m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05171
|
[1] | |||
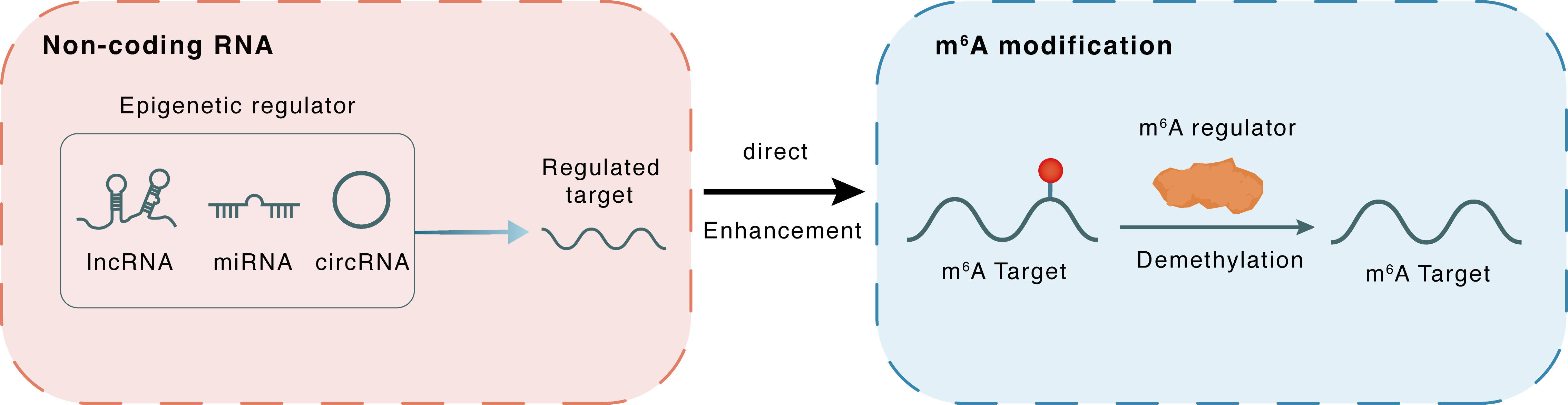 m6A modification
ESR1
ESR1
FTO
Demethylation
m6A modification
ESR1
ESR1
FTO
Demethylation
 : m6A sites
Direct
Enhancement
Non-coding RNA
miR-3492
ESR1
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
miR-3492
ESR1
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | |||
| m6A Target | Estrogen receptor (ESR1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | hsa-miR-3492 | microRNA | View Details | ||
| Regulated Target | Estrogen receptor (ESR1) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A modification regulates the functionality of ncRNAs through by altering the methylation levels of shared targets | ||||
| Crosstalk Summary | Mechanistically, the risk allele of rs67734009 increased Estrogen receptor (ESR1) expression via hsa-miR-3492 binding and m6A modification which is regulated by FTO. | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Estrogen receptor (ESR1) | 290 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Estrone | Approved | [2] | ||
| Synonyms |
Aquacrine; Crinovaryl; Cristallovar; Crystogen; Destrone; Disynformon; Endofolliculina; Esterone; Estrin; Estrol; Estron; Estrona; Estronum; Estrovarin; Estrugenone; Estrusol; Femidyn; Fermidyn; Folikrin; Folipex; Folisan; Follestrine; Follestrol; Folliculin; Folliculine; Follicunodis; Glandubolin; Hiestrone; Hormestrin; Hormofollin; Hormovarine; Kestrone; Ketodestrin; Ketohydroxyestrin; Ketohydroxyoestrin; Ketophydroxyestrin; Kolpon; Menagen; Menformon; Mestronaq; OESTRONE; Oestrin; Oestroform; Oestronum; Oestroperos; Ovifollin; Perlatan; Solliculin; Theelin; Thelestrin; Thelykinin; Thynestron; Tokokin; Unden; Unigen; Wehgen; Wynestron; Estrogenic Substance; Estrona [Spanish]; Femestrone injection; Follicular hormone; Folliculine benzoate; Hauck Brand of Estrone; Hyrex Brand of Estrone; Menformon A; Oestrone [Steroidal oestrogens]; Penncap M; Vortech Brand of Estrone; WynestronPencap M; CMC_13458; E 9750; E0026; E(sub 1); Estrona [INN-Spanish]; Estrone (E1); Estrone (TN); Estrone [USAN:INN]; Estrone-A; Estronum [INN-Latin]; Femestrone inj.; Ketohydroxy-Estratriene; NATURAL ESTROGENIC SUBSTANCE-ESTRONE; Oestrone, Estrone; Ovex (tablets); Unden (pharmaceutical); Unden (pharmaceutical) (VAN); Estrone (JAN/USP/INN); Estrone, (8 alpha)-Isomer; Estrone, (9 beta)-Isomer; Estrone, (+-)-Isomer; [2,4,6,7-3H]-E1; Delta-1,3,5-Estratrien-3beta-ol-17-one; Delta-1,3,5-Oestratrien-3beta-ol-17-one; Delta-1,3,5-estratrien-3-beta-ol-17-one; Delta-1,3,5-oestratrien-3-beta-ol-17-one; (13S)-3-hydroxy-13-methyl-7,8,9,11,12,13,15,16-octahydro-6H-cyclopenta[a]phenanthren-17(14H)-one; (8R,9S,13S,14S)-3-hydroxy-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-one; 1,3,5(10)-Estratrien-3-ol-17-one; 1,3,5(10)-Oestratrien-3-ol-17-one; 3-Hydroxy-1,3,5(10)-estratrien-17-one; 3-Hydroxy-17-keto-estra-1,3,5-triene; 3-Hydroxy-17-keto-oestra-1,3,5-triene; 3-Hydroxy-oestra-1,3,5(10)-trien-17-one; 3-Hydroxyestra-1,3,5(10)-trien-17-one; 3-Hydroxyestra-1,3,5(10)-triene-17-one; 3-hydroxy-estra-1,3,5(10)-trien-17-one
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 0.7 nM | |||
| External Link | ||||
| Conjugated estrogens b | Approved | [3] | ||
| Synonyms |
Enjuvia (TN)
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Raloxifene | Approved | [4] | ||
| Synonyms |
Raloxifene (extended-release, CDT)
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | Ki = 0.03 nM | |||
| External Link | ||||
| Ospemifene | Approved | [5] | ||
| Synonyms |
128607-22-7; Osphena; FC-1271a; Deamino-hydroxytoremifene; Fc-1271; 2-(4-(4-chloro-1,2-diphenyl-but-1-enyl)phenoxy)ethanol; UNII-B0P231ILBK; CCRIS 9205; B0P231ILBK; CHEBI:73275; 2-(p-((Z)-4-Chloro-1,2-diphenyl-1-butenyl)phenoxy)ethanol; senshio; Ethanol, 2-[4-[(1Z)-4-chloro-1,2-diphenyl-1-butenyl]phenoxy]-; 2-{4-[(1Z)-4-chloro-1,2-diphenylbut-1-en-1-yl]phenoxy}ethanol; Ophena; Ospemifene [USAN:INN:BAN]; Deaminotoremifene; Osphena (TN); Deaminohydroxytoremifene; AC1MI19T; C24H23ClO2
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Estropipate | Approved | [6] | ||
| Synonyms |
7280-37-7; Piperazine estrone sulfate; Harmogen; Ogen; Ortho-Est; UNII-SVI38UY019; Piperazine estronesulfate; Sulestrex; SVI38UY019; Estra-1,3,5(10)-trien-17-one, 3-(sulfooxy)-, compd. with piperazine (1:1); OGEN 2.5; Estropipate (USP); OGEN 5; Ogen (TN); OGEN .625; OGEN 1.25; EINECS 230-696-3; piperazine (8R,9S,13S,14S)-13-methyl-17-oxo-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-3-yl sulfate; Piperazine oestrone sulphate; Conjugated estrogens: piperazine estrone sulfate; Estrone hydrogen sulf
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Quinestrol | Approved | [7] | ||
| Synonyms |
EECPE; Eston; Estrovis; Estrovister; Plestrovis; Quilea; Quinestrolo; Quinestrolum; Quinestrolo [DCIT]; Estrovis 4000; W 3566; Estrovis (TN); Ethinyl Estradiol 3-Cyclopentyl Ether; Qui-Lea; Quinestrolum [INN-Latin]; W-3566; Quinestrol (USAN/INN); Quinestrol [USAN:INN:BAN]; Estradiol-17-beta 3-cyclopentyl ether; (8R,9S,13S,14S,17R)-3-cyclopentyloxy-17-ethynyl-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-ol; 17-alpha-Ethinylestradiol 3-cyclopentyl ether; 17ALPHA-ETHYLNYL-1,3,5[10]ESTRATRIENE-3,17BETA-DIOL 3-CYCLOPENTYL ETHER; 17alpha-Ethynyl-1,3,5(10)-estratriene-3,17beta-diol 3-cyclopentyl ether; 17alpha-Ethynylestradiol 3-cyclopentyl ether; 3-(Cyclopentyloxy)-19-nor-17-alpha-pregna-1,3,5(10)-trien-20-yn-17-ol; 3-(Cyclopentyloxy)-19-nor-17alpha-pregna-1,3,5(10)-trien-20-yn-17-ol; 3-(cyclopentyloxy)-17beta-ethynylestra-1,3,5(10)-trien-17-ol; 3-Cyclopentyloxy-17alpha-ethynylestra-1,3,5(10)-trien-17beta-ol
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Toremifene | Approved | [8] | ||
| Synonyms |
Acapodene; Estrimex; Farestone; Toremifeno; Toremifenum; Toremifene Base; Toremifeno [Spanish]; Toremifenum [Latin]; GTx 006; Acapodene (TN); FC-1157a; Fareston (TN); GTx-006; Toremifene (INN); Toremifene [INN:BAN]; Z-Toremifene; GTX-006 (Acapodene); Toremifene Citrate (1:1); {2-[4-(4-Chloro-1,2-diphenyl-but-1-enyl)-phenoxy]-ethyl}-dimethyl-amine; (Z)-2-(4-(4-Chloro-1,2-diphenyl-1-butenyl)phenoxy)-N,N-dimethylethanamine; 2-(p-[(Z)-4-chloro-1,2-diphenyl-1-butenyl]-phenoxy)-N,N-dimethyl-ethylamine citrate(1:1); 2-(para-((Z)-4-Chloro-1,2-diphenyl-1-butenyl)phenoxy)-N,N-dimethylethylamine (IUPAC); 2-({4-[(1Z)-4-chloro-1,2-diphenylbut-1-en-1-yl]phenyl}oxy)-N,N-dimethylethanamine; 2-[4-[(Z)-4-chloro-1,2-diphenylbut-1-enyl]phenoxy]-N,N-dimethylethanamine; 2-{4-[(1Z)-4-chloro-1,2-diphenylbut-1-en-1-yl]phenoxy}-N,N-dimethylethanamine
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Ethinyl Estradiol | Approved | [9] | ||
| Synonyms |
Aethinyloestradiolum; Aethinyoestradiol; Amenoron; Amenorone; Anovlar; Certostat; Cyclosa; Dicromil; Diprol; Dyloform; Ertonyl; Esteed; Estigyn; Estinyl; Estopherol; Estoral; Estorals; Ethidol; Ethinoral; Ethinylestradiolum; Ethinylestriol; Ethinyloestradiol; Ethynylestradiol; Ethynyloestradiol; Eticyclin; Eticyclol; Eticylol; Etinestrol; Etinestryl; Etinilestradiol; Etinilestradiolo; Etinoestryl; Etistradiol; Etivex; Feminone; Follicoral; Ginestrene; Inestra; Kolpolyn; Linoral; Lynoral; Menolyn; Microfollin; Novestrol; Oradiol; Orestralyn; Orestrayln; Palonyl; Perovex; Primogyn; Prosexol; Spanestrin; Ylestrol; Aethinyoestradiol [German]; Component of Demulen; Component of Oracon; Component of Ortrel; Diogyn E; Effik Brand of Ethinyl Estradiol; Estoral [Orion]; Ethinyl Estradiol Hemihydrate; Ethinyl Estradiol [USP]; Ethinyl Oestradiol Effik; Ethinylestradiol Jenapharm; Ethinyloestradiol [Steroidal oestrogens]; Ethynyl estradiol; Etinilestradiolo [DCIT]; Jenapharm Brand of Ethinyl Estradiol; Microfollin Forte; Organon Brand of Ethinyl Estradiol; PUBERTAL ETHINYL ESTRADIOL STUDY; Primogyn C; Primogyn M; Progynon C; Progynon M; Schering Brand of Ethinyl Estradiol; EE2; Ethinyl E2; Ethy 11; Diognat-E; Diogyn-E; EE(sub 2); Estinyl (TN); Eston-E; Estoral (Orion); Estoral (VAN); Estradiol, Ethinyl; Estradiol, Ethynyl; Ethinyl estradiol (USP); Ethinyl-Oestradiol Effik; Ethinyl-oestranol; Ethinylestradiolum [INN-Latin]; Ethynylestradiol, Ethinyl Estradiol; Etinilestradiol [INN-Spanish]; Hemihydrate, Ethinyl Estradiol; Jenapharm, Ethinylestradiol; Neo-Estrone; Nogest-S; OVULEN-21; OVULEN-28; Ortho-Cyclen; Chee-O-Gen; Chee-O-Genf; Ethinylestradiol (JP15/INN); Ethinylestradiol [INN:BAN:JAN]; Ethinyl Estradiol, (8 alpha)-Isomer; 17 alpha-Ethinylestradiol; 17 alpha-Ethynylestradiol; 17 alpha-Ethynyloestradiol; 17 alpha-ethinyestradiol; 17-Ethinyl-3,17-estradiol; 17-Ethinyl-3,17-oestradiol; 17-Ethinylestradiol; 17-Ethynylestradiol; 17-Ethynyloestradiol; 17-alpha-Ethinyl-17-beta-estradiol; 17-alpha-Ethynyl-17-beta-oestradiol; 17-alpha-Ethynylestradiol; 17-alpha-Ethynylestradiol-17-beta; 17-alpha-Ethynylestradiol-l7-beta; 17-alpha-Ethynyloestradiol-17-beta; 17-alpha-ethynyl estradiol; 17.alpha.-Ethinyl-17.beta.-estradiol; 17.alpha.-Ethinylestradiol; 17.alpha.-Ethynyl-17.beta.-oestradiol; 17.alpha.-Ethynylestradiol; 17.alpha.-Ethynyloestradiol; 17a-Ethynylestradiol; 17alpha-Ethinyl estradiol; 17alpha-Ethinylestradiol; 17alpha-Ethinylestradiol-17beta; 17alpha-Ethynylestradiol; 17alpha-Ethynyloestradiol; 17alpha-Ethynyloestradiol-17beta
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | IC50 = 2 nM | |||
| External Link | ||||
| Tamoxifen | Approved | [10] | ||
| Synonyms |
10540-29-1; trans-Tamoxifen; Crisafeno; Soltamox; Tamoxifene; Diemon; Tamoxifenum; Tamoxifeno; Tamizam; Istubol; Tamoxen; Citofen; Oncomox; Valodex; Retaxim; Tamoxifene [INN-French]; Tamoxifenum [INN-Latin]; Tamoxifeno [INN-Spanish]; Tamoxifen (Z); Tamoxifen and its salts; Tamoxifen [INN:BAN]; ICI-46474; ICI 47699; TRANS FORM OF TAMOXIFEN; CCRIS 3275; UNII-094ZI81Y45; HSDB 6782; CHEMBL83; EINECS 234-118-0; 1-p-beta-Dimethylaminoethoxyphenyl-trans-1,2-diphenylbut-1-ene; Nourytam; Novaldex; Tamone; Tamoxifeno;Tamoxifenum; Tomaxithen; Gen-Tamoxifen; Istubal (TN); Nolvadex (TN); Nolvadex-D; Novo-Tamoxifen; Pms-Tamoxifen; Tamoplex (TN); Tamoxifen (INN); Tamoxifen (TN); Valodex (TN); TAMOXIFEN (TAMOXIFEN CITRATE (54965-24-1)); Trans-2-[4-(1,2-Diphenyl-1-butenyl)phenoxy]-N,N-dimethylethylamine; (Z)-1-(p-Dimethylaminoethoxyphenyl)-1,2-diphenyl-1-butene; (Z)-2-(4-(1,2-Diphenyl-1-butenyl)phenoxy)-N,N-dimethylethanamine; (Z)-2-(4-(1,2-diphenylbut-1-enyl)phenoxy)-N,N-dimethylethanamine; (Z)-2-(para-(1,2-Diphenyl-1-butenyl)phenoxy)-N,N-dimethylamine (IUPAC); (Z)-2-[4-(1,2)-DIPHENYL-1-BUTENYL)-PHENOXY]-N,N-DIMETHYLETHANAMINE; (Z)-2-[p-(1,2-Diphenyl-1-butenyl)phenoxy]-N,N-dimethylethylamine; 1-p-beta-Dimethylamino-ethoxyphenyl-trans-1,2-diphenylbut-1-ene; 1-para-beta-Dimethylaminoethoxyphenyl-trans-1,2-diphenylbut-1-ene; 2-[4-[(Z)-1,2-diphenylbut-1-enyl]phenoxy]-N,N-dimethylethanamine; 2-{4-[(1Z)-1,2-diphenylbut-1-en-1-yl]phenoxy}-N,N-dimethylethanamine; Tamoxifen (Hormonal therapy); [3H]tamoxifen
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| Activity | IC50 = 8 nM | |||
| External Link | ||||
| Elacestrant | Approved | [11] | ||
| Synonyms |
ER-306323; ER-308698; SERMs, Eisai/Radius; Selective estrogen receptor modulators, Eisai/Radius
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Estradiol Valerate | Approved | [12] | ||
| Synonyms |
Delestrogen
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Estradiol | Approved | [2] | ||
| Synonyms |
Aerodiol; Alora; Altrad; Aquadiol; Bardiol; Climaderm; Climara; Compudose; Corpagen; Dermestril; Destradiol; Dihydrofolliculin; Dihydromenformon; Dihydrotheelin; Dihydroxyesterin; Dihydroxyestrin; Dihydroxyoestrin; Dimenformon; Diogyn; Diogynets; Divigel; Elestrin; Encore; Esclim; Estrace; Estraderm; Estradiolo; Estradiolum; Estradot; Estraldine; Estrasorb; Estreva; Estrifam; Estring; Estroclim; Estrodiolum; Estrogel; Estrovite; Evamist; Evorel; Extrasorb; Femanest; Femestral; Femestrol; Fempatch; Femtran; Follicyclin; Gelestra; Ginedisc; Ginosedol; GynPolar; Gynergon; Gynestrel; Gynodiol; Gynoestryl; Innofem; Lamdiol; Macrodiol; Macrol; Menest; Menorest; Microdiol; Nordicol; Oesclim; Oestergon; Oestradiol; Oestradiolum; Oestrogel; Oestroglandol; Oestrogynal; Ovahormon; Ovasterol; Ovastevol; Ovociclina; Ovocyclin; Ovocycline; Ovocylin; Perlatanol; Polyestradiol; Primofol; Profoliol; Progynon; Syndiol; Systen; Tradelia; Trocosone; Vagifem; Vivelle; Zerella; Zesteem; Zesteen; Zumenon; Climara Forte; Component of Menrium; Estraderm MX; Estraderm TTS; Estradiolo [DCIT]; Estradiolum [INN]; Estring vaginal ring; Estrofem Forte; Oestradiol Berco; Oestradiol R; Progynon DH; Sandrena Gel; Sisare Gel; Trial SAT; CMC_11154; Compudose 200; Compudose 365; E 2; E 8875; E0025; Epiestriol 50; Estraderm TTS 100; Estraderm TTS 50; Estrapak 50; Estroclim 50; Estrofem 2; Sandrena 1; [3H]]estradiol; Activella (TN); Alora (TN); Alpha-Oestradiol; AngeliQ (TN); B-Estradiol; Beta-Estradiol; Beta-Oestradiol; Cis-Estradiol; Cis-Oestradiol; Climara (TN); D-Estradiol; D-Oestradiol; Divigel (TN); E(sub 2); Elestrin (TN); Estrace (TN); Estraderm (TN); Estraderm TTS (TN); Estradiol [USAN:INN]; Estradiol acetate (TN); Estradiol cypionate (TN); Estradiol valerate (TN); Estradiol-17 beta; Estradiol-17beta; Estrasorb (TN); Estrasorb Topical (TN); Estring (TN); Estrofem (TN); Estrogel (TN); EvaMist (TN); Femring (TN); Innofem (TN); Menostar (TN); Oestradiol-17beta; Progynon-DH; Progynova (TN); S-21400; SK-Estrogens; SL-1100; VIVELLE-DOT; Vagifem (TN); Vivelle (TN); [3H]-estradiol; Estradiol-17-beta; Estradiol-3,17beta; Oestradiol-17-beta; Vivelle-Dot (TN); D-3,17beta-Estradiol; [2,4,6,7-3H]-E2; 17 beta-Estradiol; 17-.BETA.-Estradiol; 17-beta-OH-estradiol; 17-beta-estradiol; 17.beta.-Estradiol; 17.beta.-Oestradiol; 17b-Oestradiol; 17beta oestradiol; 17beta-Estradiol; 17beta-Oestradiol; 3,17-beta-Estradiol; 3,17-beta-Oestradiol; 3,17.beta.-Estradiol; 3,17beta-Estradiol
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 0.004 nM | |||
| External Link | ||||
| Danazol | Approved | [13] | ||
| Synonyms |
Anatrol; Chronogyn; Cyclomen; Danatrol; Danazant; Danazole; Danazolum; Danocrine; Danogen; Danokrin; Danol; Danoval; Danzol; Ladogal; Norciden; Panacrine; Winobanin; Alphapharm Brand of Danazol; Antigen Brand of Danazol; Kendrick Brand of Danazol; Ratiopharm Brand of Danazol; Sanofi Brand of Danazol; Sanofi Synthelabo Brand ofDanazol; Sanofi Winthrop Brand of Danazol; WIN 17757; Danazol-ratiopharm; Danazolum [INN-Latin]; Danocrine (TN); WIN 17,757; WIN-17757; Win 17, 757; Danazol (JAN/USP/INN); Danazol [USAN:BAN:INN:JAN];[1,2]oxazolo[4',5':2,3]-17alpha-pregn-4-en-20-yn-17-ol; (1R,3aS,3bR,10aR,10bS,12aS)-1-ethynyl-10a,12a-dimethyl-2,3,3a,3b,4,5,10,10a,10b,11,12,12a-dodecahydro-1H-cyclopenta[7,8]phenanthro[3,2-d]isoxazol-1-ol; (1S,2R,13R,14S,17R,18S)-17-ethynyl-2,18-dimethyl-7-oxa-6-azapentacyclo[11.7.0.0^{2,10}.0^{4,8}.0^{14,18}]icosa-4(8),5,9-trien-17-ol; 1-Ethynyl-10a,12a-dimethyl-2,3,3a,3b,4,5,10,10a,10b,11,12,12a-dodecahydro-1H-cyclopenta[7,8]phenanthro[3,2-d]isoxazol-1-ol; 17-alpha-2,4-Pregnadien-20-yno(2,3-d)isoxazol-17-ol; 17-alpha-Pregn-4-en-20-yno(2,3-d)isoxazol-17-ol; 17-alpha-Pregna-2,4-dien-20-yno(2,3-d)isoxazol-17-ol; 17.Alpha.-Pregna-2, {4-dien-20-yno[2,3-d]isoxazol-17-ol}; 17alpha-Pregna-2,4-dien-20-yno(2,3-d)isoxazol-17-ol
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| Dienestrol | Approved | [14] | ||
| Synonyms |
Agaldog; Cycladiene; Dehydrostilbestrol; Dehydrostilboestrol; Dienesterol; Dienestrolo; Dienestrolum; Dienoestrol; Dienol; Dinestrol; Dinovex; Estragard; Estraguard; Estrodienol; Estroral; Follidiene; Follormon; Gynefollin; Hormofemin; Isodienestrol; Oestrasid; Oestrodiene; Oestrodienol; Oestroral; Oestrovis; Restrol; Retalon; Sexadien; Synestrol; Teserene; Willnestrol; Dienestrolo [DCIT]; Dienoestrol [Nonsteroidal oestrogens]; Dienoestrol bp; Alpha-Dienestrol; DV (TN); Dienestrolum [INN-Latin]; Para-Dien; Restrol, Dienestrol; Dienestrol (E,E); Dienestrol (USP/INN); Dienestrol, (E,E)-Isomer; P,p'-(Diethylideneethylene)diphenol; Para,para'-(Diethylideneethylene)diphenol; Di(p-oxyphenyl)-2,4-hexadiene; Di(para-oxyphenyl)-2,4-hexadiene; Phenol, 4,4'-(1,2-diethylidene-1,2-ethanediyl)bis-, (E,E-(9CI); (E,E)-Dienestrol; 3,4-Bis(4-hydroxyphenyl)-2,4-hexadiene; 3,4-Bis(p-hydroxyphenyl)-2,4-hexadiene; 3,4-Bis(para-hydroxyphenyl)-2,4-hexadiene; 4,4'-(1,2-Diethylidene-1,2-ethanediyl)bisphenol; 4,4'-(2E,4E)-hexa-2,4-diene-3,4-diyldiphenol; 4,4'-(Diethylideneethylene)diphenol; 4,4'-Dihydroxy-gamma,delta-diphenyl-beta,delta-hexadiene; 4,4'-Hydroxy-gamma,delta-diphenyl-beta,delta-hexadiene; 4,4'-hexa-2,4-diene-3,4-diyldiphenol; 4-[(2E,4E)-4-(4-hydroxyphenyl)hexa-2,4-dien-3-yl]phenol
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Conjugated estrogens a | Approved | [15] | ||
| Synonyms |
Conestin (TN)
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Promestriene | Approved | [16] | ||
| Synonyms |
BCP9000134; VA11593; A824465; I06-0300
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Clomifene | Approved | [17] | ||
| Synonyms |
Androxal; Chlomaphene; Chloramifene; Cisclomifenum; Cisclomiphene; Clomifen; Clomifeno; Clomifenum; Clomifert; Clomiphene; Clostilbegit; Enclomifene; Enclomifeno; Enclomifenum; Enclomiphen; Enclomiphene; Klostilbegit; Transclomifenum; Transclomiphene; Zuclomifene; Zuclomifeno; Zuclomifenum; Zuclomiphene; Clomiphene B;Enclomiphene [USAN]; ISOMER B; Zuclomiphene [USAN]; Cis-Clomifene; Cis-Clomiphene; Clomid (TN); Clomifene (INN); Clomifene (TN); Clomifene [INN:BAN]; Clomifeno [INN-Spanish]; Clomifenum [INN-Latin]; En-Clomiphene; Enclomifeno [INN-Spanish]; Enclomifenum [INN-Latin]; Enclomiphene (USAN); Milophene (TN); RMI 16,289; RMI-16289; RMI-16312; Serophene (TN); Trans-Clomifene; Trans-Clomiphene; Zuclomifeno [INN-Spanish]; Zuclomifenum [INN-Latin]; Zuclomiphene (USAN); Cis-2-(p-(2-Chloro-1,2-diphenylvinyl)phenoxy)triethylamine; Trans-2-(p-(2-Chloro-1,2-diphenylvinyl)phenoxy)triethylamine; Cis-2-(4-(2-Chloro-1,2-diphenylethenyl)phenoxy)-N,N-diethylethanamine; Trans-2-(4-(2-Chloro-1,2-diphenylethenyl)phenoxy)-N,N-diethylethanamine; (E)-2-(p-(2-Chloro-1,2-diphenylvinyl)phenoxy)triethylamine; (Z)-2-(p-(2-Chloro-1,2-diphenylvinyl)phenoxy)triethylamine; (Z)-isomer; 1-(p-(beta-Diethylaminoethoxy)-phenyl)-1,2-diphenylchloroethylene; 2-(4-(2-Chloro-1,2-diphenylethenyl)phenoxy)-N,N-diethylethanamine; 2-(p-(2-Chloro-1,2-diphenylvinyl)phenoxy)triethylamine; 2-(p-(beta-Chloro-alpha-phenylstyryl)phenoxy)-triethylamine; 2-(p-(beta-chloro-alpha-phenylstyryl)phenoxy)triethylamine; 2-({4-[(Z)-2-chloro-1,2-diphenylethenyl]phenyl}oxy)-N,N-diethylethanamine; 2-[4-(2-chloro-1,2-diphenylethenyl)phenoxy]-N,N-diethylethanamine; 2-[4-[(E)-2-chloro-1,2-diphenylethenyl]phenoxy]-N,N-diethylethanamine; 2-[4-[(Z)-2-chloro-1,2-diphenylethenyl]phenoxy]-N,N-diethylethanamine; 2-{4-[(E)-2-chloro-1,2-diphenylethenyl]phenoxy}-N,N-diethylethanamine
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Clomiphene Citrate | Approved | [12] | ||
| Synonyms |
Clomid; Milophene; Serophene
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Mestranol | Approved | [18] | ||
| Synonyms |
Devocin; Menophase; Mestranolo; Mestranolum; Norquen; Ovastol; Component of Norinyl; Component of Norquen; Component of Ovulen; Ethynylestradiol methyl ether; Mestranol [Steroidal oestrogens]; Mestranolo [DCIT]; EE3ME; Ethinyl Estradiol 3 Methyl Ether; SC 4725; Component of Ortho-Novum; Delta-MVE; EE(sub3)ME; EthinylEstradiol 3-Methyl Ether; Ethinyloestradiol 3-methyl ether; Ethynylestradiol 3-methyl ether; Ethynyloestradiol 3-methyl ether; Inostral (steroid); Mestranolum [INN-Latin]; Mestranol (JP15/USP/INN); Mestranol [USAN:INN:BAN:JAN]; Alpha.-19-Norpregna-1,3,5(10)-trien-20-yn-17-ol, 3-meth; (17beta)-17-ethynyl-3-(methyloxy)estra-1,3,5(10)-trien-17-ol; 17-Ethynyl-3-methoxy-1,3,5(10)-oestratien-17-beta-ol; 17-Ethynyl-3-methoxyestra-1,3,5(10)-trien-17-ol; 17-Ethynylestradiol 3-methyl ether; 17-Ethynyloestradiol 3-methyl ether; 17-alpha-Ethinyl estradiol 3-methyl ether; 17-alpha-Ethinyl oestradiol 3-methyl ether; 17-alpha-Ethynyl-3-methoxy-1,3,5(10)-estratrien-17-beta-ol; 17-alpha-Ethynyloestradiol methyl ether; 17-ethynyl-3-methoxyestra-1(10),2,4-trien-17beta-ol; 17-ethynyl-3-methoxyoestra-1(10),2,4-trien-17beta-ol; 17alpha-Ethinyl estradiol 3-methyl ether; 17alpha-Ethinyl oestradiol 3-methyl ether; 17alpha-Ethinylestradiol 3-methyl ether; 17alpha-Ethynylestradiol 3-methyl ether; 17alpha-Ethynylestradiol methyl ether; 17alpha-Ethynyloestradiol 3-methyl ether; 17beta-Estradiol, 17-ethynyl-, 3-(methyl ether); 3-Methoxy-17-alpha-19-norpregna-1,3,5(10)-trien-20-yn-17-ol; 3-Methoxy-17-alpha-ethinylestradiol; 3-Methoxy-17-alpha-ethinyloestradiol; 3-Methoxy-17-alpha-ethynyl-1,3,5(10)-estratrien-17-beta-ol; 3-Methoxy-17-alpha-ethynyl-1,3,5(10)-oestratrien-17-beta-ol; 3-Methoxy-17-alpha-ethynylestradiol; 3-Methoxy-17-alpha-ethynyloestradiol; 3-Methoxy-17-ethynyloestradiol-17-beta; 3-Methoxy-17alpha-ethinylestradiol; 3-Methoxy-17alpha-ethinyloestradiol; 3-Methoxy-17alpha-ethynyl-1,3,5(10)-estratrien-17beta-ol; 3-Methoxy-17alpha-ethynyl-1,3,5(10)-oestratrien-17beta-ol; 3-Methoxy-17alpha-ethynylestradiol; 3-Methoxy-17alpha-ethynyloestradiol; 3-Methoxy-19-nor-17-alpha-pregna-1,3,5(10)-trien-20-yn-17-ol; 3-Methoxy-19-nor-17alpha-pregna-1,3,5(10)-trien-20-yn-17-ol; 3-Methoxy-19-norpregna-1,3,5(10)-trien-20-yn-17beta-ol; 3-Methoxyethynylestradiol; 3-Methoxyethynyloestradiol; 3-Methylethynylestradiol; 3-Methylethynyloestradiol; 3-O-Methylethynylestradiol
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Fulvestrant | Approved | [19] | ||
| Synonyms |
Faslodex; AstraZeneca brand of fulvestrant; Fulvestrant [USAN]; Ici 182780; ZD 182780; ZM 182780; Faslodex (TN); ZD-182780; ZD-9238; ZM-182780; Faslodex(ICI 182,780); Faslodex, ICI 182780, Fulvestrant; Fulvestrant (JAN/USAN/INN); (7R,13S,17S)-13-methyl-7-(9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl)-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol; (7R,8R,9S,13S,14S,17S)-13-methyl-7-[9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl]-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol; (7R,8S,9S,13S,14S,17S)-13-methyl-7-[9-(4,4,5,5,5-pentafluoropentylsulfinyl) nonyl]-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol; (7alpha,17beta)-7-{9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl}estra-1,3,5(10)-triene-3,17-diol; 7-(9-(4,4,5,5,5-pentafluoropentylsulfinyl)nonyl)estra-1,3,5(10)-triene-3,17-diol; 7alpha-(9-((4,4,5,5,5,-Pentafluoropentyl)sulfinyl)nonyl)estra-1,3,5(10)-triene-3,17beta-diol; 7alpha-(9-((4,4,5,5,5-Pentafluoropentyl)sulfinyl)nonyl)estra-1,3,5(10)-triene-3,17beta-diol; 7alpha-[9[(4,4,5,5,5-Pentafluropentyl)sulfinyl]nonyl]-estra-1,3,5(10)-triene-3, 17 beta diol; ICI
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| Activity | IC50 = 0.06 nM | |||
| External Link | ||||
| Fosfestrol | Approved | [20] | ||
| Synonyms |
Diethylstilbestrol diphosphate; 522-40-7; Phosphestrol; Phosphoestrolum; Honvan; Stilbestrol diphosphate; Desdp; Diethylstilbestryl diphosphate; Diethylstilbesterol diphosphate; ST52-Asta; Fosfestrolum [INN-Latin]; UNII-A0E0NMA80F; CCRIS 2778; (E)-Hex-3-ene-3,4-diylbis(4,1-phenylene) bis(dihydrogen phosphate); C18H22O8P2; EINECS 208-328-8; A0E0NMA80F; CHEBI:4532; Diethylstilbestrol diphosphate [USP]; NCGC00181335-01; Stilphostrol (TN); alpha,alpha'-Diethyl-(E)-4,4'-stilbenediol bis(dihydrogen phosphate)
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Bazedoxifene | Approved | [21] | ||
| Synonyms |
Brilence; Conbriza; Viviant; Bazedoxifene acetate; TSE-424; WAY-140424; WAY-TES 424
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 3.7 nM | |||
| External Link | ||||
| Gestrinone | Approved | [15] | ||
| Synonyms |
Dimetrose (TN)
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Cenestin | Approved | [22] | ||
| Synonyms |
Sodium equilin sulfate; Equilin sodium sulfate; Climopax; Estratab; 16680-47-0; Sodium equilin 3-monosulfate; Emopremarin; Novoconestron; Climestrone; Promarit; Primarin; Premarose; Premaril; Menotrol; Ganeake; Estropan; Estrifol; Premarina; Palopause; Menogen; Mannest; Hyphorin; Formatrix; Estromed; Equigyne; Prempak; Oestrilin; Menotab; Kestrin; Glyestrin; Estroate; Equilin 3-Sulfate Sodium Salt; Conjugen; Conestron; Ayerogen; Theogen; Sukingpo
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Esterified estrogens | Approved | [23] | ||
| MOA | Modulator | |||
| External Link | ||||
| Premarin/Trimegestone | Approved | [24] | ||
| Synonyms |
Conjugated estrogen tablets
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Estradiol Acetate | Approved | [12] | ||
| Synonyms |
Femring
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Estradiol Cypionate | Approved | [12] | ||
| Synonyms |
Depo-estradiol
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Nomegestrol acetate | Approved | [25] | ||
| Synonyms |
Lutenyl (TN)
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Levormeloxifene | Approved | [26] | ||
| Synonyms |
Centchroman; Centron; Ormeloxifene; Saheli; NNC-46-0020; Non-steroidal (oral, contraception/cancer); Non-steroidal (oral, contraception/cancer), Central Drug Research Institute (CDRI)
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Cyclofenil | Approved | [27] | ||
| Synonyms |
2624-43-3; Ondogyne; Cyclofenyl; Cyclophenyl; Cyclopenil; Fertodur; Sanocrisin; Cyclophenil; Sexovar; Rehibin; Sexadieno; Sexovid; Ondonid; Oginex; (Cyclohexylidenemethylene)bis(4,1-phenylene) diacetate; Neoclym; ICI 48213; Cyclofenilum [INN-Latin]; Ciclofenilo [INN-Spanish]; NSC 86464; H 3452; F 6066; UNII-J468V64WZ1; Cyclofenil [INN:BAN:DCF:JAN]; 4,4'-(Cyclohexylidenemethylene)diphenol diacetate ester; C23H24O4; EINECS 220-089-1; Bis(p-hydroxyphenyl)cyclohexyldienemethane diacetate; BRN 2014687; AI3-52271; F-6066
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | EC50 = 26 nM | |||
| External Link | ||||
| Diethylstilbestrol | Approved | [28] | ||
| Synonyms |
Acnestrol; Agostilben; Antigestil; Apstil; Bufon; Climaterine; Comestrol; Cyren; DES; Desma; Destrol; Diaethylstilboestrolum; Diastyl; Dibestrol; Dicorvin; Diethylstilbesterol; Diethylstilbestrolum; Diethylstilboesterol; Dietilestilbestrol; Dietilstilbestrolo; Distilbene; Domestrol; Dyestrol; Estril; Estrobene; Estrogenine; Estromenin; Estrosyn; Fonatol; Grafestrol; Gynopharm; Hibestrol; Idroestril; Iscovesco; Makarol; Menostilbeen; Micrest; Microest; Milestrol; OeKolp; Oestrogenine; Oestrolmensil; Oestromenin; Oestromensil; Oestromensyl; Oestromienin; Oestromon; Pabestrol; Palestrol; Protectona; Sedestran; Serral; Sexocretin; Sibol; Sintestrol; Stibilium; Stilbestroform; Stilbestrol; Stilbestrone; Stilbetin; Stilboefral; Stilboestroform; Stilboestrol; Stilbofolin; Stilbofollin; Stilbol; Stilkap; Strobene; Synestrin; Synthestrin; Synthoestrin; Synthofolin; Syntofolin; Tampovagan; Tylosterone; Vagestrol; APS Brand of Diethylstilbestrol; Anti gestil; Co Pharma Brand of Diethylstilbestrol; Comestrol estrobene; Cyren A; Diethylstilbestrol BP; Dietilestilbestrol [Spanish]; Dietilstilbestrolo [DCIT]; Estilbin MCO; Gerda Brand of Diethylstilbestrol; Oestrol vetag; Percutatrine oestrogenique iscovesco; ST IL; Stilbene Estrogen; Tampovagan stilboestrol; DiBestrol 2 Premix; MG 137; Rumestrol 1; Rumestrol 2; Cis-Des; Cis-Diethylstilbesterol; Cis-Diethylstilbestrol; Co-Pharma Brand of Diethylstilbestrol; DES (synthetic estrogen); Dawe's destrol; Di-Estryl; Diethylstilbestrol (DES); Diethylstilbestrol [USAN:INN]; Diethylstilbestrol, Disodium Salt; Diethylstilbestrolum [INN-Latin]; Dietilestilbestrol [INN-Spanish]; E-Diethylstilbestrol; Estilbin (MCO); Estrogen, Stilbene; Hi-Bestrol; Neo-Oestranol 1; Neo-Oestranol I; Neo-oe stranol 1; New-Estranol 1; Stil-Rol; Stilbestrol (TN); TRANS-DIETHYSTILBESTEROL; TRANSGENIC MODEL EVALUATION (DES); Trans-Diethylstilbesterol; Trans-Diethylstilbestrol; Trans-Diethylstilboesterol; DiBestrol "2" Premix; Dibestrol '2' premix; Diethylstilbestrol (USP/INN); Alpha,alpha'-Diethylstilbenediol; Diethylstilbestrol, (Z)-Isomer; Alpha,alpha'-Diethyl-4,4'-stilbenediol; (E)-3,4-Bis(4-hydroxyphenyl)-3-hexene; (E)-4,4'-(hex-3-ene-3,4-diyl)diphenol; (E)-Diethylstilbestrol; 3,4'(4,4'-Dihydroxyphenyl)hex-3-ene; 3,4-Bis(p-hydroxyphenyl)-3-hexene; 3,4-bis(4-hydroxyphenyl)hex-3-ene; 4,4'-(3E)-hex-3-ene-3,4-diyldiphenol; 4,4'-Dihydroxydiethylstilbene; 4,4'-hex-3-ene-3,4-diyldiphenol
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 0.007 nM | |||
| External Link | ||||
| Estriol | Approved | [2] | ||
| Synonyms |
Aacifemine; Destriol; Estratriol; Estriel; Estriolo; Gynaesan; Hemostyptanon; Holin; Hormomed; Hormonin; Klimoral; Oestratriol; Oestriol; Oestriolum; Orestin; Orgastyptin; Overstin; Ovestin; Ovestrion; Stiptanon; Synapause; Theelol; Thulol; Tridestrin; Trihydroxyestrin; Trihydroxyoestrin; Triodurin; Triovex; Deuslon A; Estriolo [Italian]; Folicular hormone; Follicular hormone hydrate; Oestriol [Steroidal oestrogens]; A 13610; E0218; OE3; Deuslon-A; Estriel (TN); Estriol [USAN:JAN]; Estriol, unconjugated; Ortho-Gynest; Estriol (JP15/USP); Estra-1,3,5(10)-trien-3,16alpha,17beta-triol; Estra-1,3,5(10)-triene-3,16,17-triol; Estra-1,3,5(10)-triene-3,16alpha,17beta-triol; Oestra-1,3,5(10)-triene-3,16alpha,17beta-triol; Oestra-1,3,5(10)-triene-3,16-alpha,17-beta-triol; Estra-1,3,5(10)-trien-3,16.alpha., 17.beta.-triol; Estra-1,3,5(10)-trien-3,16.alpha.,17.beta.-triol; Estra-1,3,5(10)-triene-3,16.alpha., 17.beta.-triol; Estra-1,3,5(10)-triene-3,16.alpha.,17.beta.-triol; Oestra-1,3,5(10)-triene-3,16.alpha., 17.beta.-triol; Oestra-1,3,5(10)-triene-3,16.alpha.,17.beta.-triol; (16.alpha.,17.beta.)-Estra-1,3,5(10)-triene-3,16,17-triol; (16.alpha.,17.beta.)-Oestra-1,3,5(10)-triene-3,16,17-triol; (16alpha,17beta)-Estra-1,3,5(10)-triene-3,16,17-triol; (16alpha,17beta)-Oestra-1,3,5(10)-triene-3,16,17-triol; 1,3,5(10)-ESTRATRIENE-3,16,17-TRIOL; 1,3,5(10)-Estratriene-3,16-alpha,17beta-triol; 1,3,5(10)-Estratriene-3,16.alpha., 17.beta.-triol; 1,3,5(10)-Estratriene-3,16.alpha.,17.beta.-triol; 1,3,5(10)-Estratriene-3,16alpha,17beta-Triol; 1,3,5-Estratriene-3.beta.,16-.alpha.,17-.beta.-triol; 1,3,5-Estratriene-3beta,16alpha,17beta-triol; 1,3,5-Oestratriene-3-.beta.,16.alpha.,17.beta.-triol; 1,3,5-Oestratriene-3beta,16alpha,17beta-triol; 16,17-Epiestriol; 16-Epiestriol; 16-Hydroxyestradiol; 16-alpha,17-beta-Estriol; 16-alpha,17-beta-Oestriol; 16-alpha-Hydroxyestradiol; 16-alpha-Hydroxyoestradiol; 16.alpha.,17.beta.-Estriol; 16.alpha.,17.beta.-Oestriol; 16.alpha.-Estriol; 16.alpha.-Hydroxy-17.beta.-estradiol; 16.alpha.-Hydroxyestradiol; 16.alpha.-Hydroxyoestradiol; 16alpha,17beta-Estriol; 16alpha,17beta-Oestriol; 16alpha-Hydroxy-17beta-estradiol; 16alpha-Hydroxyestradiol; 16alpha-Hydroxyoestradiol; 3,16-alpha,17-beta-Estriol; 3,16-alpha,17-beta-Oestriol; 3,16-alpha,17-beta-Trihydroxy-delta-1,3,5-estratriene; 3,16-alpha,17-beta-Trihydroxy-delta-1,3,5-oestratriene; 3,16-alpha,17-beta-Trihydroxyestra-1,3,5(10)-triene; 3,16-alpha,17-beta-Trihydroxyoestra-1,3,5(10)-triene; 3,16.alpha.,17.beta.-Estriol; 3,16.alpha.,17.beta.-Trihydroxy-.delta.-1,3,5-estratriene; 3,16.alpha.,17.beta.-Trihydroxy-.delta.-1,3,5-oestratriene; 3,16.alpha.,17.beta.-Trihydroxy-1,3,5(10)-estratriene; 3,16.alpha.,17.beta.-Trihydroxyestra-1,3,5(10)-triene; 3,16alpha,17beta-Estriol; 3,16alpha,17beta-Trihydroxy-1,3,5(10)-estratriene; 3,16alpha,17beta-Trihydroxy-delta-1,3,5-oestratriene; 3,16alpha,17beta-trihydroxy-Delta(1,3,5)-estratriene
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 1.995 nM | |||
| External Link | ||||
| Mitotane | Approved | [29] | ||
| Synonyms |
Chloditan; Chlodithan; Chlodithane; Khloditan; Khlodithan; Lysodren; Mitotan; Mitotano; Mitotanum; Mytotan; Bristol Myers Squibb Brand of Mitotane; C 3010; CB 313; CB313; PS694_SUPELCO; Bristol-Myers Squibb Brand of Mitotane; CB-313; Lysodren (TN); Mitotano [INN-Spanish]; Mitotanum [INN-Latin]; Ortho,para DDD; Mitotane [USAN:INN:JAN]; O,p-DDD; O,p-Tde; Ortho,para-DDD; Mitotane (JAN/USP/INN); O,p'-DDD; O,p'-Dichlorodiphenyldichloroethane; O,p'-TDE; Ethane, 1,1-dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl)-(8CI); (+-)-1,1-Dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl)ethane; (2,4'-Dichlorodiphenyl)dichloroethane; (o,p)-DDD; 1,1-Dichloro-2,2-bis(2,4'-dichlorophenyl)ethane; 1,1-Dichloro-2-(o-chlorophenyl)-2-(p-chlorophenyl)ethane; 1,1-Dichloro-2-(p-chlorophenyl)-2-(o-chlorophenyl)ethane; 1-(2-Chlorophenyl)-1-(4-chlorophenyl)-2,2-dichloroethane; 1-Chloro-2-(2,2-dichloro-1-(4-chlorophenyl)ethyl)benzene; 1-Chloro-2-[2,2-dichloro-1-(4-chlorophenyl)ethyl]benzene; 2,2-Bis(2-chlorophenyl-4-chlorophenyl)-1,1-dichloroethane; 2,4'-Ddd; 2,4'-Dichlorodiphenyldichloroethane; 2,4'-Dichlorophenyldichlorethane; 2-(2-Chlorophenyl)-2-(4-chlorophenyl)-1,1-dichloroethane; 2-(o-Chlorophenyl)-2-(p-chlorophenyl)-1,1-dichloroethane
Click to Show/Hide
|
|||
| MOA | Binder | |||
| External Link | ||||
| ARZOXIFENE | Approved | [30] | ||
| Synonyms |
182133-25-1; UNII-E569WG6E60; LY 353381; E569WG6E60; 2-(4-methoxyphenyl)-3-[4-(2-piperidin-1-ylethoxy)phenoxy]-1-benzothiophen-6-ol; 2-(4-methoxyphenyl)-3-(4-(2-(piperidin-1-yl)ethoxy)phenoxy)benzo[b]thiophen-6-ol; Arzoxifene [INN]; LY-353381; SCHEMBL285277; CHEMBL226267; BDBM19442; DTXSID10171255; ZINC1544683; AC1L4522; AN-538; AKOS030631785; SB19713; KB-05502; FT-0751607; 124708-EP2295426A1; 124708-EP2295427A1; 124708-EP2292592A1; 124708-EP2292576A2; Benzo(b)thiophene-6-ol, 2-(4-methoxyphenyl)-3-(4-(2-(1-piperidinyl)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 0.2 nM | |||
| External Link | ||||
| Lasofoxifene | Approved | [31] | ||
| Synonyms |
Oporia; 180916-16-9; rac-Lasofoxifene; CP 336156; Fablyn; UNII-337G83N988; (5R,6S)-6-phenyl-5-[4-(2-pyrrolidin-1-ylethoxy)phenyl]-5,6,7,8-tetrahydronaphthalen-2-ol; CHEMBL328190; 337G83N988; (-)-cis-5,6,7,8-Tetrahydro-6-phenyl-5-(p-(2-(1-pyrrolidinyl)ethoxy)phenyl)-2-naphthol; 180915-78-0; CP-336,156; LASOFOXIFENE HCL; Lasofoxifene [INN:BAN]; AC1L50OI; SCHEMBL26815; GTPL7542; CTK8F1062; BDBM20606; DTXSID50171037; GXESHMAMLJKROZ-IAPPQJPRSA-N; ZINC3918428; BCP03626; AKOS030241621; AN-3516; BCP9000842; DB06202; Lasofoxifene [INN]; Cis-1R-(4'-pyrrolidinoethoxyphenyl)-2S-phenyl-6-hydroxy-1,2,3,4-tetrahydronaphthalene, tartrate salt; AZD9639
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 1.3 nM | |||
| External Link | ||||
| Estrogen | Approved | [32] | ||
| MOA | Agonist | |||
| External Link | ||||
| Synthetic conjugated estrogen | Phase 3 | [33] | ||
| Synonyms |
Bijuva; Bithena; DR-2041; SCE-A Vaginal Cream; Synthetic conjugated estrogen (cream, vulvovaginal atrophy); Synthetic conjugated estrogen (cream, vulvovaginal atrophy), Teva
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Trimegestone/ethinyl estradiol | Phase 3 | [34] | ||
| MOA | Modulator | |||
| External Link | ||||
| TZTX-001 | Phase 3 | [35] | ||
| Synonyms |
Teverelix LA; TX 12-001-HR
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Acolbifene | Phase 3 | [36] | ||
| Synonyms |
EM-652; 182167-02-8; UNII-815LJ9X0D1; 815LJ9X0D1; EM 652; Acolbifene [INN:BAN]; SCH 57068; AC1L4EAA; SCHEMBL406183; CHEMBL68055; CTK4F5320; SCH-57068; HY-16023A; ( )-(2S)-3-(4-Hydroxyphenyl)-4-methyl-2-(4-(2-(piperidin-1-yl)ethoxy)phenyl)-2H-1-benzopyran-7-ol; CS-0007143; (2S)-3-(4-hydroxyphenyl)-4-methyl-2-[4-(2-piperidin-1-ylethoxy)phenyl]-2H-chromen-7-ol; (S)-3-(4-hydroxyphenyl)-4-methyl-2-(4-(2-(piperidin-1-yl)ethoxy)phenyl)-2H-chromen-7-ol
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| NE3107 | Phase 3 | [37] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Giredestrant | Phase 3 | [38] | ||
| Synonyms |
(1R,3R)-1-(2,6-DIFLUORO-4-((1-(3-FLUOROPROPYL)-3-AZETIDINYL)AMINO)PHENYL)-.BETA.,.BETA.-DIFLUORO-1,3,4,9-TETRAHYDRO-3-METHYL-2H-PYRIDO(3,4-B)INDOLE-2-PROPANOL; 1953133-47-5; 28P3DU6DB3; 2H-PYRIDO(3,4-B)INDOLE-2-PROPANOL, 1-(2,6-DIFLUORO-4-((1-(3-FLUOROPROPYL)-3-AZETIDINYL)AMINO)PHENYL)-.BETA.,.BETA.-DIFLUORO-1,3,4,9-TETRAHYDRO-3-METHYL-, (1R,3R)-; 2H-Pyrido(3,4-b)indole-2-propanol, 1-(2,6-difluoro-4-((1-(3-fluoropropyl)-3-azetidinyl)amino)phenyl)-beta,beta-difluoro-1,3,4,9-tetrahydro-3-methyl-, (1R,3R)-; 3-((1R,3R)-1-(2,6-Difluoro-4-((1-(3-fluoropropyl)azetidin-3-yl)amino)phenyl)-3-methyl-1,3,4,9-tetrahydro-2H-pyrido(3,4-b)indol-2-yl)-2,2-difluoropropan-1-ol; 3-((1R,3R)-1-(2,6-Difluoro-4-((1-(3-fluoropropyl)azetidin-3-yl)amino)phenyl)-3-methyl-1,3,4,9-tetrahydro-2H-pyrido[3,4-b]indol-2-yl)-2,2-difluoropropan-1-ol; 3-((1R,3R)-1-(2,6-difluoro-4-((1-(3-fluoropropyl)azetidin-3-yl)amino)phenyl)-3-methyl-3,4-dihydro-1H-pyrido[3,4-b]indol-2(9H)-yl)-2,2-difluoropropan-1-ol; 3-[(1R,3R)-1-(2,6-difluoro-4-{[1-(3-fluoropropyl)azetidin-3-yl]amino}phenyl)-3-methyl-1,3,4,9-tetrahydro-2H-pyrido[3,4-b]indol-2-yl]-2,2-difluoropropan-1-ol; 3-[(1R,3R)-1-[2,6-difluoro-4-[[1-(3-fluoropropyl)azetidin-3-yl]amino]phenyl]-3-methyl-1,3,4,9-tetrahydropyrido[3,4-b]indol-2-yl]-2,2-difluoropropan-1-ol; 3-[(1R,3R)-1-[2,6-difluoro-4-[[1-(3-fluoropropyl)azetidin-3-yl]amino]phenyl]-3-methyl-1,3,4,9-tetrahydropyrido[3,4-b]indol-2-yl]-2,2-difluoro-propan-1-ol; AC-37111; AKOS040733254; AT22918; BDBM50572808; CHEMBL4650316; compound 35 [PMID: 34251202]; CS-0116370; EX-A3541; GDC9545; GDC-9545; Giredestrant; Giredestrant [INN]; Giredestrant [USAN:INN]; Giredestrant [USAN]; GIREDESTRANT [WHO-DD]; GTPL12715; HY-109176; MS-29682; NSC827275; NSC-827275; RG6171; RG-6171; RO7197597; RO-7197597; SCHEMBL17839430; UNII-28P3DU6DB3; WHO 11226; ZNM
Click to Show/Hide
|
|||
| MOA | Degrader | |||
| External Link | ||||
| Imlunestrant | Phase 3 | [39] | ||
| Synonyms |
(5R)-5-(4-(2-(3-(fluoromethyl)azetidin-1-yl)ethoxy)phenyl)-8-(trifluoromethyl)-5H-(1)benzopyrano(4,3-c)quinolin-2-ol; (5R)-5-[4-[2-[3-(fluoromethyl)azetidin-1-yl]ethoxy]phenyl]-8-(trifluoromethyl)-5H-chromeno[4,3-c]quinolin-2-ol; 2408840-26-4; 5H-(1)Benzopyrano(4,3-c)quinolin-2-ol, 5-(4-(2-(3-(fluoromethyl)-1-azetidinyl)ethoxy)phenyl)-8-(trifluoromethyl)-, (5R)-; 9CXQ3PF69U; BDBM443429; CHEMBL5095183; CS-0376104; EX-A6123; Example 1B {WO2020014435A1]; GLXC-26209; GTPL12896; HY-145572; Imlunestrant; IMLUNESTRANT [INN]; Imlunestrant [USAN]; Ly 3484356; LY3484356; LY-3484356; SCHEMBL22002569; UNII-9CXQ3PF69U; US10654866, Example 1A; UVBQMXOKKDCBJN-MUUNZHRXSA-N; WHO 12039
Click to Show/Hide
|
|||
| MOA | Degrader | |||
| External Link | ||||
| NPC-01 | Phase 3 | [40] | ||
| Synonyms |
LEP pill; Low dose estrogen progestin pill (dysmenorrhea), Nobelpharma
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Premarin/Pravachol | Phase 3 | [41] | ||
| Synonyms |
Estrogen/pravastatin
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| ARN-810 | Phase 2 | [42] | ||
| MOA | Modulator | |||
| Activity | Ki = 0.37 nM | |||
| External Link | ||||
| SR 16234 | Phase 2 | [43] | ||
| Synonyms |
229634-98-4; TS 108; TAS-108; SR16234; UNII-9B29N23K7E; 9B29N23K7E; SCHEMBL2836841; DTXSID30177512; C33H47NO3.C6H8O7; (7alpha)-21-(4-((Diethylamino)methyl)-2-methoxyphenoxy)-7-methyl-19-norpregna-1,3,5(10)-trien-3-ol 2-hydroxy-1,2,3-propanetricarboxylate; Y1847; 354808-47-2; (7R,8S,9S,13R,14S,17R)-17-(2-(4-((Diethylamino)methyl)-2-methoxyphenoxy)ethyl)-7,13-dimethyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-3-ol 2-hydroxypropane-1,2,3-tricarboxylate
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Afimoxifene | Phase 2 | [4] | ||
| Synonyms |
4-Hydroxytamoxifen; (Z)-4-Hydroxytamoxifen; Hydroxytamoxifen; 4-Monohydroxytamoxifen; Tamogel; 68047-06-3; trans-4-Hydroxytamoxifen; Ici 79280; 68392-35-8; 65213-48-1; 4-OH-TAM; (Z)-4-(1-(4-(2-(Dimethylamino)ethoxy)phenyl)-2-phenylbut-1-en-1-yl)phenol; Z-4-hydroxytamoxifen; UNII-95K54647BZ; CHEMBL489; ICI 79,280; TAMOXIFEN, 4-HYDROXY-, (Z)-; BRN 4910749; (E/Z)-4-Hydroxy Tamoxifen; MLS000069742; C26H29NO2; CHEBI:44616; 95K54647BZ; SMR000058939; 4-(1-(4-(2-(Dimethylamino)ethoxy)phenyl)-2-phenylbut-1-enyl)phenol
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 0.79 nM | |||
| External Link | ||||
| GTx-758 | Phase 2 | [44] | ||
| MOA | Modulator | |||
| External Link | ||||
| ICARITIN | Phase 2 | [45] | ||
| Synonyms |
118525-40-9; UNII-UFE666UELY; UFE666UELY; CHEMBL498485; 3,5,7-trihydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-enyl)chromen-4-one; 3,5,7-Trihydroxy-2-(4-methoxyphenyl)-8-(3-methyl-2-buten-1-yl)-4H-1-benzopyran-4-one; Icartin; Anhydroicaritin; AC1NSXIV; Icaritin(Anhydroicaritin); MLS006010055; BIDD:ER0021; SCHEMBL4223542; Icaritin, > DTXSID00152154; MolPort-020-006-012; TUUXBSASAQJECY-UHFFFAOYSA-N; HY-N0678; ZINC14762797; BDBM50272527; AKOS015896858; CS-3679; DB12672; NCGC00345813-01; AK168251; SMR004701218
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Estetrol | Phase 2 | [46] | ||
| Synonyms |
Estetrol (oral, contraception/hormone deficiency/osteoporosis/cardiovascular disease/autoimmune disease/breast cancer/prostate cancer); Estetrol (oral, contraception/hormone deficiency/osteoporosis/cardiovascular disease/autoimmune disease/breast cancer/prostate cancer), Pantarhei; Estrogen receptor modulator (oral, contraception/hormone deficiency/autoimmune disease/breast cancer/prostate cancer/osteoporosis/cardiovascular disease), Pantarhei
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Endoxifen | Phase 2 | [4] | ||
| Synonyms |
Endoxifen (topical formulation, cancer); Endoxifen (topical formulation, cancer), Jina Pharmaceuticals; Tamoxifen metabolite (topical formulation, cancer), Jina Pharmaceuticals
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| AZD9833 | Phase 2 | [47] | ||
| Synonyms |
AZD-9833; UNII-JUP57A8EPZ; JUP57A8EPZ; 2222844-89-3; Camizestrant; Camizestrant [USAN]; SCHEMBL20089710; NSC828717; WHO 11592; NSC-828717; HY-136255; AZ14066724; CS-0121043; 3-Pyridinamine, N-(1-(3-fluoropropyl)-3-azetidinyl)-6-((6S,8R)-6,7,8,9-tetrahydro-8-methyl-7-(2,2,2-trifluoroethyl)-3H-pyrazolo(4,3-F)isoquinolin-6-yl)-
Click to Show/Hide
|
|||
| MOA | Degrader | |||
| External Link | ||||
| H3B-6545 | Phase 1/2 | [48] | ||
| Synonyms |
JPFTZIJTXCHJNE-HMOQVRKWSA-N; SCHEMBL18261010; HY-112596; CS-0047714; (2e)-N,N-dimethyl-4-{[2-({5-[(1z)-4,4,4-trifluoro-1-(3-fluoro-1h-indazol-5-yl)-2-phenylbut-1-en-1-yl]pyridin-2-yl}oxy)ethyl]amino}but-2-enamide
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| ZN-c5 | Phase 1/2 | [49] | ||
| MOA | Degrader | |||
| External Link | ||||
| OP-1250 | Phase 1/2 | [50] | ||
| MOA | Antagonist | |||
| External Link | ||||
| CC-8490 | Phase 1 | [51] | ||
| MOA | Modulator | |||
| External Link | ||||
| CHF-4227 | Phase 1 | [52] | ||
| Synonyms |
CHF-3316; CHF-4056; SERMs, Chiesi; Selective estrogen receptor modulators, Chiesi; CHF-3316.01
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| ATD transdermal gel | Phase 1 | [4] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| D-0502 | Phase 1 | [53] | ||
| MOA | Degrader | |||
| External Link | ||||
| TTC-352 | Phase 1 | [48] | ||
| MOA | Agonist | |||
| External Link | ||||
| AZD9496 | Phase 1 | [48] | ||
| Synonyms |
DFBDRVGWBHBJNR-BBNFHIFMSA-N; 1639042-08-2; UNII-DA9P7LN909; DA9P7LN909; CHEMBL3623004; AZD-9496; (E)-3-(3,5-difluoro-4-((1R,3R)-2-(2-fluoro-2-methylpropyl)-3-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indol-1-yl)phenyl)acrylic acid; (E)-3-(3,5-Difluoro-4-((1R,3R)-2-(2-fluoro-2-methylpropyl)-3-methyl-2,3,4,9-tetrahydro-1H-pyrido(3,4-b)indol-1-yl)phenyl)acrylic acid; SCHEMBL16266273; SCHEMBL16266275; MolPort-044-560-384; EX-A1326; s8372; BDBM50125052; ZINC219669733; AKOS030526632; AC-29011; HY-12870; Selective estrogen
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| Activity | IC50 = 0.138 nM | |||
| External Link | ||||
| G1T-48 | Phase 1 | [54] | ||
| MOA | Degrader | |||
| External Link | ||||
| LY3484356 | Phase 1 | [55] | ||
| MOA | Degrader | |||
| External Link | ||||
| AC0682 | Phase 1 | [56] | ||
| MOA | Degrader | |||
| External Link | ||||
| SCO-120 | Phase 1 | [57] | ||
| MOA | Degrader | |||
| External Link | ||||
| TTC-352 | Phase 1 | [58] | ||
| Synonyms |
Sherpa TTC-352; UNII-65ILH3Y0MI; 65ILH3Y0MI; CHEMBL3763743; 1607819-68-0; 3-(4-fluorophenyl)-2-(4-hydroxyphenoxy)-1-benzothiophene-6-ol; 3-(4-Fluorophenyl)-2-(4-hydroxyphenoxy)-benzo[b]thiophene-6-ol; SCHEMBL15658409; BDBM50145856; J3.603.123I; 2-(4-Hydroxyphenoxy)-3-(4-fluorophenyl)benzo[b]thiophene-6-ol; 3-(4-fluorophenyl)-2-(4-hydroxyphenoxy)-1-benzothiophen-6-ol; 3-(4-Fluorophenyl)-2-(4-hydroxyphenoxy)benzo(b)thiophene-6-ol; 3-(4-fluorophenyl)-2-(4-hydroxyphenoxy)benzo[b]thiophen-6-ol; 3-(4-fluorophenyl)-2-(4-hydroxyphenoxyl)benzo[b]thiophen-6-ol; Selective human estrogen-receptor alpha partial agonist TTC-352; Benzo(b)thiophene-6-ol, 3-(4-fluorophenyl)-2-(4-hydroxyphenoxy)-; V9J
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Chlorotrianisene | Withdrawn from market | [59] | ||
| Synonyms |
Anisene; Chloortrianisestrol; Chlorestrolo; Chlorotrianisenum; Chlorotrianisestrol; Chlorotrianisine; Chlorotrianizen; Chlorotrisin; Chlortrianisen; Chlortrianisene; Chlortrianisenum; Chlortrianisestrol; Chlortrianisoestrolum; Chlortrianizen; Clorestrolo; Clorotrianisene; Clorotrianiseno; Clorotrisin; Hormonisene; Khlortrianizen; Merbentul; Metace; Rianil; TACE; Triagen; Trianisestrol; Chlorotrianisene [Nonsteroidal oestrogens]; Clorotrianisene [DCIT]; Chlorotrianisene (INN); Chlorotrianisene [BAN:INN]; Chlorotrianisene [INN:BAN]; Chlorotrianisenum [INN-Latin]; Clorotrianiseno [INN-Spanish]; TACE (TN); Tace (pharmaceutical); Tace-fn; Chlorotris(p-methoxyphenyl)ethylene; Tri-p-anisylchloroethylene; Tris(p-methoxyphenyl)chloroethylene; 1,1',1''-(1-Chloro-1-ethenyl-2-ylidene)-tris(4-methoxybenzene); 1,1',1''-(2-chloroethene-1,1,2-triyl)tris(4-methoxybenzene); 1,1',1''-(2-chloroethene-1,1,2-triyl)tris[4-(methyloxy)benzene]; 1-[1-chloro-2,2-bis(4-methoxyphenyl)ethenyl]-4-methoxybenzene
Click to Show/Hide
|
|||
| MOA | Binder | |||
| External Link | ||||
| HEXESTROL | Withdrawn from market | [60] | ||
| Synonyms |
Dihydrodiethylstilbestrol; Vitestrol; Hexoestrolum; Exestrol; 5635-50-7; Stilbestrol, dihydro-; 4,4'-(1,2-Diethylethylene)diphenol; 4,4'-(hexane-3,4-diyl)diphenol; Synoestrolum; Hexanestrol; Cycloestrol; Esestrolo [DCIT]; Phenol, 4,4'-(1,2-diethyl-1,2-ethanediyl)bis-; Hexestrolum [INN-Latin]; Hexane, 3,4-bis(4-hydroxyphenyl)-; EINECS 227-082-2; 3,4-Bis(p-hydroxyphenyl)hexane; Hormoestrol; Syntrogene; Synthovo; Phenol, 4,4'-(1,2-diethylethylene)di-; CHEBI:31669; PBBGSZCBWVPOOL-UHFFFAOYSA-N; Hexestrol [INN]
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BITHIONOL | Withdrawn from market | [61] | ||
| Synonyms |
97-18-7; Actamer; Bithin; 2,2'-Thiobis(4,6-dichlorophenol); Lorothidol; Bitionol; Bithionol sulfide; Bisoxyphen; Bidiphen; Lorothiodol; Bitin; Nobacter; Bithionolate; Neopellis; Vancide BL; Usaf B-22; Bithional; Bithionolum; 2-Hydroxy-3,5-dichlorophenyl sulfide; TKhsd; Bis(2-hydroxy-3,5-dichlorophenyl) sulfide; Bis(3,5-dichloro-2-hydroxyphenyl) sulfide; 2,2'-sulfanediylbis(4,6-dichlorophenol); Caswell No. 852; Bitionol [INN-Spanish]; XL 7; Bithionolum [INN-Latin]; 2,2'-Dihydroxy-3,3',5,5'-tetrachlorodiphenyl sulfide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| IoGen | Discontinued in Phase 3 | [4] | ||
| Synonyms |
Oral iodine-based therapy (fibrocystic breast disease), Symbollon; Oral iodine-based therapy (fibrocystic breast disease), Symbollon/Gardant
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Miproxifene | Discontinued in Phase 3 | [62] | ||
| Synonyms |
Miproxifene phosphate; TAT-59
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Idoxifene | Discontinued in Phase 3 | [63] | ||
| Synonyms |
CB-7432; SB-223030
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| EM-800 | Discontinued in Phase 3 | [64] | ||
| Synonyms |
UNII-XCR716LECP; XCR716LECP; 182167-03-9; Em 800; SCH 57050; BIDD:ER0061; SCHEMBL1821303; CHEMBL308234; 189021-25-8; (s)-(+)-4-[7-(2,2-dimethyl-1-oxopropoxy)-4-meth-yl-2-[4-[2-(1-piperidinyl)-ethoxy]phenyl]-2h-1-benzopyran-3-yl]-phenyl 2,2-dimethylpropanoate
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| NP-50301 | Discontinued in Phase 2 | [65] | ||
| Synonyms |
IDESTRIN; Estrogen ester (eyedrop, dry eye syndrome), Nascent
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| SR-90067 | Discontinued in Phase 2 | [66] | ||
| Synonyms |
Estradiol spray (menopausis), Sanofi-Synthelabo
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Panomifene | Discontinued in Phase 2 | [67] | ||
| Synonyms |
Panomifine; EGIS-5650
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| SERM-3339 | Discontinued in Phase 2 | [68] | ||
| Synonyms |
HMR-3339; SERM compound, Aventis
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Droloxifene | Discontinued in Phase 2 | [69] | ||
| Synonyms |
E-droloxifene; FK-435; K-060; K-060E; RP-60850; K-21.060E; 3-hydroxytamoxifen
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| ERA-923 | Discontinued in Phase 2 | [70] | ||
| Synonyms |
Pipendoxifene; UNII-TPC5Q8496G; 198480-55-6; CHEMBL44426; TPC5Q8496G; Pipendoxifene [INN]; ERA 923; AC1O5FKF; SCHEMBL134583; JICOGKJOQXTAIP-UHFFFAOYSA-N; ZINC602799; BDBM50099587; AKOS030631784; DB05414; 2-(4-hydroxyphenyl)-3-methyl-1-(4-(2-piperidin-1-yl-ethoxy)-benzyl)-1H-indol-5-ol; US8815934, No 97; US8815934, No 107; 2-(p-Hydroxyphenyl)-3-methyl-1-(p-(2-piperidinoethoxy)benzyl)indol-5-ol; 2-(4-hydroxyphenyl)-3-methyl-1-[[4-(2-piperidin-1-ylethoxy)phenyl]methyl]indol-5-ol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 0.1 nM | |||
| External Link | ||||
| HRT | Discontinued in Phase 1 | [71] | ||
| Synonyms |
HRT (oral); IP-1162; IP-1163; IP-1164; HRT (oral), Shire
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| MX-4509 | Discontinued in Phase 1 | [72] | ||
| Synonyms |
17alpha-Estradiol; 57-91-0; Alfatradiol; Epiestradiol; 17-alpha-Estradiol; 3,17-Dihydroxyestratriene; 17a-estradiol; Estradiol-17alpha; Epiestrol; 17-Epiestradiol; Estra-1,3,5(10)-triene-3,17alpha-diol; 17alpha estradiol; NSC 20293; UNII-3VQ38D63M7; Alfatradiol [INN]; Oestradiol-17alpha; 17alpha-Oestradiol; Estradiol, 17alpha-; CHEBI:17160; VOXZDWNPVJITMN-SFFUCWETSA-N; 3VQ38D63M7; 17-epi-Estradiol; Alfatradiol (INN); Oestradiol-17-alpha; MITO-4509; Parkinsons therapeutics, MitoKor; Drug screening (Parkinsons), MitoKor; Estrogen analogs (Parkinsons), Mitokor
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 1.5 nM | |||
| External Link | ||||
| HE2100 | Discontinued in Phase 1 | [73] | ||
| Synonyms |
Androstenediol; Hermaphrodiol; 5-Androstenediol; Androst-5-enediol; 521-17-5; Androst-5-ene-3beta,17beta-diol; Neumune; 3beta,17beta-Dihydroxyandrost-5-ene; delta(sup 5)-Androstenediol; Androstenediol [JAN]; UNII-95PS51EMXY; Androst-5-enediol (VAN); (3beta,17beta)-androst-5-ene-3,17-diol; 5-AED; 3beta,17beta-Dihydroxy-5-androstene; androst-5-en-3beta,17beta-diol; EINECS 208-306-8; NSC 12163; 95PS51EMXY; ANDROST-5-ENE-3-beta,17-beta-DIOL; CHEMBL440283; CHEBI:2710; (3-beta,17-beta)-Androst-5-ene-3,17-diol; delta(sup
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 26 nM | |||
| External Link | ||||
| BN-AO-014 | Preclinical | [74] | ||
| Synonyms |
Selective estrogen receptor modulators (genitourinary atrophy); Selective estrogen receptor modulators (genitourinary atrophy), Bionovo
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| BN-OD-026 | Preclinical | [75] | ||
| Synonyms |
Selective estrogen receptor modulators (colon/breast cancer); BN-RP-006-OHA; Selective estrogen receptor modulators (colon/breast cancer), Bionovo
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| BN-DF-037 | Preclinical | [74] | ||
| Synonyms |
Selective estrogen receptor modulators (osteoporosis); BN-EU-036; Selective estrogen receptor modulators (osteoporosis), Bionovo; BN-AM-008-XG; BN-PC-049-BK
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| BN-AA-003-NY | Preclinical | [74] | ||
| Synonyms |
Selective estrogen receptor modulators (menopause symptoms); Selective estrogen receptor modulators (menopause symptoms), Bionovo
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| BN-CB-045 | Preclinical | [74] | ||
| Synonyms |
Selective estrogen receptor modulators (female sexual dysfunction); BN-ES-022; Selective estrogen receptor modulators (female sexual dysfunction), Bionovo
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| BN-GU-005-DHP | Preclinical | [74] | ||
| Synonyms |
Selective estrogen receptor modulators (arthralgia); Selective estrogen receptor modulators (arthralgia), Bionovo
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Tamoxifen methyl iodide | Terminated | [76] | ||
| Synonyms |
Tamoxifen methiodide; CHEMBL1213783; MLS002701640; AC1MHWIV; N-Methyltamoxifen iodide; 107256-99-5; PXJJOGITBQXZEQ-JTHROIFXSA-M; NSC630510; NSC-630510; (Z)-2-(4-(1,2-Diphenyl-1-butenyl)phenoxy)-N,N,N-trimethylethanaminium iodide; 2-[4-[(Z)-1,2-diphenylbut-1-enyl]phenoxy]ethyl-trimethylazanium iodide; Ethanaminium, 2-(4-(1,2-diphenyl-1-butenyl)phenoxy)-N,N,N-trimethyl-, iodide, (Z)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 46.29 nM | |||
| External Link | ||||
| Zindoxifene | Terminated | [77] | ||
| Synonyms |
D-16726
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| ICI-164384 | Terminated | [78] | ||
| Synonyms |
Ici 164384; 98007-99-9; UNII-84LT43726C; ICI M164384; ICI 164,384; CHEMBL1222035; N-n-Butyl-N-methyl-11-(3,17beta-dihydroxyestra-1,3,5(10)-trien-7alpha-yl)undecanamide; 84LT43726C; N-BUTYL-11-[(7R,8R,9S,13S,14S,17S)-3,17-DIHYDROXY-13-METHYL-7,8,9,11,12,13,14,15,16,17-DECAHYDRO-6H-CYCLOPENTA[A]PHENANTHREN-7-YL]-N-METHYLUNDECANAMIDE; Estra-1,3,5(10)-triene-7-undecanamide, N-butyl-3,17-dihydroxy-N-methyl-, (7alpha,17beta)-; AOE
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 50 nM | |||
| External Link | ||||
| ZK-119010 | Terminated | [79] | ||
| Synonyms |
ZK 119010; UNII-80Z22M837F; CHEMBL46903; 80Z22M837F; 127451-69-8; AC1L2YAG; 2-(4-Hydroxyphenyl)-3-methyl-1-(6-(1-pyrrolidinyl)hexyl)indol-5-ol; SCHEMBL9028173; DTXSID50155574; zk119010; BDBM50099586; 1H-Indol-5-ol, 2-(4-hydroxyphenyl)-3-methyl-1-(6-(1-pyrrolidinyl)hexyl)-; LS-191850; 2-(4-hydroxyphenyl)-3-methyl-1-(6-pyrrolidin-1-ylhexyl)indol-5-ol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 25 nM | |||
| External Link | ||||
| LY-117018 | Terminated | [80] | ||
| Synonyms |
Lilly 117018; LY 117018; 63676-25-5; CHEBI:90187; LY 139478; [6-hydroxy-2-(4-hydroxyphenyl)-1-benzothiophen-3-yl]{4-[2-(pyrrolidin-1-yl)ethoxy]phenyl}methanone; 6-Hydroxy-2-(4-hydroxyphenyl)benzo(b)thien-3-yl 4-(2-(1-pyrrolidinyl)ethoxy) phenyl ketone; Methanone, (6-hydroxy-2-(4-hydroxyphenyl)benzo(b)thien-3-yl)(4-(2-(1-pyrrolidinyl)ethoxy)phenyl)-; PHENETHIPYLONE; 6-hydroxy-2-(4-hydroxyphenyl)benzo[b]thien-3-yl 4-[2-(1-pyrrolidinyl)ethoxy] phenyl ketone; LY117018; AC1L2XRN; AC1Q5D9H; CHEMBL10030
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| JNJ-17148066 | Investigative | [81] | ||
| Synonyms |
CHEMBL1088485; SCHEMBL14277842; BDBM50310388
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 11 nM | |||
| External Link | ||||
| N-benzyl-4-hydroxy-N-phenylbenzenesulfonamide | Investigative | [82] | ||
| Synonyms |
CHEMBL203860
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 242 nM | |||
| External Link | ||||
| 2-AMINO-1-METHYL-6-PHENYLIMIDAZO[4,5-B]PYRIDINE | Investigative | [83] | ||
| Synonyms |
PhIP; 105650-23-5; 1-methyl-6-phenyl-1H-imidazo[4,5-b]pyridin-2-amine; 2-Amino-1-methyl-6-phenylimidazo(4,5-b)pyridine; UNII-909C6UN66T; CHEBI:76290; 909C6UN66T; 1-Methyl-6-phenyl-1H-imidazo(4,5-b)pyridin-2-amine; PIQ; 2-AMINO-1-METHYL-6-PHENYL-IMIDAZO [4,5-b] PYRIDINE; 1H-Imidazo(4,5-b)pyridin-2-amine, 1-methyl-6-phenyl- (9CI); CCRIS 2954; HSDB 7768; BRN 5951264; ACMC-1BRGI; AC1Q4WMI; (3H)PhIP; AC1L1BO3; SCHEMBL151718; CHEMBL1213271; DTXSID3037628; CTK0I0185
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SNG-8006 | Investigative | [4] | ||
| Synonyms |
Estrogen receptor alpha-36 antagonist (osteoporosis), Shenogen Pharma
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| N-cyclohexyl-4-hydroxy-N-phenylbenzenesulfonamide | Investigative | [82] | ||
| Synonyms |
CHEMBL382873
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 534 nM | |||
| External Link | ||||
| 3-CHLORO-2-(4-HYDROXYPHENYL)-2H-INDAZOL-5-OL | Investigative | [83] | ||
| Synonyms |
848142-62-1; 3-chloro-2-(4-hydroxyphenyl)indazol-5-ol; CHEMBL180071; CTK2I5101; DTXSID40463812; Indazole-Cl, > ZINC13609473; AKOS030536245; DB07708; 2-(p-Hydroxyphenyl)-3-chloro-2H-indazole-5-ol; 2H-Indazol-5-ol, 3-chloro-2-(4-hydroxyphenyl)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2,4-diisobutylamino-6-isopentylpyrimidine | Investigative | [84] | ||
| Synonyms |
CHEMBL347927; ZINC13559143; BDBM50138886
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 2800 nM | |||
| External Link | ||||
| JNJ-26529152 | Investigative | [81] | ||
| Synonyms |
CHEMBL1088483; BDBM50310372
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| SNG-8033 | Investigative | [4] | ||
| Synonyms |
Estrogen receptor alpha 36 inhibitor (leukemia), Shenogen Pharma
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2,4-Dibenzylamino-6-isopentylpyrimidine | Investigative | [84] | ||
| Synonyms |
CHEMBL494121
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 4100 nM | |||
| External Link | ||||
| SNG-8023 | Investigative | [4] | ||
| Synonyms |
Estrogen receptor alpha 36 inhibitor (breast cancer), Shenogen Pharma; Monoclonal antibody (breast cancer), Shenogen Pharma
Click to Show/Hide
|
|||
| External Link | ||||
| LTERHKILHRLLQEGSPSD | Investigative | [85] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| GW7604 | Investigative | [86] | ||
| MOA | Antagonist | |||
| Activity | EC50 = 1.7 nM | |||
| External Link | ||||
| 4-benzyl-2,6-diisobutylamino-pyrimidine | Investigative | [84] | ||
| Synonyms |
CHEMBL493703
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki > 7300 nM | |||
| External Link | ||||
| 7-Cyclopentyloxy-3-(4-hydroxyphenyl)chromen-4-one | Investigative | [87] | ||
| Synonyms |
SCHEMBL1700973
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N,N,N-Triisobutyl-pyrimidine-2,4,6-triamine | Investigative | [84] | ||
| Synonyms |
CHEMBL494706; BDBM50253380
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2400 nM | |||
| External Link | ||||
| Carboron Cluster with phenol | Investigative | [88] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-[1-(4-hydroxyphenyl)-2-phenylvinyl]phenol | Investigative | [89] | ||
| Synonyms |
CHEMBL415864; 1,1-Bis(4-hydroxyphenyl)-2-phenylethylene; 4,4'-(2-Phenylethene-1,1-Diyl)diphenol; 66422-18-2; Bhpe-1,1; SCHEMBL641985; AC1MI565; CTK5C4724; 4-(1,2-diphenyl-vinyl)-phenol; BDBM50023749; ZINC13492380; Phenol, 4,4'-(phenylethenylidene)bis-; 1,1-bis(4-hydroxyphenyl)-2-phenylethene; 4-[1-(4-hydroxyphenyl)-2-phenylethenyl]phenol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 150 nM | |||
| External Link | ||||
| MPrP | Investigative | [90] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 20 nM | |||
| External Link | ||||
| Tamoxifen isopropyl bromide | Investigative | [76] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 25.43 nM | |||
| External Link | ||||
| 17-METHYL-17-ALPHA-DIHYDROEQUILENIN | Investigative | [83] | ||
| Synonyms |
2b1z; ZINC5048688; DB06871; 17beta-Methyl-17alpha-dihydroequilenin; 17M; (13alpha,17beta)-17-methylestra-1(10),2,4,6,8-pentaene-3,17-diol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-hydroxy-N-neopentyl-N-phenylbenzenesulfonamide | Investigative | [82] | ||
| Synonyms |
CHEMBL203515
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1371 nM | |||
| External Link | ||||
| SNG-163 | Investigative | [4] | ||
| Synonyms |
SGIC-163; ER-a36 inhibitor (cancer), Shenogen
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| GSK-5182 | Investigative | [91] | ||
| Synonyms |
GSK5182; GSK 5182; 877387-37-6; (Z)-4-(1-{4-[2-(DIMETHYLAMINO)ETHOXY]PHENYL}-5-HYDROXY-2-PHENYLPENT-1-ENYL)PHENOL; TXF; 4-[(1Z)-1-{4-[2-(dimethylamino)ethoxy]phenyl}-5-hydroxy-2-phenylpent-1-en-1-yl]phenol; 2ewp; AC1OA9V8; GTPL8908; CHEMBL201013; SCHEMBL20534177; BDBM22435; AOB1629; EX-A2580; ZINC14978677; 4-hydroxytamoxifen (4-OHT) analog, 15; 4-[(z)-1-[4-(2-dimethylaminoethyloxy)phenyl]-hydroxy-2-phenylpent-1-enyl]phenol; 4-[(Z)-1-[4-(2-dimethylaminoethyloxy)phenyl]-5-hydroxy-2-phenylpent-1-enyl]phenol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 320 nM | |||
| External Link | ||||
| N-ethyl-4-hydroxy-N-phenylbenzenesulfonamide | Investigative | [82] | ||
| Synonyms |
CHEMBL379234; Enamine_001944; Oprea1_552384; AC1M697W; SCHEMBL10904389; MolPort-004-000-970; HMS1399I08; ZINC3251814; BDBM50177761; AKOS008967166; MCULE-9434872926; N-ethyl-4-hydroxy-N-phenylbenzene-1-sulfonamide; Z56821525
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 404 nM | |||
| External Link | ||||
| JNJ-19398990 | Investigative | [81] | ||
| Synonyms |
CHEMBL1085429; BDBM50310424
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 64 nM | |||
| External Link | ||||
| 4-(1-benzyl-7-chloro-1H-indazol-3-yl)phenol | Investigative | [92] | ||
| MOA | Inhibitor | |||
| Activity | EC50 = 390 nM | |||
| External Link | ||||
| 3-ETHYL-2-(4-HYDROXYPHENYL)-2H-INDAZOL-5-OL | Investigative | [83] | ||
| Synonyms |
CHEMBL180517
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-allyl-4-hydroxy-N-phenylbenzenesulfonamide | Investigative | [82] | ||
| Synonyms |
CHEMBL426849
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 235 nM | |||
| External Link | ||||
| 4-(7-chloro-1-propyl-1H-indazol-3-yl)phenol | Investigative | [92] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 52 nM | |||
| External Link | ||||
| Tamoxifen ethyl bromide | Investigative | [76] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 13.66 nM | |||
| External Link | ||||
| 6-butyl-2,4-dipropylaminopyrimidine | Investigative | [84] | ||
| Synonyms |
CHEMBL352024; BDBM50138883; ZINC13559144
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 13000 nM | |||
| External Link | ||||
| RG6046 | Investigative | [93] | ||
| MOA | Modulator | |||
| External Link | ||||
| STX | Investigative | [4] | ||
| Synonyms |
Selective estrogen receptor modulator (menopause-associated symptoms), Oregon Health & Science University
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| 4-(1-cyclopentyl-7-fluoro-1H-indazol-3-yl)phenol | Investigative | [92] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 18 nM | |||
| External Link | ||||
| 3-(4-Hydroxyphenyl)-7-isobutoxychromen-4-one | Investigative | [87] | ||
| Synonyms |
SCHEMBL1701136
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SERMs | Investigative | [4] | ||
| Synonyms |
SERMs (cancer); SERMs (cancer), CEAMED; Selective estrogen receptor modulators (cancer), CEAMAD
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| 6-ethyl-2,4-diisobutylaminopyrimidine | Investigative | [84] | ||
| Synonyms |
CHEMBL484451
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7300 nM | |||
| External Link | ||||
| 4-(7-chloro-1-cyclohexyl-1H-indazol-3-yl)phenol | Investigative | [92] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 37 nM | |||
| External Link | ||||
| Org-37663 | Investigative | [94] | ||
| MOA | Modulator | |||
| External Link | ||||
| 4-hydroxy-N-isopropyl-N-phenylbenzenesulfonamide | Investigative | [82] | ||
| Synonyms |
CHEMBL204664
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 1985 nM | |||
| External Link | ||||
| 4-(7-methyl-1-propyl-1H-indazol-3-yl)phenol | Investigative | [92] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 40 nM | |||
| External Link | ||||
| 4-(6-HYDROXY-1H-INDAZOL-3-YL)BENZENE-1,3-DIOL | Investigative | [83] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 48 nM | |||
| External Link | ||||
| 4-[1-(4-hydroxyphenyl)-2-phenylbut-1-enyl]phenol | Investigative | [89] | ||
| Synonyms |
91221-46-4; 1,1-Bis(4-hydroxyphenyl)-2-phenylbut-1-ene; 1,1-Bis(4-hydroxyphenyl)-2-phenyl-1-butene; CHEMBL149791; 4,4'-(2-phenylbut-1-ene-1,1-diyl)diphenol; Tamoxifen bis-phenol; 1,1-Bhpe; Tamoxifenbisphenol; AC1L1YAG; SCHEMBL639225; CTK5G9076; DTXSID50238452; BPKSDMHGDYTXLI-UHFFFAOYSA-N; ZINC1849484; BDBM50121317; AKOS030254689; KB-09832; 4,4'-(2-Phenyl-1-butenylidene)bisphenol; B5114; FT-0663304; Phenol, 4,4'-(2-phenyl-1-butenylidene)bis-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 0.015 nM | |||
| External Link | ||||
| 4-hydroxy-N-phenyl-N-propylbenzenesulfonamide | Investigative | [82] | ||
| Synonyms |
CHEMBL205070
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 1430 nM | |||
| External Link | ||||
| N-butyl-4-hydroxy-N-phenylbenzenesulfonamide | Investigative | [82] | ||
| Synonyms |
CHEMBL203810; BDBM50177754
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 705 nM | |||
| External Link | ||||
| 3-(4-Hydroxyphenyl)-7-isopropoxychromen-4-one | Investigative | [87] | ||
| Synonyms |
CHEMBL115109; 97846-18-9; 4H-1-Benzopyran-4-one, 3-(4-hydroxyphenyl)-7-(1-methylethoxy)-; ACMC-20m1ro; SCHEMBL1701110; CTK3G8070; DTXSID70440494; 7-Isopropoxy-4'-hydroxyisoflavone; BDBM50104874; ZINC13864225; AKOS030553087; 3-(4-Hydroxy-phenyl)-7-isopropoxy-chromen-4-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-[1-(4-hydroxyphenyl)-2-phenylpent-1-enyl]phenol | Investigative | [89] | ||
| Synonyms |
CHEMBL357824; SCHEMBL19158863; BDBM50121321
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 50 nM | |||
| External Link | ||||
| 4-(1-butyl-7-chloro-1H-indazol-3-yl)phenol | Investigative | [92] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 25 nM | |||
| External Link | ||||
| 4-[1-(4-hydroxyphenyl)-2-phenylprop-1-enyl]phenol | Investigative | [89] | ||
| Synonyms |
CHEMBL150461; SCHEMBL18882768
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 30 nM | |||
| External Link | ||||
| Tamoxifen butyl bromide | Investigative | [76] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 13.01 nM | |||
| External Link | ||||
| Estriol E3 | Investigative | [95] | ||
| Synonyms |
Estriol E3 (oral formulation, multiple sclerosis)
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| 4-(1,2-Diphenyl-but-1-enyl)-phenol | Investigative | [89] | ||
| Synonyms |
CHEMBL50995; 4-(1,2-Diphenyl-1-butenyl)phenol; Monophenoltamoxifen; 4-[(Z)-1,2-diphenylbut-1-enyl]phenol; AC1O4GB8; SCHEMBL5354173; BDBM50121319; ZINC29469549; 69967-80-2; LS-104581
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7 nM | |||
| External Link | ||||
| 4-(7-chloro-1-cyclopentyl-1H-indazol-3-yl)phenol | Investigative | [92] | ||
| MOA | Inhibitor | |||
| Activity | EC50 = 98 nM | |||
| External Link | ||||
| TSERaM | Investigative | [4] | ||
| Synonyms |
TSERaM (menopausal weight gain); TSERaM (menopausal weight gain), Bionovo; Tissue-selective estrogen receptor alpha modulators (menopausal weight gain), Bionovo
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| JNJ-26529126 | Investigative | [81] | ||
| Synonyms |
CHEMBL1088343; BDBM50310407
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 76 nM | |||
| External Link | ||||
| 5-hydroxy-2-phenylisoindoline-1,3-dione | Investigative | [96] | ||
| Synonyms |
CHEMBL276030; 4-Hydroxy-N-phenylphthalimide; 3975-50-6; 5-hydroxy-2-phenyl-isoindole-1,3-dione; SCHEMBL8615277; CTK1B3719; DTXSID80445430; HCUAWJNHCZEMJS-UHFFFAOYSA-N; ZINC26020837; BDBM50088673; 5-hydroxy-2-phenyl-1H-isoindole-1,3(2H)-dione; 1H-Isoindole-1,3(2H)-dione, 5-hydroxy-2-phenyl-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2700 nM | |||
| External Link | ||||
| 4-[1-(4-hydroxyphenyl)-2-phenylhex-1-enyl]phenol | Investigative | [89] | ||
| Synonyms |
CHEMBL356693
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| methyl-piperidino-pyrazole | Investigative | [97] | ||
| MOA | Antagonist | |||
| Activity | Ki = 2.7 nM | |||
| External Link | ||||
| propylpyrazoletriol | Investigative | [98] | ||
| MOA | Agonist | |||
| Activity | EC50 = 0.1 nM | |||
| External Link | ||||
| 4-hydroxy-N,N-diphenylbenzenesulfonamide | Investigative | [82] | ||
| Synonyms |
CHEMBL203061; SCHEMBL2114599
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 1543 nM | |||
| External Link | ||||
| 3-[1-ethyl-2-(3-hydroxyphenyl)butyl]phenol | Investigative | [99] | ||
| Synonyms |
Metahexes trol; 3,3'-Hexestrol; Metahexestrol; 3,3'-Hes; 68266-24-0; meso-3,4-Bis(3'-hydroxyphenyl)hexane; BRN 3971661; NSC-297,170; meso-3,3'-(1,2-Diethylethylene)diphenol; NSC-297170; (R*,S*)-3,3'-(1,2-Diethyl-1,2-ethanediyl)bisphenol; Phenol, 3,3'-(1,2-diethyl-1,2-ethanediyl)bis-, (R*,S*)-; Phenol, 3,3'-((1R,2S)-1,2-diethyl-1,2-ethanediyl)bis-, rel-; UNII-DSF584X94B; DSF584X94B; NSC 297170; 3-[4-(3-hydroxyphenyl)hexan-3-yl]phenol; AC1L2OPY; 1,2-Diethyl-1,2-bis(3'-hydroxyphenyl)ethane; AC1Q79WV; CHEMBL18268; SCHEMBL5014485
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(4-Hydroxy-phenyl)-quinolin-6-ol | Investigative | [100] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 310 nM | |||
| External Link | ||||
| Nafoxidine | Investigative | [101] | ||
| Synonyms |
1845-11-0; Nafoxidine [INN]; Nafoxidinum [INN-Latin]; Nafoxidina [INN-Spanish]; UNII-4RIY10WM82; U-11000A; BRN 1440873; CHEMBL28211; 4RIY10WM82; CHEBI:34881; NSC 70735; C29H31NO2; JEYWNNAZDLFBFF-UHFFFAOYSA-N; 1-(2-(p-(3,4-Dihydro-6-methoxy-2-phenyl-1-naphthyl)phenoxy)ethyl)pyrrolidine; Pyrrolidine, 1-(2-(4-(3,4-dihydro-6-methoxy-2-phenyl-1-naphthalenyl)phenoxy)ethyl)-; 1-[2-[4-(6-methoxy-2-phenyl-3,4-dihydronaphthalen-1-yl)phenoxy]ethyl]pyrrolidine; Pyrrolidine, 1-(2-(p-(3,4-dihydro-6-methoxy-2-phenyl-1-naphthyl
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| [1,1':2',1'']Terphenyl-4'-carbaldehyde oxime | Investigative | [102] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(6-Hydroxy-naphthalen-2-yl)-benzooxazol-6-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 780 nM | |||
| External Link | ||||
| 6-ethyl-4,7-dimethyl-6H-benzo[c]chromene-3,8-diol | Investigative | [104] | ||
| Synonyms |
CHEMBL206547; SCHEMBL6828050
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 101 nM | |||
| External Link | ||||
| 4-(3-phenyl-1H-indol-2-yl)phenol | Investigative | [105] | ||
| Synonyms |
CHEMBL199766; SCHEMBL7040440; BDBM50175418
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 8310 nM | |||
| External Link | ||||
| 4-[1,2-bis(4-hydroxyphenyl)vinyl]phenol | Investigative | [106] | ||
| Synonyms |
CHEMBL34709
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4,10-dimethyl-6H-benzo[c]chromene-3,8-diol | Investigative | [104] | ||
| Synonyms |
CHEMBL206582; SCHEMBL6924719
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1030 nM | |||
| External Link | ||||
| 7-Allyl-2-(4-hydroxy-phenyl)-benzooxazol-5-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 727 nM | |||
| External Link | ||||
| 6-(4-Hydroxy-2-methyl-phenyl)-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL195389; SCHEMBL4077781; YIKDKHNOMWERET-UHFFFAOYSA-N; ZINC13645062; BDBM50168377
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 40 nM | |||
| External Link | ||||
| 1-CHLORO-6-(4-HYDROXYPHENYL)-2-NAPHTHOL | Investigative | [107] | ||
| Synonyms |
CHEMBL364092; 1-chloro-6-(4-hydroxyphenyl)naphthalen-2-ol; 4NA; 1yy4; AC1O0UUG; 1-Chloro-6-(4-hydroxy-phenyl)-naphthalen-2-ol; SCHEMBL3933824; YHEHVRSGKUYDON-UHFFFAOYSA-N; ZINC13645009; BDBM50168323
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 91 nM | |||
| External Link | ||||
| 5-Chloro-2-(4-hydroxy-phenyl)-benzooxazol-6-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2765 nM | |||
| External Link | ||||
| 2-(6-Hydroxy-naphthalen-1-yl)-benzooxazol-5-ol | Investigative | [103] | ||
| Synonyms |
CHEMBL183367; SCHEMBL3933889; GLVGKXBQPYTMGJ-UHFFFAOYSA-N; ZINC3817709; BDBM50154056; 2-(6-hydroxynaphthalen-1-yl)benzo[d]oxazol-5-ol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 153 nM | |||
| External Link | ||||
| 2-(3-hydroxyphenyl)-1,2'-spirobi[1H-indene]-5-ol | Investigative | [108] | ||
| Synonyms |
CHEMBL24950; SCHEMBL6286195; BDBM50123206; 2-(3-hydroxyphenyl)-1,2''-spirobi[1H-indene]-5-ol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 35 nM | |||
| External Link | ||||
| 3-chloro-4-(4-hydroxyphenyl)salicylaldoxime | Investigative | [109] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(4-HYDROXY-PHENYL)BENZOFURAN-5-OL | Investigative | [103] | ||
| Synonyms |
2-(4-Hydroxy-phenyl)-benzofuran-5-ol; 2-(4-hydroxyphenyl)-1-benzofuran-5-ol; CHEMBL367588; 52814-86-5; 2-(4-Hydroxyphenyl)benzofuran-5-ol; AC1LCVQT; 1u9e; SCHEMBL3922677; CTK1G1983; DTXSID60349669; SNNNDCMXZYWCCI-UHFFFAOYSA-N; ZINC3816519; BDBM50152627; SBB096959; AKOS030555876; DB07032; 2-(4-hydroxyphenyl)benzo[b]furan-5-ol; 5-Benzofuranol,
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 176 nM | |||
| External Link | ||||
| COUMESTROL | Investigative | [110] | ||
| Synonyms |
479-13-0; Cumoestrol; Cumoesterol; Cumostrol; Coumesterol; 3,9-Dihydroxy-6H-[1]benzofuro[3,2-c]chromen-6-one; 7,12-Dihydroxycoumestan; 3,9-Dihydroxycoumestan; UNII-V7NW98OB34; NSC 22842; CCRIS 7311; NSC22842; 6H-Benzofuro[3,2-c][1]benzopyran-6-one, 3,9-dihydroxy-; EINECS 207-525-6; BRN 0266702; CHEMBL30707; MLS000738006; V7NW98OB34; CHEBI:3908; ZZIALNLLNHEQPJ-UHFFFAOYSA-N; 3,9-Dihydroxy-6H-benzofuro(3,2-c)(1)benzopyran-6-one; 6H-Benzofuro(3,2-c)(1)benzopyran-6-one, 3,9-dihydroxy-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 11 nM | |||
| External Link | ||||
| 4-[1,2-bis(4-hydroxyphenyl)pent-1-enyl]phenol | Investigative | [106] | ||
| Synonyms |
CHEMBL38266
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(4-Hydroxy-phenyl)-7-propenyl-benzooxazol-5-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 775 nM | |||
| External Link | ||||
| 5-Chloro-2-(4-hydroxy-phenyl)-quinolin-6-ol | Investigative | [100] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 632 nM | |||
| External Link | ||||
| 2-(6-Hydroxy-naphthalen-2-yl)-benzooxazol-5-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 3800 nM | |||
| External Link | ||||
| 2-(4-Hydroxy-phenyl)-7-isopropyl-benzooxazol-5-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1200 nM | |||
| External Link | ||||
| 6-(2-Chloro-4-hydroxy-phenyl)-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL195311; SCHEMBL3932692; FNSIACDWDZGUHN-UHFFFAOYSA-N; BDBM50168347; ZINC13645053
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| DIHYDRORALOXIFENE | Investigative | [111] | ||
| Synonyms |
CHEMBL14955; BDBM50217538; ZINC15919388
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(3-hydroxyphenyl)-1,2'-spirobi[1H-indene]-6-ol | Investigative | [108] | ||
| Synonyms |
CHEMBL144431; SCHEMBL6283932; BDBM50123207; 2-(3-hydroxyphenyl)-1,2''-spirobi[1H-indene]-6-ol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 31 nM | |||
| External Link | ||||
| 4-[2,2-bis(4-hydroxyphenyl)-1-methylvinyl]phenol | Investigative | [106] | ||
| Synonyms |
Ici-3188; 1,1,2-Tris(4-hydroxyphenyl)prop-1-ene; 68373-13-7; 4-[1,1-bis(4-hydroxyphenyl)prop-1-en-2-yl]phenol; Phenol, 4,4',4''-(1-methyl-1-ethenyl-2-ylidene)tris-; Ici 3188; AC1MJ4DX; CHEMBL38103; CTK2F3093; DTXSID20218504; AKOS030547870
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Butyl-2-(4-hydroxy-phenyl)-benzooxazol-5-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 498 nM | |||
| External Link | ||||
| 8-(2,2-dimethylpropyl)naringenin | Investigative | [112] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 216 nM | |||
| External Link | ||||
| 4-(6-Hydroxy-benzooxazol-2-yl)-benzene-1,3-diol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 82 nM | |||
| External Link | ||||
| 2-(4-Hydroxy-phenyl)-7-methoxy-benzofuran-5-ol | Investigative | [113] | ||
| Synonyms |
CHEMBL361226; SCHEMBL3928011; KEWMBPCQWDVBAS-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 483 nM | |||
| External Link | ||||
| 3-(2-Hydroxy-phenyl)-benzo[d]isoxazol-6-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 3000 nM | |||
| External Link | ||||
| R,R-THC | Investigative | [114] | ||
| Synonyms |
(R,R)-THC; 138090-06-9; (R,R)-cis-Diethyl tetrahydro-2,8-chrysenediol; CHEMBL282489; 221368-54-3; (5R,11R)-5,11-diethyl-5,6,11,12-tetrahydrochrysene-2,8-diol; (R,R)-5,11-DIETHYL-5,6,11,12-TETRAHYDRO-2,8-CHRYSENEDIOL; (R,R)-cis-Diethyltetrahydro-2,8-chrysenediol; (R,R)-5,11-CIS-DIETHYL-5,6,11,12-TETRAHYDROCHRYSENE-2,8-DIOL; Lopac-D-8690; AC1L9K5Y; Lopac0_000463; MLS002153151; BIDD:ER0043; GTPL2822; DTXSID6040749; SCHEMBL13352540; CTK4E8744; MolPort-003-941-174; HMS3268D03; HMS3261M08; HMS2232C18; ZINC3940885; Tox21_500463
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| 2-(4-Hydroxy-phenyl)-7-propyl-benzooxazol-5-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 390 nM | |||
| External Link | ||||
| 1,8-Dichloro-6-(4-hydroxy-phenyl)-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL192876; SCHEMBL4456831; YTFVBUBYMJJWQZ-UHFFFAOYSA-N; ZINC13645103; BDBM50168322
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 43 nM | |||
| External Link | ||||
| 6-Chloro-2-(4-hydroxy-phenyl)-benzooxazol-5-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 464 nM | |||
| External Link | ||||
| 2-(4-Hydroxy-phenyl)-7-methoxy-benzooxazol-5-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2557 nM | |||
| External Link | ||||
| 8-n-undecylnaringenin | Investigative | [112] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1017 nM | |||
| External Link | ||||
| 7-Chloro-2-(4-hydroxy-phenyl)-benzofuran-5-ol | Investigative | [113] | ||
| Synonyms |
CHEMBL184448; SCHEMBL3922992
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 6.8 nM | |||
| External Link | ||||
| BROUSSONIN A | Investigative | [115] | ||
| Synonyms |
73731-87-0; CHEMBL465879; Biooussonin A; AC1NST4K; 2-[3-(4-hydroxyphenyl)propyl]-5-methoxyphenol; SCHEMBL774802; MSNVBURPCQDLEP-UHFFFAOYSA-N; MolPort-019-937-120; BDBM50251012; ZINC13341109; MCULE-7164788732
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 1590 nM | |||
| External Link | ||||
| 4-[1,2-bis(4-hydroxyphenyl)hex-1-enyl]phenol | Investigative | [106] | ||
| Synonyms |
CHEMBL38222
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Ethyl-2-(4-hydroxy-phenyl)-benzooxazol-5-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 537 nM | |||
| External Link | ||||
| WAY200070 | Investigative | [103] | ||
| Synonyms |
WAY 200070; 440122-66-7; WAY-200070; CHEMBL188528; 7-BROMO-2-(4-HYDROXYPHENYL)-1,3-BENZOXAZOL-5-OL; 7-Bromo-2-(4-hydroxy-phenyl)-benzooxazol-5-ol; SCHEMBL3930187; ZINC9308; GTPL4052; CTK1D2551; DTXSID80432027; MolPort-023-276-777; BAAILVWEAXFTSF-UHFFFAOYSA-N; BCP28989; WAY-00005; BDBM50154088; ZINC138816775; AKOS024457585; CS-6398; HY-101271; RT-016254; B7386; 7-bromo-2-(4-hydroxyphenyl)-5-benzoxazolol; 5-Benzoxazolol, 7-bromo-2-(4-hydroxyphenyl)-; 7-bromo-2-(4-hydroxyphenyl)benzo[d]oxazol-5-ol; WAY-200070, >
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 155 nM | |||
| External Link | ||||
| 6-(4-Hydroxy-phenyl)-naphthalen-2-ol | Investigative | [100] | ||
| Synonyms |
CHEMBL191974; 6-(4-hydroxyphenyl)-2-naphthol; SCHEMBL2699234; SKPPZWVFPOQPOT-UHFFFAOYSA-N; ZINC13644983
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 211 nM | |||
| External Link | ||||
| 2-(4-Hydroxy-phenyl)-7-phenyl-benzooxazol-5-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1300 nM | |||
| External Link | ||||
| 6-(4-Hydroxy-phenyl)-1-methyl-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL372030; SCHEMBL3927148; FGIUXCLAHODWKV-UHFFFAOYSA-N; BDBM50168320; ZINC13645024
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 282 nM | |||
| External Link | ||||
| 8-methylnaringenin | Investigative | [112] | ||
| Synonyms |
CHEMBL426154; MolPort-039-339-073; ZINC14814929; 5,7,4'-Trihydroxy-8-methylflavanone
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5245 nM | |||
| External Link | ||||
| 3-(4-Hydroxy-phenyl)-benzo[d]isoxazol-5-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 819 nM | |||
| External Link | ||||
| 2-(3-Chloro-4-hydroxy-phenyl)-benzooxazol-6-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5000 nM | |||
| External Link | ||||
| 8-Chloro-6-(4-hydroxy-phenyl)-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL363367; SCHEMBL3923941; IVJIWGTXXHDRCU-UHFFFAOYSA-N; BDBM50168384; ZINC13645099
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 30 nM | |||
| External Link | ||||
| 4-Benzo[d]isoxazol-3-yl-benzene-1,3-diol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6100 nM | |||
| External Link | ||||
| 2-(4-Hydroxy-phenyl)-benzooxazol-6-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 902 nM | |||
| External Link | ||||
| 7-(4-Hydroxy-phenyl)-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL191715; 7-(4-Hydroxyphenyl)-2-naphthol; SCHEMBL3932603; NSCZTZNRMFWAIY-UHFFFAOYSA-N; ZINC13645002; BDBM50168330
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 442 nM | |||
| External Link | ||||
| 8-(3-methylbutyl)naringenin | Investigative | [112] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 140 nM | |||
| External Link | ||||
| 4,7-dimethyl-6H-benzo[c]chromene-3,8-diol | Investigative | [104] | ||
| Synonyms |
CHEMBL378798; SCHEMBL6830103
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4610 nM | |||
| External Link | ||||
| 2-(3-Fluoro-4-hydroxy-phenyl)-benzooxazol-5-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1570 nM | |||
| External Link | ||||
| 3'-Methoxy-4'Hydroxyclomiphene | Investigative | [116] | ||
| Synonyms |
CHEMBL955; 3-Methoxy-4-hydroxyclomiphene; AC1MIWC3; SCHEMBL20193223; 3''-Methoxy-4''Hydroxyclomiphene; BDBM50020285; 4-{2-Chloro-1-[4-(2-diethylamino-ethoxy)-phenyl]-2-phenyl-vinyl}-2-methoxy-phenol; Phenol, 4-(2-chloro-1-(4-(2-(diethylamino)ethoxy)phenyl)-2-phenylethenyl)-2-methoxy-; 4-[(Z)-2-chloro-1-[4-(2-diethylaminoethyloxy)phenyl]-2-phenylethenyl]-2-methoxyphenol; 4-(2-Chloro-1-(4-(2-(diethylamino)ethoxy)phenyl)-2-phenylethenyl)-2-methoxyphenol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(3-Fluoro-4-hydroxy-phenyl)-benzooxazol-6-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 8430 nM | |||
| External Link | ||||
| 3-(4-Hydroxy-phenyl)-benzo[d]isoxazol-6-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 815 nM | |||
| External Link | ||||
| Trans-hydroxytamoxifen | Investigative | [97] | ||
| MOA | Antagonist | |||
| External Link | ||||
| 3,8-dihydroxy-4-methyl-6H-benzo[c]chromen-6-one | Investigative | [104] | ||
| Synonyms |
CHEMBL206600; SCHEMBL6828078
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 654 nM | |||
| External Link | ||||
| 2-(4-Hydroxy-phenyl)-7-methyl-benzofuran-5-ol | Investigative | [113] | ||
| Synonyms |
CHEMBL365290; SCHEMBL1119325; FUWUZPRNKPGRGX-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 114 nM | |||
| External Link | ||||
| 4-(6-Hydroxy-benzooxazol-2-yl)-benzene-1,2-diol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2410 nM | |||
| External Link | ||||
| 6-(2-Fluoro-4-hydroxy-phenyl)-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL195878; SCHEMBL3926652; MAFQDFZNSVYFEW-UHFFFAOYSA-N; ZINC13645034; BDBM50168350
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 24 nM | |||
| External Link | ||||
| 8-benzylnaringenin | Investigative | [112] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 503 nM | |||
| External Link | ||||
| 2-(3-Butoxy-4-hydroxy-phenyl)-benzooxazol-6-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6240 nM | |||
| External Link | ||||
| 8-Fluoro-6-(4-hydroxy-phenyl)-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL192386; SCHEMBL3924505; KBQSRNQTSRTYGN-UHFFFAOYSA-N; ZINC13645086; BDBM50168353
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 22 nM | |||
| External Link | ||||
| 2-Naphthalen-1-yl-benzooxazol-6-ol | Investigative | [103] | ||
| Synonyms |
CHEMBL188527; 2-(naphthalen-1-yl)benzo[d]oxazol-6-ol; SCHEMBL6311736; LYJKLNQQSXCGDI-UHFFFAOYSA-N; BDBM50154077; ZINC12353755
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1880 nM | |||
| External Link | ||||
| 6-(4-Hydroxy-phenyl)-1-nitro-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL195788; SCHEMBL3935673; MYVOVZDBAWARDP-UHFFFAOYSA-N; ZINC13645031; BDBM50168340
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 709 nM | |||
| External Link | ||||
| 4,6,6,7-tetramethyl-6H-benzo[c]chromene-3,8-diol | Investigative | [104] | ||
| Synonyms |
CHEMBL204025; SCHEMBL6827956
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 468 nM | |||
| External Link | ||||
| 3,8-dihydroxy-7-methyl-6H-benzo[c]chromen-6-one | Investigative | [104] | ||
| Synonyms |
CHEMBL206226
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1170 nM | |||
| External Link | ||||
| 6-(3-Hydroxy-phenyl)-naphthalen-1-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL193010; 6-(3-hydroxyphenyl)-1-naphthol; SCHEMBL3010784; HXKYTCGOCJUQSE-UHFFFAOYSA-N; ZINC13644987
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1452 nM | |||
| External Link | ||||
| 4-[1,2-bis(4-hydroxyphenyl)but-1-enyl]phenol | Investigative | [106] | ||
| Synonyms |
CHEMBL37775
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 0.15 nM | |||
| External Link | ||||
| 4-(2-phenyl-1H-benzo[d]imidazol-1-yl)phenol | Investigative | [105] | ||
| Synonyms |
CHEMBL200483; SCHEMBL6440186
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1800 nM | |||
| External Link | ||||
| 5-Bromo-2-(4-hydroxy-phenyl)-quinolin-6-ol | Investigative | [100] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1140 nM | |||
| External Link | ||||
| 8-n-heptylnaringenin | Investigative | [112] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 392 nM | |||
| External Link | ||||
| 4-(2-phenyl-1H-indol-3-yl)phenol | Investigative | [105] | ||
| Synonyms |
CHEMBL371012; SCHEMBL7041571; OITCYCYIUVYFKQ-UHFFFAOYSA-N; BDBM50175419; 4-(2-Phenyl-1H-indol-3-yl)-phenol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 24 nM | |||
| External Link | ||||
| 2-(3-Chloro-4-hydroxy-phenyl)-benzooxazol-5-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5280 nM | |||
| External Link | ||||
| 6-(3-Chloro-4-hydroxy-phenyl)-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL365999; SCHEMBL4012132; UVRXGDFVCKYIGU-UHFFFAOYSA-N; BDBM50168370; ZINC13645074
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 107 nM | |||
| External Link | ||||
| 7-Bromo-2-(4-hydroxy-phenyl)-benzofuran-5-ol | Investigative | [113] | ||
| Synonyms |
CHEMBL183782; SCHEMBL3927964; UBYHIUUMRHTEFF-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1.5 nM | |||
| External Link | ||||
| 4-Naphthalen-2-yl-phenol | Investigative | [107] | ||
| Synonyms |
4-(naphthalen-2-yl)phenol; 6336-82-9; 4-(2-Naphthyl)phenol; 4-naphthalen-2-ylphenol; CHEMBL194772; NSC-39892; 2-(4-HYDROXYPHENYL)NAPHTHALENE; NSC39892; AC1L5XK9; AC1Q7B9I; SCHEMBL234529; KS-00000ORV; CTK5B8841; DTXSID70284931; NIRHUNSXEDESLN-UHFFFAOYSA-N; MolPort-015-145-553; ZINC1671629; MFCD18312886; KM0737; BDBM50168385; ANW-73541; AKOS016007751; IMED1575423883; KB-40006; AK-50357; AJ-29221; TC-162286; AX8212912; 4CH-024303
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 638 nM | |||
| External Link | ||||
| 2-(4-Hydroxy-phenyl)-4-methoxy-quinolin-6-ol | Investigative | [100] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6630 nM | |||
| External Link | ||||
| 8-n-pentylnaringenin | Investigative | [112] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 383 nM | |||
| External Link | ||||
| 6-(4-Hydroxy-phenyl)-1-methoxy-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL195786; SCHEMBL5980579; DSBWWPDVDPKMHN-UHFFFAOYSA-N; ZINC13645027; BDBM50168344
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 884 nM | |||
| External Link | ||||
| 6-(3,5-Difluoro-4-hydroxy-phenyl)-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL191716; SCHEMBL3934682; DZGRIHNLLUZLTL-UHFFFAOYSA-N; BDBM50168366; ZINC13645080
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 92 nM | |||
| External Link | ||||
| 2-(4-Hydroxy-phenyl)-benzooxazol-5-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1227 nM | |||
| External Link | ||||
| 4',5,7-trihydroxy-6,8-dimethylisoflavone | Investigative | [117] | ||
| Synonyms |
CHEMBL512579; AC1LCTEG; SCHEMBL571136; BDBM50250906; 4'',5,7-trihydroxy-6,8-dimethylisoflavone; 5,7-dihydroxy-3-(4-hydroxyphenyl)-6,8-dimethylchromen-4-one; 5,7-dihydroxy-3-(4-hydroxyphenyl)-6,8-dimethyl-4H-chromen-4-one; 5,7-Dihydroxy-3-(4-hydroxy-phenyl)-6,8-dimethyl-chromen-4-one; 4H-1-benzopyran-4-one, 5,7-dihydroxy-3-(4-hydroxyphenyl)-6,8-dimethyl-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10200 nM | |||
| External Link | ||||
| 2-phenyl-1,2'-spirobi[1H-indene]-5'-ol | Investigative | [108] | ||
| Synonyms |
CHEMBL439806; SCHEMBL6283895; BDBM50123212; 2-phenyl-1,2''-spirobi[1H-indene]-5''-ol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 58 nM | |||
| External Link | ||||
| 4,6,7-trimethyl-6H-benzo[c]chromene-3,8-diol | Investigative | [104] | ||
| Synonyms |
CHEMBL381337; SCHEMBL6828067
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 335 nM | |||
| External Link | ||||
| 4,6,7,10-tetramethyl-6H-benzo[c]chromene-3,8-diol | Investigative | [104] | ||
| Synonyms |
CHEMBL206584; SCHEMBL6830345
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 237 nM | |||
| External Link | ||||
| ZK-164015 | Investigative | [118] | ||
| Synonyms |
ZK 164015; 177583-70-9; 2-(4-HYDROXYPHENYL)-3-METHYL-1-[10-(PENTYLSULFONYL)DECYL]-1H-INDOL-5-OL; CHEMBL184074; SCHEMBL7133598; CHEBI:92087; CTK4D6535; DTXSID00430964; MolPort-006-822-606; HMS3268P09; ZINC8022535; AKOS024456974; NCGC00092322-01; RT-016336; B6960; J-011295; BRD-K05151076-001-01-8
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-(4-Hydroxy-2-methoxy-phenyl)-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL427180; SCHEMBL3932725; TWSSGPRRAGPVKD-UHFFFAOYSA-N; BDBM50168373; ZINC13645059
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 174 nM | |||
| External Link | ||||
| 2,3-diphenyl-1H-indole | Investigative | [105] | ||
| Synonyms |
2,3-Diphenylindole; 3469-20-3; 1H-Indole, 2,3-diphenyl-; CHEMBL200719; GYGKJNGSQQORRG-UHFFFAOYSA-N; Indole, 2,3-diphenyl-; NSC17363; EINECS 222-432-0; Indole,3-diphenyl-; Cyto5A2; AC1Q1HUE; 1H-Indole,3-diphenyl-; AC1Q4W1P; AC1L2S6L; Oprea1_738570; 2,3-Diphenyl-1H-indole #; SCHEMBL3864662; CTK1C3291; KS-00000FNK; DTXSID50188241; MolPort-001-804-408; ZINC1036850; STK080838; BDBM50175411; RW3400; NSC-17363; CCG-46312; AKOS001022174; ACM3469203; MCULE-8938765915; VI30149; ACN-036136; 7J-655S; QC-2111; CC-07034; AK-81890
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 6950 nM | |||
| External Link | ||||
| 4-(5-Hydroxy-benzooxazol-2-yl)-benzene-1,3-diol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 190 nM | |||
| External Link | ||||
| Doxorubicin-Formaldehyde Conjugate | Investigative | [119] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Ethynyl-2-(4-hydroxy-phenyl)-benzooxazol-5-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 481 nM | |||
| External Link | ||||
| 3-(6-Hydroxy-benzooxazol-2-yl)-benzene-1,2-diol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2353 nM | |||
| External Link | ||||
| 1-Bromo-6-(4-hydroxy-phenyl)-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL194936; SCHEMBL3936480; IPBYYNRPJBGKET-UHFFFAOYSA-N; ZINC13645011; BDBM50168334
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 266 nM | |||
| External Link | ||||
| 4,6,10-trimethyl-6H-benzo[c]chromene-3,8-diol | Investigative | [104] | ||
| Synonyms |
CHEMBL380362
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1210 nM | |||
| External Link | ||||
| 7-Phenyl-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL194718; 7-Phenyl-2-naphthol; 7-Phenylnaphthalene-2-ol; SCHEMBL5981104; MLFRZWHPLMDWES-UHFFFAOYSA-N; BDBM50168338
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3405 nM | |||
| External Link | ||||
| 3-hydroxy-8,10-dimethyl-6H-benzo[c]chromen-6-one | Investigative | [104] | ||
| Synonyms |
CHEMBL380720
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 585 nM | |||
| External Link | ||||
| 2-(5-Hydroxy-naphthalen-1-yl)-benzooxazol-6-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 117 nM | |||
| External Link | ||||
| 4-(3-(4-hydroxyphenyl)-1H-indol-2-yl)phenol | Investigative | [105] | ||
| Synonyms |
Phenol, 4,4'-indole-2,3-diyldi-; BRN 1541731; 4,4'-Indole-2,3-diyldiphenol; CHEMBL200232; 5890-93-7; SCHEMBL7037980; AC1L46D4; DTXSID90207642; ZINC3638330; BDBM50175412; LS-104733; 4,4'-[1H-Indole-2,3-diyl]bis(phenol)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 94.8 nM | |||
| External Link | ||||
| 6-Phenyl-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
6-phenyl-2-naphthol; 6-Phenylnaphthalene-2-ol; CHEMBL191714; SCHEMBL3019399; PZOXFBPKKGTQDZ-UHFFFAOYSA-N; ZINC13644999
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1345 nM | |||
| External Link | ||||
| 6-(2,6-Difluoro-4-hydroxy-phenyl)-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL192621; SCHEMBL3924515; AUZLISXBMGKYGA-UHFFFAOYSA-N; ZINC13645047; BDBM50168333
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10.3 nM | |||
| External Link | ||||
| 2-(2-Chloro-4-hydroxy-phenyl)-benzooxazol-5-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 139 nM | |||
| External Link | ||||
| 6-(4-Hydroxy-phenyl)-1-phenyl-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL192102; SCHEMBL3927262; INNGVLJQICTRAF-UHFFFAOYSA-N; BDBM50168362
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1231 nM | |||
| External Link | ||||
| 8-n-propylnaringenin | Investigative | [112] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 155 nM | |||
| External Link | ||||
| 3-(5-Hydroxy-benzooxazol-2-yl)-benzene-1,2-diol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5110 nM | |||
| External Link | ||||
| 2-(4-Hydroxy-naphthalen-1-yl)-benzooxazol-6-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1900 nM | |||
| External Link | ||||
| EFFUSOL | Investigative | [104] | ||
| Synonyms |
73166-28-6; NSC 371300; 5-ethenyl-1-methyl-9,10-dihydrophenanthrene-2,7-diol; UNII-S436Y000RU; CHEMBL205119; S436Y000RU; 1-methyl-5-vinyl-9,10-dihydrophenanthrene-2,7-diol; NSC-371300; 2,7-Phenanthrenediol, 5-ethenyl-9,10-dihydro-1-methyl-; NSC371300; AC1L2PII; AC1Q7B8H; CTK8D4698; DTXSID00223419; MolPort-042-624-482; ZINC1588038; BDBM50180512; FT-0667825
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 240 nM | |||
| External Link | ||||
| 8-n-nonylnaringenin | Investigative | [112] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 436 nM | |||
| External Link | ||||
| 2-(4-hydroxyphenyl)-1,2'-spirobi[1H-indene]-5-ol | Investigative | [108] | ||
| Synonyms |
CHEMBL281499; SCHEMBL6286186; BDBM50123205; 2-(4-hydroxyphenyl)-1,2''-spirobi[1H-indene]-5-ol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4.3 nM | |||
| External Link | ||||
| 6-(3-Fluoro-4-hydroxy-phenyl)-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL193727; SCHEMBL3924993; QTDYCKGQBCKMGC-UHFFFAOYSA-N; ZINC13645068; BDBM50168372
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 92 nM | |||
| External Link | ||||
| 6-(2,5-Difluoro-4-hydroxy-phenyl)-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL195289; SCHEMBL3930290; VLJUOFGVEJAFFK-UHFFFAOYSA-N; ZINC13645040; BDBM50168359
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 27 nM | |||
| External Link | ||||
| 8-(2-methylpropyl)naringenin | Investigative | [112] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 80 nM | |||
| External Link | ||||
| WAY-169916 | Investigative | [92] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 93 nM | |||
| External Link | ||||
| 2-(6-Hydroxy-naphthalen-1-yl)-benzooxazol-6-ol | Investigative | [103] | ||
| Synonyms |
CHEMBL189077; 2-(6-hydroxynaphthalen-1-yl)benzo[d]oxazol-6-ol; SCHEMBL3926798; ZRGCERZQAHMXNC-UHFFFAOYSA-N; BDBM50154135; 595566-69-1; 6-Benzoxazolol,2-(6-hydroxy-1-naphthalenyl)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 35 nM | |||
| External Link | ||||
| 2-(4-Hydroxy-phenyl)-7-vinyl-benzooxazol-5-ol | Investigative | [103] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 447 nM | |||
| External Link | ||||
| 1,2-Bis-(4-hydroxy-phenyl)-3H-inden-5-ol | Investigative | [120] | ||
| Synonyms |
CHEMBL26865
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 7 nM | |||
| External Link | ||||
| 1-Fluoro-6-(4-hydroxy-phenyl)-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
CHEMBL192782; SCHEMBL3925203; VQATUPWMFYRKSD-UHFFFAOYSA-N; BDBM50168380; ZINC13645014
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 77 nM | |||
| External Link | ||||
| 7-(3-Hydroxy-phenyl)-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
7-(3-hydroxyphenyl)-2-naphthol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2642 nM | |||
| External Link | ||||
| 4-Chloro-2-(4-hydroxy-phenyl)-quinolin-6-ol | Investigative | [100] | ||
| Synonyms |
4-chloro-2-(4-hydroxyphenyl)quinolin-6-ol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 213 nM | |||
| External Link | ||||
| 4-Bromo-2-(4-hydroxy-phenyl)-quinolin-6-ol | Investigative | [100] | ||
| Synonyms |
4-bromo-2-(4-hydroxyphenyl)quinolin-6-ol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 212 nM | |||
| External Link | ||||
| 4-Ethynyl-2-(4-hydroxy-phenyl)-quinolin-6-ol | Investigative | [100] | ||
| Synonyms |
4-ethynyl-2-(4-hydroxyphenyl)quinolin-6-ol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1500 nM | |||
| External Link | ||||
| 4-Ethyl-2-(4-hydroxy-phenyl)-quinolin-6-ol | Investigative | [100] | ||
| Synonyms |
4-ethyl-2-(4-hydroxyphenyl)quinolin-6-ol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 634 nM | |||
| External Link | ||||
| 6-(4-Hydroxy-phenyl)-naphthalen-1-ol | Investigative | [107] | ||
| Synonyms |
6-(4-hydroxyphenyl)-1-naphthol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 22 nM | |||
| External Link | ||||
| 2-(4-Hydroxy-phenyl)-4-vinyl-quinolin-6-ol | Investigative | [100] | ||
| Synonyms |
2-(4-hydroxyphenyl)-4-vinylquinolin-6-ol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 504 nM | |||
| External Link | ||||
| SOPHORAFLAVANONE B | Investigative | [112] | ||
| Synonyms |
8-Prenylnaringenin; 53846-50-7; Flavaprenin; (-)-(2S)-8-dimethylallylnaringenin; (S)-8-dimethylallylnaringenin; YS04; CHEMBL376915; CHEBI:50207; (-)-(2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-8-(3-methylbut-2-enyl)chroman-4-one; (2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-8-(3-methylbut-2-en-1-yl)-2,3-dihydro-4H-chromen-4-one; (s)-8-prenylnaringenin; 8-dimethylallylnaringenin; AC1LA3DM; UNII-5L872SZR8X; 8-prenylnaringenin (8-PN); BIDD:ER0149; cid_509245; SCHEMBL1171435; 5L872SZR8X; ZINC39452; CTK5J8800; BDBM19460
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 57 nM | |||
| External Link | ||||
| Geldanamycin-estradiol hybrid | Investigative | [121] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| CP-409069 | Investigative | [122] | ||
| Synonyms |
CHEMBL78625; SCHEMBL3114566; BDBM50113780; (2R,4aS,10aR)-4a-benzyl-2-(prop-1-ynyl)-1,2,3,4,4a,9,10,10a-octahydrophenanthrene-2,7-diol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CP-394531 | Investigative | [122] | ||
| Synonyms |
UNII-227D9ED2SI; CHEMBL77779; 227D9ED2SI; 305822-63-3; SCHEMBL3111189; BDBM50113783; 2,7-Phenanthrenediol, 2-(chloroethynyl)-1,2,3,4,4a,9,10,10a-octahydro-4a-(phenylmethyl)-, (2R,4aS,10aR)-; 2,7-Phenanthrenediol, 2-(2-chloroethynyl)-1,2,3,4,4a,9,10,10a-octahydro-4a-(phenylmethyl)-, (2R,4aS,10aR)-; 4a-Benzyl-2-chloroethynyl-1,2,3,4,4a,9,10,10a-octahydro-phenanthrene-2,7-diol(CP-394531)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-(3-Hydroxy-phenyl)-naphthalen-2-ol | Investigative | [107] | ||
| Synonyms |
6-(3-hydroxyphenyl)-2-naphthol; 6-(3-hydroxyphenyl)naphthalen-2-ol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 230 nM | |||
| External Link | ||||
| daidzein | Investigative | [117] | ||
| Synonyms |
486-66-8; 4',7-Dihydroxyisoflavone; Daidzeol; 7,4'-Dihydroxyisoflavone; 7-hydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one; 4H-1-Benzopyran-4-one, 7-hydroxy-3-(4-hydroxyphenyl)-; 7-Hydroxy-3-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; 7-hydroxy-3-(4-hydroxyphenyl)chromen-4-one; 7-Hydroxy-3-(4-hydroxyphenyl)-4-benzopyrone; UNII-6287WC5J2L; CCRIS 7600; K 251b; 4,7-Dihydroxyisoflavone; EINECS 207-635-4; BRN 0231523; CHEMBL8145; 4',7-Dihydroxy-iso-flavone; d-(+)-alpha-methylbenzylamine; ,7-Dihydroxyisoflavone; DIADZEIN
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 450 nM | |||
| External Link | ||||
| 2B91: Colorectal cancer | 25 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Retifanlimab | Approved | [123] | ||
| Synonyms |
INCMGA0012; Retifanlimab
Click to Show/Hide
|
|||
| External Link | ||||
| Aflibercept | Approved | [93] | ||
| Synonyms |
Ziv-Aflibercept; Zaltrap (TN); VEGF Trap; VEGF Trap-Eye
Click to Show/Hide
|
|||
| External Link | ||||
| Regorafenib | Approved | [124] | ||
| Synonyms |
755037-03-7; BAY 73-4506; Regorafenibum; Stivarga; 4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide; BAY73-4506; Regorafenib (BAY 73-4506); UNII-24T2A1DOYB; 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide; BAY-73-4506; 24T2A1DOYB; CHEMBL1946170; CHEBI:68647; Stivarga (TN); BAY73-4506 hydrochloride; Regorafenib [USAN:INN]
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [48] | ||
| Synonyms |
Bevacizumab (ophthalmic slow-release tissue tablet)
Click to Show/Hide
|
|||
| External Link | ||||
| SYM-004 | Phase 3 | [48] | ||
| Synonyms |
Chimeric IgG1 antibody 1024 (cancer), Symphogen; Chimeric IgG1 antibody 992 (cancer), Symphogen; Chimeric IgG1 antibodies992 + 1024 (cancer), Symphogen
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab + Erlotinib | Phase 3 | [125] | ||
| External Link | ||||
| CPI-613 | Phase 3 | [48] | ||
| Synonyms |
95809-78-2; 6,8-bis(benzylthio)octanoic acid; CPI 613; MLS006010202; SCHEMBL1062218; 6,8-Bis(benzylsulfanyl)octanoic acid; Octanoic acid, 6,8-bis((phenylmethyl)thio)-; Octanoic acid, 6,8-bis[(phenylMethyl)thio]-; 6,8-Bis[(phenylmethyl)thio]octanoic acid; CPI613; CHEMBL3186849; QCR-193; AOB1058; MolPort-023-219-128; HMS3656L06; C22H28O2S2; BCP04663; EX-A2043; s2776; AKOS025142095; BCP9000552; DB12109; RL06062; CS-0961; NCGC00344764-01; SMR004701300; AS-16613; BC261916; AK174899; HY-15453; BCP0726000030; KB-293127; AB0035874
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [93] | ||
| External Link | ||||
| AlloStim | Phase 2/3 | [126] | ||
| Synonyms |
AlloStim (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Sibrotuzumab | Phase 2 | [127] | ||
| External Link | ||||
| CV301 | Phase 2 | [128] | ||
| External Link | ||||
| Efatutazone | Phase 2 | [129] | ||
| Synonyms |
Inolitazone; 223132-37-4; 5-[[4-[[6-(4-amino-3,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-yl]methoxy]phenyl]methyl]-2,4-Thiazolidinedione; Efatutazone [INN]; RS5444; CS-7017; SCHEMBL3246054; CHEMBL3545280; JCYNMRJCUYVDBC-UHFFFAOYSA-N; Efatutazone;CS-7017;RS5444; BCP07478; AKOS030526729; DB11894; CS-0778; KB-77905; DA-07988; HY-14792; QC-10456; 4CA-1384; FT-0737589; 5-[4-[6-(4-amino-3 ,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-ylmethoxy]benzyl]thiazolidine-2,4-dione
Click to Show/Hide
|
|||
| External Link | ||||
| LOR-2040 | Phase 2 | [130] | ||
| External Link | ||||
| RG7221 | Phase 2 | [131] | ||
| External Link | ||||
| PEG-SN38 | Phase 2 | [132] | ||
| Synonyms |
EZN-2208
Click to Show/Hide
|
|||
| External Link | ||||
| MEGF0444A | Phase 2 | [133] | ||
| External Link | ||||
| Encapsulated cell therapy | Phase 1/2 | [134] | ||
| External Link | ||||
| AB928 | Phase 1/2 | [135] | ||
| External Link | ||||
| MGD007 | Phase 1 | [131] | ||
| External Link | ||||
| BNC-101 | Phase 1 | [136] | ||
| External Link | ||||
| Navicixizumab | Phase 1 | [48] | ||
| External Link | ||||
| RG7160 | Discontinued in Phase 2 | [137] | ||
| External Link | ||||
| Nimesulide | Terminated | [138] | ||
| Synonyms |
51803-78-2; N-(4-Nitro-2-phenoxyphenyl)methanesulfonamide; Mesulid; Flogovital; Sulidene; Nimed; R-805; 4-NITRO-2-PHENOXYMETHANESULFONANILIDE; Nisulid; Nimesulidum [INN-Latin]; Nimesulida [INN-Spanish]; R 805; UNII-V4TKW1454M; 4-Nitro-2-phenoxy-methanesulfonanilide; 4'-Nitro-2'-phenoxymethanesulfonanilide; Methanesulfonamide, N-(4-nitro-2-phenoxyphenyl)-; EINECS 257-431-4; 4'-Nitro-2'-phenoxymethansulfonanilid; BRN 2421175; CHEMBL56367; MLS000069680; V4TKW1454M; Methanesulfonanilide, 4'-nitro-2'-phenoxy-; CHEBI:44445; Dulanermin
Click to Show/Hide
|
|||
| External Link | ||||
| Saracatinib | Phase 2 | [139] | ||
| External Link | ||||
| G3139 + Irinotecan | Investigative | [140] | ||
| External Link | ||||
References