase 1 | [238] | ||
| Synonyms |
Anti-CD6-bR; Anti-T12-bR
Click to Show/Hide
|
|||
| External Link | ||||
| Eltenac | Discontinued in Phase 1 | [239] | ||
| Synonyms |
BY-820; B-788-20
Click to Show/Hide
|
|||
| External Link | ||||
| RG-4934 | Discontinued in Phase 1 | [240] | ||
| Synonyms |
IL-17 huMAb (psoriatic arthritis), Roche
Click to Show/Hide
|
|||
| External Link | ||||
| AC-100 | Discontinued in Phase 1 | [241] | ||
| Synonyms |
Bicyclo[4.2.0]octa-1,3,5-trien-3-ylboronic acid; BENZOCYCLOBUTANE-4-BORONIC ACID; Boronic acid, bicyclo[4.2.0]octa-1,3,5-trien-3-yl-; SCHEMBL12759201; KS-00000SRU; MolPort-005-942-889; ZINC196051840; AKOS025146995; DS-8536; BC225113; AK164312; 1,2-Dihydrobenzocyclobutene-4-ylboranic acid; CS-0043999; J3.522.588I; 4-bbcb benzocyclobutene-4-boronic acid
Click to Show/Hide
|
|||
| External Link | ||||
| IL-18BP | Discontinued in Phase 1 | [242] | ||
| External Link | ||||
| Roquinimex | Discontinued in Phase 1 | [243] | ||
| Synonyms |
Linomide
Click to Show/Hide
|
|||
| External Link | ||||
| D-1367 | Discontinued in Phase 1 | [244] | ||
| Synonyms |
167692-94-6; 2-(3,3-dimethylbutyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane; SCHEMBL15923700; DTXSID80570615; ZINC196470483; SC-92964; 2-(3,3-dimethylbutyl)-4,4,5,5,-tetramethyl-1,3,2-d; 1,3,2-Dioxaborolane, 2-(3,3-dimethylbutyl)-4,4,5,5-tetramethyl-
Click to Show/Hide
|
|||
| External Link | ||||
| SB-242235 | Discontinued in Phase 1 | [245] | ||
| Synonyms |
193746-75-7; SB242235; SB 242235; 4-(4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidazol-5-yl)-2-methoxypyrimidine; CHEMBL95692; 4-[5-(4-fluorophenyl)-3-piperidin-4-ylimidazol-4-yl]-2-methoxypyrimidine; 4-[4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidazol-5-yl]-2-methoxypyrimidine; Kinome_3169; SCHEMBL2267209; BDBM15458; SYN1076; PDTYLGXVBIWRIM-UHFFFAOYSA-N; MolPort-028-720-427; HMS3244I18; HMS3244J17; HMS3244I17; EX-A1881; BCP05992; ZINC1487129; 3254AH; RS0056; AKOS027323444; CS-2097; NCGC00345831-01; NCGC00345831-03
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-2315 | Discontinued in Phase 1 | [246] | ||
| Synonyms |
ZD-2315
Click to Show/Hide
|
|||
| External Link | ||||
| AVE-9940 | Discontinued in Phase 1 | [247] | ||
| External Link | ||||
| AZD-8309 | Discontinued in Phase 1 | [248] | ||
| External Link | ||||
| MLN0415 | Discontinued in Phase 1 | [249] | ||
| Synonyms |
SCHEMBL3805703
Click to Show/Hide
|
|||
| External Link | ||||
| AZD8566 | Discontinued in Phase 1 | [250] | ||
| External Link | ||||
| LY-231617 | Discontinued in Phase 1 | [251] | ||
| Synonyms |
2,6-Di-tert-butyl-4-(ethylaminomethyl)phenol hydrochloride
Click to Show/Hide
|
|||
| External Link | ||||
| CI-972 | Discontinued in Phase 1 | [252] | ||
| External Link | ||||
| R-1487 | Discontinued in Phase 1 | [253] | ||
| Synonyms |
449811-92-1; 6-(2,4-difluorophenoxy)-8-methyl-2-((tetrahydro-2H-pyran-4-yl)amino)pyrido[2,3-d]pyrimidin-7(8H)-one; UNII-IO0DCY55NQ; IO0DCY55NQ; CHEMBL1230122; 6-(2,4-difluorophenoxy)-8-methyl-2-[(tetrahydro-2H-pyran-4-yl)amino]-Pyrido[2,3-d]pyrimidin-7(8H)-one; 6-(2,4-difluorophenoxy)-8-methyl-2-(tetrahydro-2h-pyran-4-ylamino)pyrido[2,3-d]pyrimidin-7(8h)-one; Kinome_3762; SCHEMBL5120612; KKKRKRMVJRHDMG-UHFFFAOYSA-N; HMS3401C13; BDBM50341342; ZINC58633224; AKOS027420928; NCGC00262195-02; KB-80224; ACM449811921
Click to Show/Hide
|
|||
| External Link | ||||
| R1295 | Discontinued in Phase 1 | [23] | ||
| Synonyms |
CHEMBL2146615
Click to Show/Hide
|
|||
| External Link | ||||
| KB-2683 | Discontinued in Phase 1 | [254] | ||
| External Link | ||||
| AZD-6703 | Discontinued in Phase 1 | [255] | ||
| Synonyms |
P38 MAP kinase inhibitors (rheumatoid arthritis), AstraZeneca
Click to Show/Hide
|
|||
| External Link | ||||
| PF-251802 | Discontinued in Phase 1 | [256] | ||
| Synonyms |
PF-00251802; Selective glucocorticoid receptor agonist (1), Pfizer
Click to Show/Hide
|
|||
| External Link | ||||
| SC-57666 | Discontinued in Phase 1 | [257] | ||
| Synonyms |
CHEMBL274893; AC1L9EJK; SC57666; CHEBI:8982; 1-fluoro-4-[2-(4-methylsulfonylphenyl)cyclopenten-1-yl]benzene; SureCN213008; SCHEMBL213008; ZINC18527; GJGZQTGPOKPFES-UHFFFAOYSA-N; HY-U00129; BDBM50029614; DNC003789; CS-7166; C11706; 1-[2(4-Flurophenyl)-1-cyclopentyl]4-methylsulfonylbenzene; 1-[2-(4-fluorophenyl)cyclopenten-1-yl]-4-(methylsulfonyl)benzene; 1-[2-(4-fluorophenyl) cyclopenten-1-yl]-4- (methylsulfonyl)benzene; 1-fluoro-4-[2-(4-methylsulfonylphenyl)-1-cyclopentenyl]benzene
Click to Show/Hide
|
|||
| External Link | ||||
| C-5997 | Discontinued in Phase 1 | [258] | ||
| Synonyms |
7709-58-2; 4-(Chloromethyl)thiazole hydrochloride; 4-(chloromethyl)-1,3-thiazole Hydrochloride; 4-(chloromethyl)thiazole hcl; 4-chloromethylthiazole hydrochloride; 4-chloromethylthiazole hcl; 4-(chloromethyl)thiazolehcl; C4H4ClNS.ClH; ACMC-1BBSC; chloromethylthiazole-4 hcl; AC1Q3BH9; AC1MC82W; SCHEMBL122926; 4-(Chloromethyl)thiazole, HCl; CTK6H6834; DTXSID60376874; NVTBASMQHFMANH-UHFFFAOYSA-N; MolPort-000-139-475; 4-(chloromethyl)thiazole HCl salt; ACT02312; KS-000004ND; ANW-36949; 4-chloromethyl thiazole hydrochloride
Click to Show/Hide
|
|||
| External Link | ||||
| PF-755616 | Discontinued in Phase 1 | [259] | ||
| External Link | ||||
| GP2015 | Application submitted | [19] | ||
| External Link | ||||
| CLIK-181 | Preclinical | [260] | ||
| Synonyms |
J3.633.044I
Click to Show/Hide
|
|||
| External Link | ||||
| SR1555 | Preclinical | [261] | ||
| Synonyms |
CHEMBL3218917; SR-1555; 1386439-51-5; 1-(4-((4'-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)-[1,1'-biphenyl]-4-yl)methyl)piperazin-1-yl)ethan-1-one; 1-[4-[[4-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]phenyl]methyl]piperazin-1-yl]ethanone; SCHEMBL13974905; GTPL10145; BDBM50044227; ZINC95537675
Click to Show/Hide
|
|||
| External Link | ||||
| CLIK-148 | Preclinical | [260] | ||
| Synonyms |
SCHEMBL7207304
Click to Show/Hide
|
|||
| External Link | ||||
| MCP-1 | Preclinical | [262] | ||
| External Link | ||||
| Romazarit | Preclinical | [263] | ||
| Synonyms |
ORE-5007; Metabolic modulator (obesity/hyperlipidemia), Ore Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| NPC-17923 | Terminated | [264] | ||
| Synonyms |
147960-65-4; N-(9H-(2,7-Dichlorofluorenyl)-9-ethoxycarbonyl)-4-aminobenzoic acid; Benzoic acid, 4-((3-((2,7-dichloro-9H-fluoren-9-yl)oxy)-1-oxopropyl)amino)-
Click to Show/Hide
|
|||
| External Link | ||||
| TM-31 | Terminated | [265] | ||
| External Link | ||||
| BCX-25 | Terminated | [266] | ||
| Synonyms |
BCX-14
Click to Show/Hide
|
|||
| External Link | ||||
| BB-1433 | Terminated | [267] | ||
| Synonyms |
BB-2014; BB-2116; BB-2284; BB-2633; BB-3029; BB-3050; BB-3103; BB-3241
Click to Show/Hide
|
|||
| External Link | ||||
| ZK-90695 | Terminated | [268] | ||
| External Link | ||||
| RP-54745 | Terminated | [269] | ||
| Synonyms |
RP 54745; 135330-08-4; AC1L2PX8; SCHEMBL4362761; rp54745; CS-6750; 4-Chloro-5-(3,4-dihydro-1-methyl-2(1H)-isoquinolinyl)-3H-1,2-dithiol-3-one; HY-101716; 4-chloro-5-(1-methyl-3,4-dihydro-1H-isoquinolin-2-yl)dithiol-3-one; 3H-1,2-Dithiol-3-one, 4-chloro-5-(3,4-dihydro-1-methyl-2(1H)-isoquinolinyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| FR-123826 | Terminated | [270] | ||
| Synonyms |
COX-2 inhibitor, Fujisawa
Click to Show/Hide
|
|||
| External Link | ||||
| Seprilose | Terminated | [271] | ||
| Synonyms |
GW-80126
Click to Show/Hide
|
|||
| External Link | ||||
| NOX-200 | Terminated | [272] | ||
| Synonyms |
2-Amino-3-(1H-indol-3-yl)-2-methylpropanoic acid; alpha-Methyl-DL-tryptophan; 153-91-3; NSC9948; 13510-08-2; alpha-Methyl-L-tryptophan; M-5098; Tryptophan, alpha-methyl-; 16709-25-4; DL-alpha-Methyltryptophan; alpha-methyl-D,L-tryptophan; NSC-9948; NSC 9948; Tryptophan,a-methyl-; ACMC-1BNRO; alpha-Methyltryptophan; AC1L3UBI; AC1Q5S1L; (S)-alpha-Methyl pryptophan; SCHEMBL343309; CHEMBL559578; GTPL4693; CTK4C8033; ZTTWHZHBPDYSQB-UHFFFAOYSA-N; MolPort-003-958-850; KS-000001AA; ANW-21478
Click to Show/Hide
|
|||
| External Link | ||||
| SAN-18 | Terminated | [273] | ||
| External Link | ||||
| CI-986 | Terminated | [274] | ||
| Synonyms |
CI 986; 5-(3,5-Di-tert-butyl-4-hydroxyphenyl)-1,3,4-thiadiazole-2(3H)-thione; 5-(3,5-Bis(1,1-dimethylethyl)-4-(hydroxyphenyl))-1,3,4-thiadiazole-2(3H)-thione choline salt; Ethanaminium, 2-hydroxy-N,N,N-trimethyl-, salt with 5-(3,5-bis(1,1-dimethylethyl)-4-hydroxyphenyl)-1,3,4-thiadiazole-2(3H)-thione (1:1); 130116-16-4; AC1MI25H; C16H22N2OS2.C5H14NO; LS-172752; 2,6-ditert-butyl-4-(2-sulfanylidene-3H-1,3,4-thiadiazol-5-yl)phenolate
Click to Show/Hide
|
|||
| External Link | ||||
| SDZ-224-015 | Terminated | [275] | ||
| Synonyms |
VE-13045
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-6942 | Terminated | [276] | ||
| Synonyms |
CCR2b antagonists, AstraZeneca; CCR2b, AstraZeneca
Click to Show/Hide
|
|||
| External Link | ||||
| 3-DEAZAADENOSINE | Terminated | [277] | ||
| Synonyms |
Cc3Ado; BW-91Y78; 3DZA; 4-Amino-1-beta-D-ribofuranosyl-1H-imidazo[4,5-c]pyridine
Click to Show/Hide
|
|||
| External Link | ||||
| WY-28342 | Terminated | [278] | ||
| External Link | ||||
| AG-2024 | Terminated | [279] | ||
| External Link | ||||
| AZD-0902 | Terminated | [280] | ||
| External Link | ||||
| Ro-31-4724 | Terminated | [281] | ||
| Synonyms |
CHEMBL92608; [[1-[N-HYDROXY-ACETAMIDYL]-3-METHYL-BUTYL]-CARBONYL-LEUCINYL]-ALANINE ETHYL ESTER; RO4; 112105-54-1; Ro 314724; ro 31-4724; AC1NUPD6; ZINC3801503; ro-314724; BDBM50146631; DB08482; RO314724; (R)-N-(N-(2-(2-(Hydroxyamino)-2-oxoethyl)-4-methyl-1-oxopentyl)-L-leucyl)-L-alanine ethyl ester; ethyl N-{(2R)-2-[2-(hydroxyamino)-2-oxoethyl]-4-methylpentanoyl}-L-leucyl-L-alaninate; ethyl (2S)-2-[[(2S)-2-[[(2R)-2-[2-(hydroxyamino)-2-oxoethyl]-4-methylpentanoyl]amino]-4-methylpentanoyl]amino]propanoate
Click to Show/Hide
|
|||
| External Link | ||||
| RO-319790 | Terminated | [282] | ||
| Synonyms |
Ro-31-9790; CHEMBL16520; Ro 31-9790; (R)-N1-((S)-3,3-dimethyl-1-(methylamino)-1-oxobutan-2-yl)-N4-hydroxy-2-isobutylsuccinamide; SCHEMBL4633699; QRXOZHSEEGNRFC-ZYHUDNBSSA-N; ZINC1534591; BDBM50063920; CS-6682; HY-101703
Click to Show/Hide
|
|||
| External Link | ||||
| S-33516 | Terminated | [283] | ||
| External Link | ||||
| VE-16084 | Terminated | [284] | ||
| Synonyms |
ICE inhibitors, Sanofi Winthrop; WIN-67694
Click to Show/Hide
|
|||
| External Link | ||||
| D-5410 | Terminated | [285] | ||
| Synonyms |
CH-138; CH-263
Click to Show/Hide
|
|||
| External Link | ||||
| SKF-104351 | Terminated | [286] | ||
| Synonyms |
111908-94-2; Skf 104351; F 104351; 5H-Pyrrolo[1,2-a]imidazole,2-(4-fluorophenyl)-6,7-dihydro-3-(4-pyridinyl)-; 2-(4-fluorophenyl)-3-(4-pyridyl)-6,7-dihydro-5H-pyrrolo[1,2-a]imidazole; F-104351; AC1L3V0R; SCHEMBL7378515; DTXSID90149775; GJFVAEMLAFFGDZ-UHFFFAOYSA-N; ZINC3815711; 5H-Pyrrolo(1,2-a)imidazole, 2-(4-fluorophenyl)-6,7-dihydro-3-(4-pyridinyl)-; KB-292516; 2-p-Fluorophenyl-3-(4-pyridyl)-6,7-dihydro[5H]-pyrrolo[1,2-a]imidazole
Click to Show/Hide
|
|||
| External Link | ||||
| SB220025 | Terminated | [287] | ||
| Synonyms |
3erk; sb 220025; SB-220025; CHEMBL274064; 165806-53-1; CHEBI:82713; 4-[4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidazol-5-yl]pyrimidin-2-amine; 4-(4-FLUOROPHENYL)-1-(4-PIPERIDINYL)-5-(2-AMINO-4-PYRIMIDINYL)-IMIDAZOLE; SB4; 5-(2-Amino-4-pyrimidinyl)-4-(4-fluorophenyl)-1-(4-piperidinlyl)imidazole; 4-[5-(4-fluorophenyl)-3-(4-piperidyl)imidazol-4-yl]pyrimidin-2-amine; SB-220025-A; 1-(4-piperidinyl)-4-(4-fluorophenyl)-5-(2-(amino)-4-pyrimidinyl)imidazole
Click to Show/Hide
|
|||
| External Link | ||||
| RWJ-63556 | Terminated | [288] | ||
| External Link | ||||
| YM-26734 | Terminated | [289] | ||
| Synonyms |
YM 26734; 144337-18-8; CHEMBL444450; 1-Dodecanone,1,1'-[5-[3,4-dihydro-7-hydroxy-2-(4-hydroxyphenyl)-2H-1-benzopyran-4-yl]-2,4,6-trihydroxy-1,3-phenylene]bis-; 1,1'-[5-[3,4-Dihydro-7-hydroxy-2-(4-hydroxyphenyl)-2H-1-benzopyran-4-yl]-2,4,6-trihydroxy-1,3-phenylene]bis-1-dodecanone; ACMC-20n3vr; CTK4C4039; AOB2291; SYN5086; MolPort-023-276-467; BDBM50274336; AKOS024457146; 4-(3,5-Didodecanoyl-2,4,6-trihydroxyphenyl)-7-hydroxy-2-(4-hydroxyphenyl)chroman; KB-276131; YM-26734, > J-007943; 1-Dodecanone, 1
Click to Show/Hide
|
|||
| External Link | ||||
| AZD5672 | Terminated | [290] | ||
| Synonyms |
CHEMBL1951914; SCHEMBL2767780; GTPL7686; QOSMEMHKXNNIGG-SSEXGKCCSA-N; AZD 5672; BDBM50364743; N-[1-[(3R)-3-(3,5-difluorophenyl)-3-(1-methylsulfonylpiperidin-4-yl)propyl]piperidin-4-yl]-N-ethyl-2-(4-methylsulfonylphenyl)acetamide
Click to Show/Hide
|
|||
| External Link | ||||
| S-16276-1 | Terminated | [291] | ||
| External Link | ||||
| FR-111142 | Terminated | [292] | ||
| Synonyms |
WF-2015A
Click to Show/Hide
|
|||
| External Link | ||||
| BW A4C | Terminated | [293] | ||
| Synonyms |
BW4C; Bwa-4C; Bwa 4C; 106328-57-8; BW-A4C; BWA4C; CHEMBL314360; C17H17NO3; N-(E)-3-(3-Phenoxyphenyl)prop-2-enylacetohydroxamine acid; Acetamide, N-hydroxy-N-(3-(3-phenoxyphenyl)-2-propenyl)-; N-[(E)-3-(3-Phenoxyphenyl)prop-2-enyl]acetohydroxamic acid; Acetohydroxamine acid, N-(E)-3-(3-phenoxyphenyl)prop-2-enyl-; Acetamide, N-hydroxy-N-(3-(3-phenoxyphenyl)-2-propenyl)-, (E)-; BW-4AC; AC1O5PJ5; BW-A-4C; BW-A 4C; JMC515449 Compound 7; BDBM22334; ZINC5138195; MFCD00869694; N-(3-phenoxycinnamyl)-N-hydroxyacetamide
Click to Show/Hide
|
|||
| External Link | ||||
| WY-48989 | Terminated | [294] | ||
| Synonyms |
WY 48989; 136917-40-3; 4-((2-(7-Chloro-2-phenyl-2H-pyrazolo(4,3-c)quinolin-4-yl)ethyl)amino)benzonitrile; 4-[[2-(7-chloro-2-phenyl-2h-pyrazolo[4,3-c]quinolin-4-yl)ethyl]amino]benzonitrile; WY-48,989; AC1L307T; SCHEMBL10625720; DTXSID90160006; GYVYUQVVKBZPRY-UHFFFAOYSA-N; 4[[2-(7-Chloro-2-phenyl-2H-pyrazolo[4,3-c]quinolin-4-yl)ethyl]amino]benzonitrile
Click to Show/Hide
|
|||
| External Link | ||||
| FR-133605 | Terminated | [295] | ||
| External Link | ||||
| SR-26831 | Terminated | [296] | ||
| Synonyms |
SR 26831; AC1L30OS; ((5-(2-Chlorobenzyl-2-tert-butyloxycarbonyl))-4,5,6,7-tetrahydrothieno(3,2-c)pyridine)-N-oxide; Thieno(3,2-c)pyridinium, 5-((2-chlorophenyl)methyl)-2-(2,2-dimethyl-1-oxopropoxy)-4,5,6,7-tetrahydro-5-hydroxy-; [5-[(2-chlorophenyl)methyl]-5-hydroxy-6,7-dihydro-4H-thieno[3,2-c]pyridin-5-ium-2-yl] 2,2-dimethylpropanoate
Click to Show/Hide
|
|||
| External Link | ||||
| GDC-0834 | Terminated | [297] | ||
| Synonyms |
1133432-49-1; UNII-FM7JG3L4SR; FM7JG3L4SR; CHEMBL2057915; N-{3-[6-({4-[(2r)-1,4-Dimethyl-3-Oxopiperazin-2-Yl]phenyl}amino)-4-Methyl-5-Oxo-4,5-Dihydropyrazin-2-Yl]-2-Methylphenyl}-4,5,6,7-Tetrahydro-1-Benzothiophene-2-Carboxamide; GDC0834; CDOOFZZILLRUQH-GDLZYMKVSA-N; 2VL; GTPL9263; SCHEMBL1205333; GDC 0834; GDC 0834;GDC0834; ZINC59185874; BDBM50388183; AKOS030526267; SB18925; CS-3123; Benzo(b)thiophene-2-carboxamide, N-(3-(6-((4-((2R)-1,4-dimethyl-3-oxo-2-piperazinyl)phenyl)amino)-4,5-dihydro-4-methyl-5-oxo-2-pyraz
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-0275 | Terminated | [298] | ||
| External Link | ||||
| RPR 200765A | Terminated | [299] | ||
| Synonyms |
CHEMBL68211; SCHEMBL5770288; SCHEMBL13819552; SCHEMBL20555598; SCHEMBL18797648
Click to Show/Hide
|
|||
| External Link | ||||
| LY-221068 | Terminated | [300] | ||
| Synonyms |
LY 221068; 132392-39-3; AC1O5RGH; SCHEMBL2746042; ly221068; 5-((3,5-Bis(1,1,-dimethylethyl)-4-hydroxyphenyl)methylene)-3-(dimethylamino)-4-thiazolidinone; 4-Thiazolidinone, 5-((3,5-bis(1,1-dimethylethyl)-4-hydroxyphenyl)methylene)-3-(dimethylamino)-
Click to Show/Hide
|
|||
| External Link | ||||
| DPC-333 | Terminated | [301] | ||
| Synonyms |
BMS-561392; UNII-2X066A8676; BMS 561392; CHEMBL489100; BMS561392; 2X066A8676; DPC 333; 611227-74-8; SCHEMBL6350418; GTPL6509; BDBM50247606; C476910000; (2R)-2-[(3R)-3-amino-3-[4-[(2-methylquinolin-4-yl)methoxy]phenyl]-2-oxopyrrolidin-1-yl]-N-hydroxy-4-methylpentanamide
Click to Show/Hide
|
|||
| External Link | ||||
| PXS-2076 | Terminated | [302] | ||
| Synonyms |
PXS-2000; PXS-2030; Cannabinoid CB2 agonist (rheumatoid arthritis), Pharmaxis; TNFrelease inhibitor (rheumatoid arthritis), Pharmaxis
Click to Show/Hide
|
|||
| External Link | ||||
| ETP-46321 | Investigative | [303] | ||
| Synonyms |
1252594-99-2; CHEMBL2087474; 5-(2-((4-(methylsulfonyl)piperazin-1-yl)methyl)-8-morpholinoimidazo[1,2-a]pyrazin-6-yl)pyrimidin-2-amine; 5-[2-[(4-methylsulfonylpiperazin-1-yl)methyl]-8-morpholin-4-ylimidazo[1,2-a]pyrazin-6-yl]pyrimidin-2-amine; SCHEMBL10100315; BCP19935; ETP46321; EX-A1284; BDBM50420714; ZINC68247289; CS-3350; ETP 46321;ETP46321; HY-12340; 5-[2-[(4-methylsulfonylpiperazin-1-yl)methyl]-8-morpholin-4-yl-imidazo[1,2-a]pyrazin-6-yl]pyrimidin-2-amine
Click to Show/Hide
|
|||
| External Link | ||||
| ODN-1411 | Investigative | [304] | ||
| External Link | ||||
| PAC-10649 | Investigative | [305] | ||
| Synonyms |
PAC-10549; COX-2 inhibitors (pain/inflammation), Pacific Corp
Click to Show/Hide
|
|||
| External Link | ||||
| Silicon-modified indomethacin | Investigative | [306] | ||
| Synonyms |
Silexsyn; RND-100; SiM-100; Silicon-modified indomethacin (cancer); Silicon-modified indometacin (cancer), Silamed; Silicon-modified indomethacin (cancer), Silamed
Click to Show/Hide
|
|||
| External Link | ||||
| BFPT-16864 | Investigative | [111] | ||
| Synonyms |
BFPT-6864; BRFD-104
Click to Show/Hide
|
|||
| External Link | ||||
| Arthromir | Investigative | [111] | ||
| External Link | ||||
| NM-2014 | Investigative | [307] | ||
| External Link | ||||
| SPX-601 | Investigative | [111] | ||
| External Link | ||||
| CIGB-814 | Investigative | [111] | ||
| Synonyms |
Hsp60 peptide (juvenile idiopathic arthritis), CIGB
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-CD40-XTEN | Investigative | [308] | ||
| Synonyms |
CD40 ligand receptor antagonist (XTEN, rheumatoid arthritis/transplant rejection), Amunix
Click to Show/Hide
|
|||
| External Link | ||||
| TNFcept | Investigative | [309] | ||
| Synonyms |
TNFcept (rheumatoid arthritis)
Click to Show/Hide
|
|||
| External Link | ||||
| X-071-NAB | Investigative | [111] | ||
| Synonyms |
NATHMAB (systemic lupus erythematosus/multiple myeloma/arthritis), XBiotech; Natural/true human monoclonal antibody (systemic lupus erythematosus/multiple myeloma/arthritis), XBiotech
Click to Show/Hide
|
|||
| External Link | ||||
| TENOSAL | Investigative | [310] | ||
| Synonyms |
MR-Y134; 2-(2-Thienoyloxy)benzoic acid; 2-Thiophenecarboxylic acid salicyl ester
Click to Show/Hide
|
|||
| External Link | ||||
| DW-908 | Investigative | [111] | ||
| Synonyms |
DW-0908
Click to Show/Hide
|
|||
| External Link | ||||
| Recombinant human TNF receptor | Investigative | [309] | ||
| Synonyms |
Recombinant human TNF receptor (arthritis); RhTNFR (arthritis), Shanghai/Taizhou; Recombinant human TNFreceptor (arthritis), Taizhou Fudan-Zhangjiang Pharmaceutical/ Shanghai Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| XGP-110 | Investigative | [306] | ||
| Synonyms |
Synoviocyte/tumor cell inhibitor + COX-2 inhibitor (arthritis/cancer/cancer pain), Xaragen
Click to Show/Hide
|
|||
| External Link | ||||
| MDDR 217769 | Investigative | [111] | ||
| Synonyms |
(E)-2-(4,6-Difluoroindan-1-ylidene)acetamide
Click to Show/Hide
|
|||
| External Link | ||||
| FKB327 | Investigative | [19] | ||
| External Link | ||||
| DMARDs | Investigative | [111] | ||
| Synonyms |
DMARDs (oral, rheumatoid arthritis); DMARDs (oral, rheumatoid arthritis), Abbott; Disease-modifying antirheumatic drugs (oral, rheumatoid arthritis), Abbott
Click to Show/Hide
|
|||
| External Link | ||||
| TX-RAD | Investigative | [111] | ||
| Synonyms |
RA1-TX; RA1-TX (ankylosing spondylitis), TxCell; RA1-TX (rheumatoid arthritis), TxCell; RA1-TX (psoriatic arthritis,inflammatory disease), TxCell
Click to Show/Hide
|
|||
| External Link | ||||
| UR-5269 | Investigative | [111] | ||
| Synonyms |
Rheumatoid arthritis therapy, UBE Industries
Click to Show/Hide
|
|||
| External Link | ||||
| AVX-002 | Investigative | [111] | ||
| External Link | ||||
| XGP-125 | Investigative | [111] | ||
| Synonyms |
Synoviocyte inhibitor + glucocorticoid (arthritis), Xaragen
Click to Show/Hide
|
|||
| External Link | ||||
| DWJ-425 | Investigative | [111] | ||
| Synonyms |
DW-Ab2; MAb (RA), Daewoong; Monoclonal antibody (rheumatoid arthritis), Daewoong
Click to Show/Hide
|
|||
| External Link | ||||
| HL-1020 | Investigative | [111] | ||
| Synonyms |
Grape seed proanthocyanidin extract (rheumatoid arthritis), Hanlim
Click to Show/Hide
|
|||
| External Link | ||||
| XGP-120 | Investigative | [111] | ||
| Synonyms |
Synoviocyte inhibitor + DMARD (arthritis), Xaragen
Click to Show/Hide
|
|||
| External Link | ||||
| XGP-115 | Investigative | [111] | ||
| Synonyms |
Synoviocyte inhibitor + NSAID (arthritis), Xaragen
Click to Show/Hide
|
|||
| External Link | ||||
| CTLA-4-XTEN | Investigative | [311] | ||
| Synonyms |
Cytotoxic T-lymphocyte protein 4 modulator (XTEN, rheumatoid arthritis/transplant rejection), Amunix
Click to Show/Hide
|
|||
| External Link | ||||
| Nano-steroid | Investigative | [111] | ||
| Synonyms |
Steroidal anti-inflammatory (slow release nanoparticle formulation), LTT Bio-Pharma; Steroids (sustained release nanoparticles), LTT Bio-Pharma/Cipra; Steroidal anti-inflammatory (rheumatoid arthritis/collagen disease), LTT Bio-Pharma
Click to Show/Hide
|
|||
| External Link | ||||
| TNFmab | Investigative | [309] | ||
| Synonyms |
TNFmab (rheumatoid arthritis)
Click to Show/Hide
|
|||
| External Link | ||||
| YD-101 | Investigative | [111] | ||
| External Link | ||||
| KI-0906 | Investigative | [111] | ||
| External Link | ||||
| FHT-401 | Investigative | [111] | ||
| Synonyms |
PTD-conjugated methotrexate (transdermal, rheumatoid arthritis), ForHumanTech/Hyundai
Click to Show/Hide
|
|||
| External Link | ||||
| TCH-013 | Investigative | [111] | ||
| Synonyms |
NF-kB inhibitors (rheumatoid arthritis); NF-kB inhibitors (rheumatoid arthritis), TCH Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-CD97 mab | Investigative | [111] | ||
| Synonyms |
Anti-CD97 monoclonal antibody (rheumatoid arthritis); Anti-CD97 mAb (RA), Organon/AMC; Anti-CD97 monoclonal antibody (rheumatoid arthritis), Organon/AMC; Anti-CD97 mAb (RA), Schering-Plough/AMC; Anti-CD97 monoclonal antibody (rheumatoid arthritis), Merck & Co/AMC; Anti-CD97 monoclonal antibody (rheumatoid arthritis), Schering-Plough/AMC
Click to Show/Hide
|
|||
| External Link | ||||
| CIGB-55 | Investigative | [111] | ||
| External Link | ||||
| NM-9405 | Investigative | [307] | ||
| Synonyms |
Humanized Yalciomab (rheumatoid arthritis), Novelmed Therapeutics Inc; H-Yalciomab (rheumatoid arthritis), NovelmedTherapeutics Inc
Click to Show/Hide
|
|||
| External Link | ||||
| Dendritic cell-based immunotherapy | Investigative | [111] | ||
| Synonyms |
Dendritic cell-based immunotherapy (rheumatoid arthritis); Dendritic cell-based immunotherapy (rheumatoid arthritis), Dendright; Dendritic cell-based immunotherapy (rheumatoid arthritis, injectable formulation), Dendright
Click to Show/Hide
|
|||
| External Link | ||||
| RT-401 | Investigative | [111] | ||
| Synonyms |
BoNTA protein (topical, rhinitis), Revance
Click to Show/Hide
|
|||
| External Link | ||||
| VVZ-138 | Investigative | [111] | ||
| Synonyms |
VVZ-000138
Click to Show/Hide
|
|||
| External Link | ||||
| IG-AI-025 | Investigative | [111] | ||
| External Link | ||||
| CHR-4487 | Investigative | [111] | ||
| Synonyms |
Esterase-sensitive motif compounds (inflammatory disease); Esterase-sensitive motif compounds (inflammatory disease), Chroma Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| Ankinara | Investigative | [262] | ||
| External Link | ||||
| ROSMARINIC ACID | Investigative | [312] | ||
| Synonyms |
20283-92-5; Rosemary acid; (R)-rosmarinic acid; UNII-MQE6XG29YI; Rosmarinate; MQE6XG29YI; CHEMBL324842; CHEBI:50371; Labiatenic acid; C18H16O8; (R)-O-(3,4-Dihydroxycinnamoyl)-3-(3,4- dihydroxyphenyl)lactic acid; 3,4-Dihydroxycinnamic acid (R)-1-carboxy-2-(3,4-dihydroxyphenyl)ethyl ester; (2R)-3-(3,4-dihydroxyphenyl)-2-[(2E)-3-(3,4-dihydroxyphenyl)prop-2-enoyloxy]propanoic acid; 537-15-5; Rosemaric acid; (2R)-3-(3,4-dihydroxyphenyl)-2-[(E)-3-(3,4-dihydroxyphenyl)prop-2-enoyl]oxy-propanoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| CT-340 | Investigative | [111] | ||
| Synonyms |
TrkA kinase inhibitor (polyoxyethylene conjugated, LSE, arthritis, scarring, pain), Creabilis
Click to Show/Hide
|
|||
| External Link | ||||
| ADR58 | Investigative | [313] | ||
| External Link | ||||
| CEL-2000 | Investigative | [111] | ||
| Synonyms |
T-cell modulating vaccine (LEAPS, rheumatoid arthritis), CEL-SCI
Click to Show/Hide
|
|||
| External Link | ||||
| FM-201 | Investigative | [111] | ||
| External Link | ||||
| BMS-345541 | Investigative | [314] | ||
| Synonyms |
445430-58-0; BMS345541; BMS-345541 free base; BMS-345541 (free base); UNII-26SU0NEF5F; N1-(1,8-dimethylimidazo[1,2-a]quinoxalin-4-yl)ethane-1,2-diamine; 26SU0NEF5F; IKK Inhibitor III, BMS-345541; C14H17N5; 4-(2& -Aminoethyl)amino-1,8-dimethylimidazo[1,2-a]quinoxaline; BMS-345541(free base); IKK Inhibitor III; Kinome_3215; SCHEMBL118886; GTPL5669; CHEMBL249697; CTK8E9618; KS-00001CRN; CHEBI:91340; BDBM25919; DTXSID60196216; PSPFQEBFYXJZEV-UHFFFAOYSA-N; MolPort-035-395-836; HMS3653F06; HMS2043P05
Click to Show/Hide
|
|||
| External Link | ||||
| CAT-2200 | Investigative | [315] | ||
| Synonyms |
MEDI-571; Anti-IL-17 antibody (rheumatoid arthritis/systemic lupus erythematosus (SLE)), MedImmune; Anti-IL-17 antibody (rheumatoid arthritis/systemic lupus erythematosus (SLE)), Cambridge Antibody Technology (CAT)
Click to Show/Hide
|
|||
| External Link | ||||
| OCT-SG815 | Investigative | [111] | ||
| Synonyms |
OPG secretagogues (osteoporosis/arthritis), Oscotec; Osteoprotegerin secretagogues (osteoporosis/arthritis), Oscotec; OPG secretagogues (osteoporosis/arthritis), KT&G; Osteoprotegerin secretagogues (osteoporosis/arthritis), KT&G
Click to Show/Hide
|
|||
| External Link | ||||
References