m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05083
|
[1] | |||
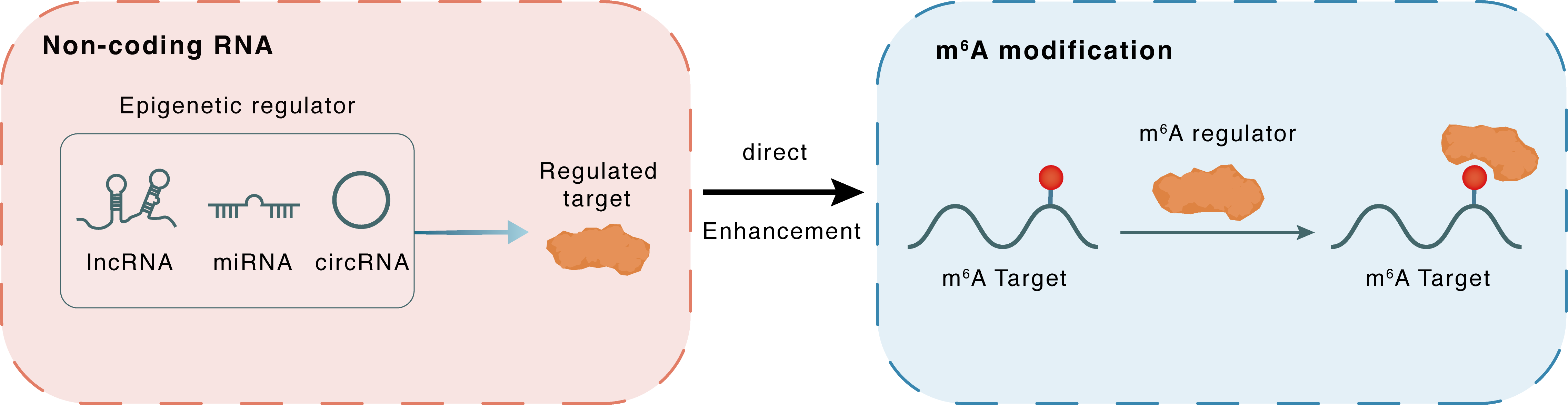 Non-coding RNA
DRAIC
HNRNPA2B1
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
IGF1R
IGF1R
hnRNPA2B1
Non-coding RNA
DRAIC
HNRNPA2B1
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
IGF1R
IGF1R
hnRNPA2B1
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Heterogeneous nuclear ribonucleoproteins A2/B1 (HNRNPA2B1) | READER | |||
| m6A Target | Insulin-like growth factor 1 receptor (IGF1R) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | Downregulated RNA in cancer, inhibitor of cell invasion and migration (DRAIC) | LncRNA | View Details | ||
| Regulated Target | Heterogeneous nuclear ribonucleoprotein A2/B1 (HNRNPA2B1) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Enhancement | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through recruiting m6A regulator | ||||
| Crosstalk Summary | DRAIC mediates HNRNPA2B1 stability and m6A-modified Insulin-like growth factor 1 receptor (IGF1R) instability to inhibit tumor progression | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Insulin-like growth factor 1 receptor (IGF1R) | 38 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Teprotumumab | Approved | [2] | ||
| Synonyms |
RV001
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| Mecasermin | Approved | [3] | ||
| Synonyms |
Increlex (TN)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Somatomedin-1 | Approved | [4] | ||
| Synonyms |
Igef (TN)
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| OSI-906 | Phase 3 | [5] | ||
| Synonyms |
Linsitinib; 867160-71-2; Linsitinib(OSI-906); OSI906; OSI 906; OSI-906AA; OSI-906 (Linsitinib); UNII-15A52GPT8T; Kinome_3532; ASP-7487; 3-[8-amino-1-(2-phenylquinolin-7-yl)imidazo[1,5-a]pyrazin-3-yl]-1-methylcyclobutan-1-ol; 15A52GPT8T; CHEMBL1091644; MMV676605; cis-3-[8-Amino-1-(2-phenyl-7-quinolinyl)imidazo[1,5-a]pyrazin-3-yl]-1-methylcyclobutanol; C26H23N5O; cis-3-(8-amino-1-(2-phenyl-7-quinolinyl)imidazo(1,5-a)pyrazin-3-yl)-1-methylcyclobutanol; Linsitinib [USAN:INN]; OSI906/Linsitinib/
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 13 nM | |||
| External Link | ||||
| Rinfabate | Phase 2/3 | [6] | ||
| Synonyms |
RhIGFBP-3; Rinfabate, Insmed; RhIGF-BP3, Insmed; Insulin-like growth factor binding protein-3, Insmed
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| AMG 479 | Phase 2 | [7] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| VPI-2690B | Phase 2 | [8] | ||
| MOA | Antagonist | |||
| External Link | ||||
| AXL-1717 | Phase 2 | [9] | ||
| Synonyms |
BVT-51004; IGF-1 inhibitors, Axelar/Biovitrum; IGF-1 inhibitors, Karolinska/Biovitrum; Insulin-like growth factor 1 inhibitors, Axelar/Biovitrum
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| R1507 | Phase 2 | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Cixutumumab | Phase 2 | [11] | ||
| Synonyms |
LY3012217
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| TT-100 | Phase 2 | [12] | ||
| Synonyms |
TT-100, TriAct; Dual IGF-1/EGFR inhibitor (non-small-cell lung cancer), TriAct
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MM-141 | Phase 2 | [13] | ||
| MOA | Modulator | |||
| External Link | ||||
| Cyclolignan picropodophyllin | Phase 1 | [14] | ||
| Synonyms |
PPP; IGF-1R inhibitor (cancer), Karolinska
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RG-7010 | Phase 1 | [15] | ||
| Synonyms |
R-7010; PEGylated IGF1 (amyotrophic lateral sclerosis), Roche
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| HF-0299 | Phase 1 | [16] | ||
| MOA | Modulator | |||
| External Link | ||||
| BIIB 022 | Phase 1 | [17] | ||
| MOA | Antagonist | |||
| External Link | ||||
| FPI-1434 | Phase 1 | [18] | ||
| External Link | ||||
| AEW-541 | Phase 1 | [19] | ||
| Synonyms |
AECDBHGVIIRMOI-UHFFFAOYSA-N; NVP-AEW541; 475489-16-8; 475488-34-7; AEW541; NVP-AEW 541; UNII-97QB5037VR; AEW 541; AVP-AEW541; 7-((1s,3s)-3-(azetidin-1-ylmethyl)cyclobutyl)-5-(3-(benzyloxy)phenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine; CHEMBL1614712; 97QB5037VR; 7-[TRANS-3-(1-AZETIDINYLMETHYL)CYCLOBUTYL]-5-[3-(PHENYLMETHOXY)PHENYL]-7H-PYRROLO[2,3-D]PYRIMIDIN-4-AMINE; C27H29N5O; 7H-PYRROLO[2,3-D]PYRIMIDIN-4-AMINE, 7-[CIS-3-(1-AZETIDINYLMETHYL)CYCLOBUTYL]-5-[3-(PHENYLMETHOXY)PHENYL]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 60 nM | |||
| External Link | ||||
| SAR446159 | Phase 1 | [20] | ||
| External Link | ||||
| AVE-1642 | Discontinued in Phase 2 | [21] | ||
| Synonyms |
EM-164; Anti-IGF-1 receptor antibody (cancer), Aventis; Anti-IGF-1 receptor antibody (cancer), ImmunoGen; Anti-insulin-like growth factor-1 receptor antibody, Aventis; Anti-insulin-like growth factor-1 receptor antibody, ImmunoGen; Anti-IGF-1 receptor antibody (cancer), sanofi-aventis; Anti-insulin-like growthfactor-1 receptor antibody, sanofi-aventis
Click to Show/Hide
|
|||
| External Link | ||||
| KW-2450 | Discontinued in Phase 1/2 | [22] | ||
| MOA | Modulator | |||
| External Link | ||||
| Figitumumab | Discontinued in Phase 1 | [23] | ||
| Synonyms |
AC1OCENC; (2R)-3-[(4S,6R,7R,10S)-4-[(E,2R)-4-[(2S,2'S,4R,4aS,6R,8aR)-4-hydroxy-2-[(1S,3S)-1-hydroxy-3-[(6S,9R,10S)-9-methyl-5,11-dioxaspiro[5.5]undecan-10-yl]butyl]-3-methylidenespiro[4a,7,8,8a-tetrahydro-4H-pyrano[3,2-b]pyran-6,5'-oxolane]-2'-yl]but-3-en-2-yl]-7-hydroxy-2-methyl-5,11-dioxaspiro[5.5]undec-1-en-10-yl]-2-hydroxy-2-methylpropanoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BMS-695735 | Preclinical | [24] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 34 nM | |||
| External Link | ||||
| EGFR/IGFR tandem adnectin | Preclinical | [25] | ||
| Synonyms |
BMS-964210
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NVP-ADW742 | Investigative | [26] | ||
| Synonyms |
475488-23-4; ADW-742; 475489-15-7; UNII-MXS2N5862L; ADW742; MXS2N5862L; CHEMBL399021; 5-(3-(Benzyloxy)phenyl)-7-((1r,3r)-3-(pyrrolidin-1-ylmethyl)-cyclobutyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine; 5-(3-(Benzyloxy)phenyl)-7-(cis-3-(pyrrolidin-1-ylmethyl)cyclobutyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine; C28H31N5O; 5-(3-Benzyloxyphenyl)-7-[trans-3-[(pyrrolidin-1-yl)methyl]cyclobutyl]-7H-pyrrolo[2,3-d]pyrimidin-4-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 323 nM | |||
| External Link | ||||
| JB-1 | Investigative | [26] | ||
| Synonyms |
JB1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-((naphthalen-2-ylamino)methyl)benzene-1,2-diol | Investigative | [27] | ||
| Synonyms |
CHEMBL1240677; BDBM50326006
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4500 nM | |||
| External Link | ||||
| 4-((1H-indazol-6-ylamino)methyl)benzene-1,2-diol | Investigative | [27] | ||
| Synonyms |
CHEMBL1240676; BDBM50326004
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1500 nM | |||
| External Link | ||||
| AMP-PNP | Investigative | [28] | ||
| Synonyms |
Phosphoaminophosphonic acid-adenylate ester; gamma-Imino-ATP; ADENYLYL IMIDODIPHOSPHATE; AMPPNP; Adenyl imidodiphosphate; 25612-73-1; adenyl-5'-yl imidodiphosphate; CHEBI:47785; App(NH)p; O(5')-(1,2-dihydroxy-2-phosphonoaminodiphosphoryl)adenosine; 5'-O-(hydroxy{[hydroxy(phosphonoamino)phosphoryl]oxy}phosphoryl)adenosine; [[[[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]amino]phosphonic acid; p(NH)Ppf; beta,gamma-Imido-ATP; beta,gamma-Imidoadenosine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BMS-536924 | Investigative | [29] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| Fucose | Investigative | [30] | ||
| Synonyms |
L-galactomethylose; 6-Desoxygalactose; SCHEMBL13092958; AKOS030212707
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Alpha-D-Mannose | Investigative | [30] | ||
| Synonyms |
alpha-D-Mannopyranose; alpha-Mannose; alpha-D-Man; UNII-W3F28J9G0W; CHEBI:28729; 7296-15-3; W3F28J9G0W; 3h-mannose; Manalpha1,; 1rdl; 1rin; 29696-75-1; Epitope ID:130701; AC1Q59RC; AC1L4HD7; SCHEMBL76882; CHEMBL365590; WQZGKKKJIJFFOK-PQMKYFCFSA-N; ZINC3860903; FT-0773891; C00936; WURCS=2.0/1,1,0/[a1122h-1a_1-5]/1/
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ATL-1101 | Preclinical | [31] | ||
| External Link | ||||
| AG 1024 | Investigative | [31] | ||
| Synonyms |
tyrphostin AG 1024; AG-1024; AG1024
Click to Show/Hide
|
|||
| External Link | ||||
| PQ401 | Investigative | [31] | ||
| Synonyms |
PQ 401; IGF-1R inhibitor II; PQ-401
Click to Show/Hide
|
|||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| GSK1511931 | Investigative | [31] | ||
| Synonyms |
GSK1511931A; GSK-1511931; compound 14 [PMID: 19081716]
Click to Show/Hide
|
|||
| Activity | IC50 = 250 nM | |||
| External Link | ||||
| AZD3463 | Investigative | [31] | ||
| Synonyms |
AZD-3463; CS-1382; HY-15609; KB-154896
Click to Show/Hide
|
|||
| External Link | ||||
| GSK-1838705A | Investigative | [31] | ||
| Synonyms |
GSK 1838705A; GSK1838705A
Click to Show/Hide
|
|||
| Activity | IC50 = 2 nM | |||
| External Link | ||||
References