m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05120
|
[1] | |||
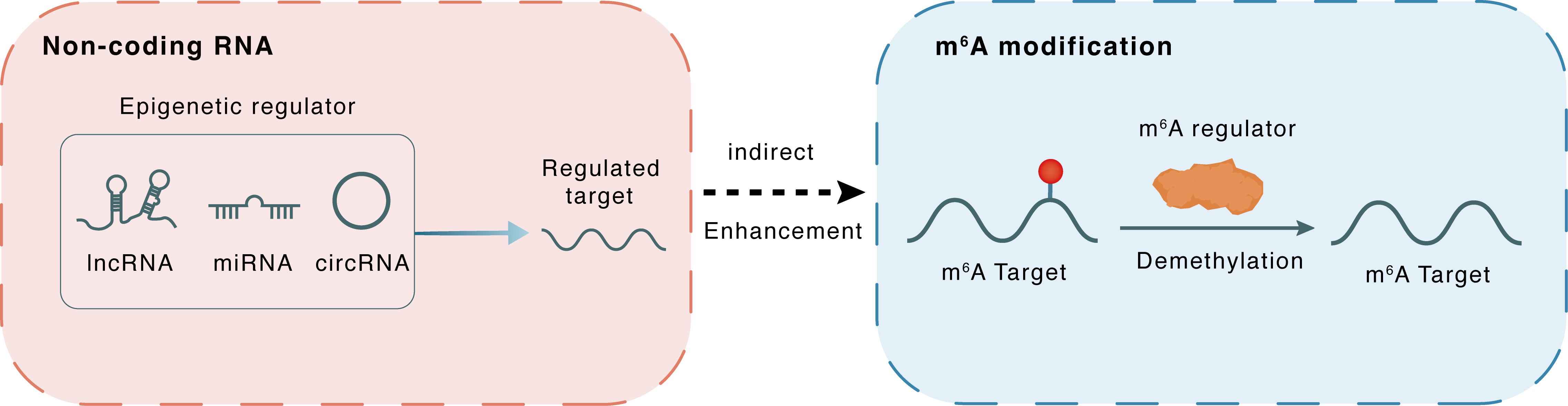 Non-coding RNA
CircGPR137B
miR-4739
lncRNA miRNA circRNA
Indirect
Enhancement
m6A modification
Circ_GPR137B
Circ_GPR137B
FTO
Demethylation
Non-coding RNA
CircGPR137B
miR-4739
lncRNA miRNA circRNA
Indirect
Enhancement
m6A modification
Circ_GPR137B
Circ_GPR137B
FTO
Demethylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | |||
| m6A Target | Circ_GPR137B | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | Circ_GPR137B | circRNA | View Details | ||
| Regulated Target | hsa-miR-4739 | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Enhancement | |||
| Crosstalk Mechanism | ncRNAs indirectly impacts m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | Circ_GPR137B, co-localized with hsa-miR-4739 in the cytoplasm, acted as a sponge for miR-4739 to upregulate its target FTO, which mediated m6A demethylation of circGPR137B and promoted its expression. Thus, a feedback loop comprising circGPR137B/miR-4739/FTO axis was formed. FTO suppressed cell growth and indicated favorable survival in patients with HCC. | ||||
| Responsed Disease | Liver cancer | ICD-11: 2C12 | |||
In-vitro Model |
L-02 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 | ||
| Hep 3B2.1-7 | Childhood hepatocellular carcinoma | Homo sapiens | CVCL_0326 | ||
| HuH-6 | Hepatoblastoma | Homo sapiens | CVCL_4381 | ||
| Huh-7 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0336 | ||
| SK-HEP-1 | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | ||
| In-vivo Model | The mice were subcutaneously inoculated with 6 × 107 HepG2 cells stably transfected with pLV-circGPR137B and empty vectors or Sk-hep-1 cells stably transfected with pLV-sh-circGPR137B/sh-NC. HepG2 cells stably transfected with circGPR137B or pcDNA3.1 were cultured in complete medium. When the cells were 70% confluent, the medium was replaced with fresh medium to remove dead and detached cells. Subsequently, 6 × 107 cells were used to inject into mice via the tail vein. Each mouse was injected with 100ul (6× 106 cells). | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| 2C12: Liver cancer | 49 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| 90Y-loaded resin microspheres | Approved | [2] | ||
| External Link | ||||
| Thymalfasin | Phase 2 | [3] | ||
| Synonyms |
Zadaxin; 62304-98-7; Thymosin alpha1; Thymosin alpha 1; Thymosin alpha1 (ox); Thymosin alpha1 (human); Thymalfasin [USAN:INN]; UNII-W0B22ISQ1C; Thymosin-alpha-1; 69440-99-9; Thymosin alpha1 (cattle); C129H215N33O55; W0B22ISQ1C; Zadaxin (TN); Thymalfasin alfa 1
Click to Show/Hide
|
|||
| External Link | ||||
| Ferumoxides | Approved | [4] | ||
| Synonyms |
AMI-25; 119683-68-0; Feridex; Feridex IV; Superparamagnetic iron oxide; UNII-G6N3J05W84; Ferumoxides [USAN:USP:BAN]; CCRIS 6722; HSDB 8072; AC1O5DID; G6N3J05W84; iron(2+); iron(3+); Iron oxide crystal is inverse spinel (X-ray data); Fe(II) and Fe(III) are present (Mossbauer Spectroscopy; Physical form is a colloidal particle of nonstoichiometric
Click to Show/Hide
|
|||
| External Link | ||||
| DTI-015 | Approved | [5] | ||
| Synonyms |
Carmustine; 154-93-8; 1,3-Bis(2-chloroethyl)-1-nitrosourea; BCNU; Carmustin; Nitrumon; Carmubris; Gliadel; BiCNU; Bi CNU; Carmustinum; Bischlorethylnitrosurea; Bischlorethylnitrosourea; Carmustina; Becenun; Becenum; Bischloroethyl nitrosourea; N,N'-BIS(2-CHLOROETHYL)-N-NITROSOUREA; Bis(2-chloroethyl)nitrosourea; Urea, N,N'-bis(2-chloroethyl)-N-nitroso-; Gliadel Wafer; FDA 0345; Bischloroethylnitrosourea; SRI 1720; 1,3-Bis(2-chloroethyl)nitrosourea; BiCNU (TN); Carmustinum [INN-Latin]; Carmustina [INN-Spanish]; DTI 015; NCI-C04773; SK; Injectable carmustine, Direct Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| Nofazinlimab | Phase 3 | [6] | ||
| Synonyms |
CS1003; EQ176
Click to Show/Hide
|
|||
| External Link | ||||
| PV-10 | Phase 3 | [7] | ||
| Synonyms |
632-69-9; Rose bengal sodium; Rose bengal disodium salt; R105 sodium; Rose-bengal (131 I) natrium; Food Red No 105, sodium salt; EINECS 211-183-3; Food Red Color No 105, sodium salt; Sel disodique de rose bengale iodee (131 I); Rose bengale (131 I) sodique [INN-French]; Rosa bengala sodica (131 I) [INN-Spanish]; Roseum bengalense (131 I) natricum [INN-Latin]; 2,4,5,7-Tetraido(m,p,o',m')tetrachlorofluorescein, disodium salt; Fluorescein, 4,5,6,7-tetrachloro-2',4',5',7'-tetraiodo-, disodium salt; Disodium
Click to Show/Hide
|
|||
| External Link | ||||
| Brivanib | Phase 3 | [8] | ||
| Synonyms |
649735-46-6; BMS-540215; Brivanib (BMS-540215); BMS 540215; UNII-DDU33B674I; Brivanib [USAN]; BMS540215; DDU33B674I; CHEMBL377300; (2R)-1-[4-(4-Fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy]propanol; Brivanib (USAN); (2R)-1-[4-[(4-FLUORO-2-METHYL-1H-INDOL-5-YL)OXY]-5-METHYL-PYRROLO[2,1-F][1,2,4]TRIAZIN-6-YL]OXYPROPAN-2-OL; (2R)-1-({4-[(4-fluoro-2-methyl-1H-indol-5-yl)oxy]-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yl}oxy)propan-2-ol
Click to Show/Hide
|
|||
| External Link | ||||
| JX-594 | Phase 3 | [9] | ||
| Synonyms |
Pexastimogene devacirepvec
Click to Show/Hide
|
|||
| External Link | ||||
| ABH001 | Phase 3 | [10] | ||
| External Link | ||||
| MTC-DOX | Phase 2/3 | [11] | ||
| Synonyms |
MTC-doxorubicin
Click to Show/Hide
|
|||
| External Link | ||||
| KD018 | Phase 2 | [12] | ||
| External Link | ||||
| Doxorubicin-eluting beads | Phase 2 | [13] | ||
| Synonyms |
DC Bead (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| 32-P BioSilicon | Phase 2 | [14] | ||
| Synonyms |
BrachySil
Click to Show/Hide
|
|||
| External Link | ||||
| Cixutumumab | Phase 2 | [15] | ||
| Synonyms |
LY3012217
Click to Show/Hide
|
|||
| External Link | ||||
| [131I]-Metuximab | Phase 2 | [16] | ||
| External Link | ||||
| Darinaparsin | Phase 2 | [17] | ||
| Synonyms |
ZIO-101
Click to Show/Hide
|
|||
| External Link | ||||
| Tigatuzumab | Phase 2 | [18] | ||
| External Link | ||||
| CMC-001 | Phase 2 | [19] | ||
| Synonyms |
Manganese-based MRI contrast agent (liver tumor imaging), CMC Contrast
Click to Show/Hide
|
|||
| External Link | ||||
| OBP-301 | Phase 1/2 | [20] | ||
| External Link | ||||
| MBO7133 | Phase 1/2 | [21] | ||
| External Link | ||||
| INCB62079 | Phase 1/2 | [7] | ||
| External Link | ||||
| NV-1020 | Phase 1/2 | [22] | ||
| External Link | ||||
| DCVax-Liver | Phase 1/2 | [23] | ||
| Synonyms |
Dendritic cell-based immunotherapy (liver cancer), Northwest Biotherapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| SRF388 | Phase 1 | [24] | ||
| External Link | ||||
| ET140202 | Phase 1 | [25] | ||
| External Link | ||||
| ADP-A2AFP | Phase 1 | [26] | ||
| External Link | ||||
| SM04755 | Phase 1 | [27] | ||
| External Link | ||||
| Anti-CEA CAR-T therapy | Phase 1 | [7] | ||
| External Link | ||||
| PI-166 | Phase 1 | [28] | ||
| External Link | ||||
| CRS-100 | Phase 1 | [29] | ||
| External Link | ||||
| Autologous ET1402L1-CART cells | Phase 1 | [30] | ||
| External Link | ||||
| Anti-CD133-CAR vector-transduced T cells | Phase 1 | [31] | ||
| External Link | ||||
| MRX34 | Phase 1 | [32] | ||
| External Link | ||||
| ALN-VSP | Phase 1 | [33] | ||
| External Link | ||||
| EPCAM-targeted CAR-T cells | Clinical trial | [34] | ||
| External Link | ||||
| ADI | Discontinued in Phase 3 | [35] | ||
| Synonyms |
Arginine deiminase
Click to Show/Hide
|
|||
| External Link | ||||
| GN-1140 | Discontinued in Phase 2 | [36] | ||
| External Link | ||||
| OGT-719 | Discontinued in Phase 2 | [37] | ||
| Synonyms |
OGS-719
Click to Show/Hide
|
|||
| External Link | ||||
| AFP-Scan | Discontinued in Phase 2 | [38] | ||
| External Link | ||||
| SR1078 | Preclinical | [39] | ||
| Synonyms |
1246525-60-9; SR 1078; SR-1078; N-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]-4-(trifluoromethyl)benzamide; CHEMBL3094387; N-(4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl)-4-(trifluoromethyl)benzamide; N-[4-[2,2,2-Trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-4-(trifluoromethyl)benzamide; SCHEMBL4880524; C17H10F9NO2; DTXSID30591895; BCP09203; EX-A2215; 4063AH; BDBM50444350; s5775; ZINC98052696; AKOS024458390; CS-1045; NCGC00379222-02; AK547149; AS-55921; HY-14422; W-5797; SR-03000001078; SR-03000001078-1; SR-03000001078-2
Click to Show/Hide
|
|||
| External Link | ||||
| Occlusin | Preclinical | [40] | ||
| Synonyms |
Occlusin 50 Injection; Occlusin 500 injection
Click to Show/Hide
|
|||
| External Link | ||||
| HRC-201 | Terminated | [41] | ||
| Synonyms |
Hemoglobin-imaging conjugate (HepSelect), Hemosol
Click to Show/Hide
|
|||
| External Link | ||||
| 1,2,3,4,5,6-hexabromocyclohexane | Investigative | [42] | ||
| Synonyms |
1837-91-8; Benzene hexabromide; Cyclohexane, 1,2,3,4,5,6-hexabromo-; Hexabromocyclohexane; JAK2 Inhibitor II; ACMC-1BQJT; SCHEMBL459442; trans-alpha-Benzene hexabromide; CHEMBL444236; DTXSID4052687; CHEBI:93940; NSC7908; HMS3268H22; HMS3413C10; HMS3677C10; NSC-7908; ZINC1586309; ANW-23174; MFCD00059127; s5902; Cyclohexane,2,3,4,5,6-hexabromo-; AKOS015836040; 1,2,3,4,5,6-Hexabromo-cyclohexane; 1,2,3,4,5,6-Hexabromocyclohexane #; NCGC00092358-01; NCGC00092358-02; 1,2,3,4,5,6-hexakis(bromanyl)cyclohexane; A4510; FT-0633875; JAK2 Inhibitor II - CAS 1837-91-8; 1,2,3,4,5,6-Hexabromocyclohexane;NSC7908; A812818; 1,2,3,4,5,6-Hexabrom-cyclohexan (I(2)-Form); J-011778; 1,2,3,4,5,6-Hexabromocyclohexane, >=98% (HPLC); BRD-K06817181-001-01-5; Q27165694
Click to Show/Hide
|
|||
| External Link | ||||
| STP-322 | Investigative | [43] | ||
| Synonyms |
Multi-targeted siRNA therapeutic cocktail (nanoparticle, liver tumor), Sirnaomics
Click to Show/Hide
|
|||
| External Link | ||||
| AMB-8LK | Investigative | [43] | ||
| Synonyms |
Cancer therapy (monoclonal antibody), MAT Biopharma; Y90 anti-ferritin monoclonal antibody (cancer), MAT Biopharma; Yttrium-90 anti-ferritin mAb (cancer), MAT Biopharma; Yttrium-90 anti-ferritin mAb (Hodgkin's disease/pancreatic/liver cancer), MAT Biopharma; 90Y-AMB8LK mAb (cancer), MAT Biopharma; 90Y-AMB8LK monoclonal antibody (cancer), MAT Biopharma; 90Y-labelled anti-ferritin monoclonal antibody (cancer), MAT Biopharma
Click to Show/Hide
|
|||
| External Link | ||||
| MiR-34a mimics | Investigative | [43] | ||
| Synonyms |
MiR-34a mimics (liver cancer)
Click to Show/Hide
|
|||
| External Link | ||||
| P53 fusion protein | Investigative | [43] | ||
| Synonyms |
P53 fusion protein (pancreatic/liver cancer)
Click to Show/Hide
|
|||
| External Link | ||||
| OP-05 | Investigative | [43] | ||
| Synonyms |
OP-05 program (prodrug, liver tumor); OP-05 program (prodrug, liver tumor), Onco-Pharmakon
Click to Show/Hide
|
|||
| External Link | ||||
| GR-DD1 | Investigative | [43] | ||
| Synonyms |
Cytotoxin (hepatic metastasis), ERYtech
Click to Show/Hide
|
|||
| External Link | ||||
References