m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03646
|
[1], [2] | |||
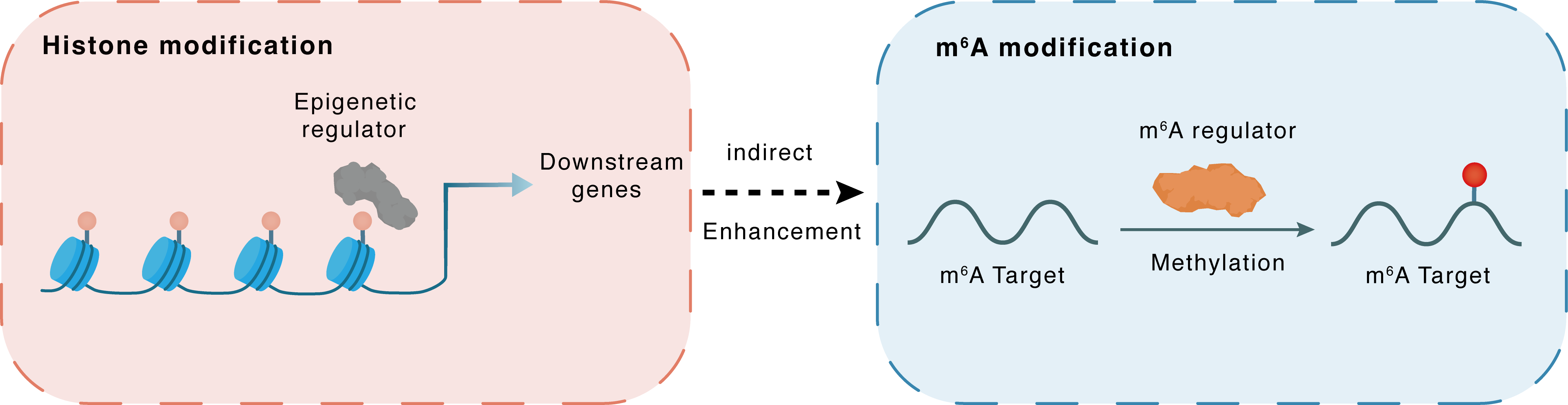 Histone modification
H3K27me3
EZH2
miR-338-5p
Indirect
Enhancement
m6A modification
ZMYM1
ZMYM1
METTL3
Methylation
Histone modification
H3K27me3
EZH2
miR-338-5p
Indirect
Enhancement
m6A modification
ZMYM1
ZMYM1
METTL3
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Zinc finger MYM-type protein 1 (ZMYM1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone-lysine N-methyltransferase EZH2 (EZH2) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 27 trimethylation (H3K27me3) | View Details | |||
| Downstream Gene | miR-338-5p | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | Collectively, EZH2 downregulated hsa-miR-338-5p through Histone H3 lysine 27 trimethylation (H3K27me3), which in turn impaired miR-338-5p-dependent METTL3 inhibition and enhanced CDCP1 translation, therefore contributing to the development of GC. The m6A modification of Zinc finger MYM-type protein 1 (ZMYM1) mRNA by METTL3 enhanced its stability relying on the "reader" protein HuR (also known as ELAVL1) dependent pathway.The study uncover METTL3/ZMYM1/E-cadherin signaling as a potential therapeutic target in anti-metastatic strategy against Gastric cancer. | ||||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | |||
| Pathway Response | Cytokine-cytokine receptor interaction | hsa04060 | |||
| Cell Process | Epithelial-mesenchymal transition | ||||
In-vitro Model |
GES-1 | Normal | Homo sapiens | CVCL_EQ22 | |
| BGC-823 | Gastric carcinoma | Homo sapiens | CVCL_3360 | ||
| AGS | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | ||
| MGC-803 | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
| SGC-7901 | Gastric carcinoma | Homo sapiens | CVCL_0520 | ||
| MKN28 | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_1416 | ||
| In-vivo Model | The luciferase signal intensity from days 7 to 42 is on equivalent scales in the models. Bioluminescent flux (photons/s/cm2/steradian) was determined for the lung metastases. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Histone-lysine N-methyltransferase EZH2 (EZH2) | 74 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Tazemetostat | Approved | [3] | ||
| Synonyms |
EPZ-6438; 1403254-99-8; EPZ6438; UNII-Q40W93WPE1; N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(morpholinomethyl)-[1,1'-biphenyl]-3-carboxamide; Q40W93WPE1; EPZ 6438; E7438; (1,1'-Biphenyl)-3-carboxamide, N-((1,2-dihydro-4,6-dimethyl-2-oxo-3-pyridinyl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(4-morpholinylmethyl)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 2.5 nM | |||
| External Link | ||||
| DS-3201b | Phase 2 | [4] | ||
| Synonyms |
Valemetostat; 1809336-39-7; UNII-60RD0234VE; 60RD0234VE; 1809336-39-7 (free base); DS-3201; (2R)-7-chloro-2-[trans-4-(dimethylamino)cyclohexyl]-N-[(4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl]-2,4-dimethyl-1,3-benzodioxole-5-carboxamide; Valemetostat 2HCl; Valemetostat [INN]; Valemetostat (DS-3201); CHEMBL4597193; EZH1/2 inhibitor DS-3201; SCHEMBL18393626; SCHEMBL18393627; SCHEMBL18639210; EX-A3423; DS3201; NSC813381; s8926; NSC-813381; HY-109108; CS-0039740; D11551
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CPI-1205 | Phase 1/2 | [5] | ||
| Synonyms |
HPODOLXTMDHLLC-QGZVFWFLSA-N; 1621862-70-1; UNII-455J2479FY; CPI1205; CPI 1205; 455J2479FY; (R)-N-((4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-methyl-1-(1-(1-(2,2,2-trifluoroethyl)piperidin-4-yl)ethyl)-1H-indole-3-carboxamide; GTPL9115; SCHEMBL17329268; MolPort-044-560-382; KS-000006BA; EX-A1068; s8353; AKOS030628484; ZINC220982768; CS-7648; compound 13 [PMID: 27739677]; HY-100021; J3.556.402K; N-[(4-methoxy-6-methyl-2-oxo-1H-pyridin-3-yl)methyl]-2-methyl-1-[(1R)-1-[1-(2,2,2-trifluoroethyl)piperidin-4-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SHR2554 | Phase 1/2 | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| CPI-0209 | Phase 1/2 | [7] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| GSK2816126 | Phase 1 | [8] | ||
| Synonyms |
GSK 126; GSK-126
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | Ki = 0.5 nM | |||
| External Link | ||||
| PF-06821497 | Phase 1 | [9] | ||
| Synonyms |
UNII-S4L4MM20B6; S4L4MM20B6; CHEMBL4080228; PF06821497; 1844849-10-0; 5,8-dichloro-2-[(4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl]-7-[(R)-methoxy(oxetan-3-yl)methyl]-3,4-dihydroisoquinolin-1(2H)-one; SCHEMBL17330426; GTPL10516; BDBM50246967; NSC800019; DB14799; HY-101571A; NSC-800019; compound 23a [PMID: 29211475]; CS-0092626; Q29209799; 1(2H)-Isoquinolinone, 5,8-dichloro-2-((1,2-dihydro-4-methoxy-6-methyl-2-oxo-3-pyridinyl)methyl)-3,4-dihydro-7-((R)-methoxy-3-oxetanylmethyl)-; 5,8-dichloro-2-[(4-methoxy-6-methyl-2-oxo-1H-pyridin-3-yl)methyl]-7-[(R)-methoxy(oxetan-3-yl)methyl]-3,4-dihydroisoquinolin-1-one; CJD
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| DS-3201 | Phase 1 | [5] | ||
| Synonyms |
QTGYNKYZRZATJB-UHFFFAOYSA-N; 701899-63-0; 2-(2-(2-Methyl-1H-imidazol-5-yl)ethyl)isoindoline-1,3-dione; 2-(2-(2-Methyl-1H-imidazol-5-yl)-ethyl)isoindoline-1,3-dione; SCHEMBL7743461; MolPort-035-945-474; MolPort-035-690-353; ZINC98086069; AKOS024459002; AKOS024262663; MCULE-2708350770; FCH4077443; AK158834; AX8292467; ST2403812; 2-[2-(2-methyl-1H-imidazol-5-yl)ethyl]-2,3-dihydro-1H-isoindole-1,3-dione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| HH2853 | Phase 1 | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-33 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID28394193-Compound-Figure3bI | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-35 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| PMID28394193-Compound-54 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-24 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-27 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-25 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-50 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-47 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2 nM; Ki < 1 nM | |||
| External Link | ||||
| PMID28394193-Compound-21 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-41 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-53 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 80 nM | |||
| External Link | ||||
| PMID28394193-Compound-Figure5aVIII | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-38 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-51 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-31 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 20 nM | |||
| External Link | ||||
| PMID28394193-Compound-42 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-15 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-52 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-32 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| PMID28394193-Compound-23 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-29 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID28394193-Compound-30 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 16 nM | |||
| External Link | ||||
| PMID28394193-Compound-39 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-49 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-43 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-40 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-Figure3bIII | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-36 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 316 nM | |||
| External Link | ||||
| PMID28394193-Compound-28 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 32 nM | |||
| External Link | ||||
| PMID28394193-Compound-22 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-18 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-16 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-44 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-20 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-19 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-37 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-Figure3bII | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-26 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-17 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-34 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID28394193-Compound-56 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.93 nM | |||
| External Link | ||||
| PMID28394193-Compound-46 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| PMID28394193-Compound-57 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6.45 nM | |||
| External Link | ||||
| PMID28394193-Compound-14 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 21900 nM | |||
| External Link | ||||
| PMID28394193-Compound-12 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 10 nM | |||
| External Link | ||||
| PMID26882240-Compound-1 | Patented | [12] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-55 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 8.13 nM | |||
| External Link | ||||
| PMID28394193-Compound-45 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 168000 nM | |||
| External Link | ||||
| PMID28394193-Compound-13 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1470 nM | |||
| External Link | ||||
| EPZ005687 | Investigative | [13] | ||
| Synonyms |
EPZ-005687; EPZ 005687
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| EI1 | Investigative | [14] | ||
| Synonyms |
KB-145943
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| UNC1999 | Investigative | [15] | ||
| Synonyms |
UNC 1999; UNC-1999
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MS1943 | Investigative | [6] | ||
| Synonyms |
2225938-17-8; SCHEMBL21271666; EX-A3962; s8918; HY-133129; CS-0112146; 6-(6-(4-(2-(2-((3r,5r,7r)-adamantan-1-yl)acetamido)ethyl)piperazin-1-yl)pyridin-3-yl)-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-1-isopropyl-1H-indazole-4-carboxamide; 6-(6-(4-(2-(2-(Adamantan-1-yl)acetamido)ethyl)piperazin-1-yl)pyridin-3-yl)-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-1-isopropyl-1H-indazole-4-carboxamide
Click to Show/Hide
|
|||
| MOA | Degrader | |||
| External Link | ||||
| GSK343 | Investigative | [16] | ||
| Synonyms |
compound 6 [PMID 24900432]; GSK 343
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 174 nM | |||
| External Link | ||||
| PMID28394193-Compound-11 | Patented | [17] | ||
| External Link | ||||
| PMID28394193-Compound-10 | Patented | [17] | ||
| External Link | ||||
| PMID28394193-Compound-14 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 21900 nM | |||
| External Link | ||||
| PMID28394193-Compound-12 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 10 nM | |||
| External Link | ||||
| PMID28394193-Compound-13 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1470 nM | |||
| External Link | ||||
| PMID28394193-Compound-56 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.93 nM | |||
| External Link | ||||
| PMID28394193-Compound-46 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| PMID28394193-Compound-57 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6.45 nM | |||
| External Link | ||||
| PMID28394193-Compound-55 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 8.13 nM | |||
| External Link | ||||
| PMID28394193-Compound-45 | Patented | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 168000 nM | |||
| External Link | ||||
| 2B72: Gastric cancer | 81 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Leniolisib | Approved | [18] | ||
| Synonyms |
1354690-24-6; Leniolisib free base; UNII-L22772Z9CP; (S)-1-(3-((6-(6-methoxy-5-(trifluoromethyl)pyridin-3-yl)-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4-yl)amino)pyrrolidin-1-yl)propan-1-one; L22772Z9CP; 1354690-24-6 (free base); leniolisib(CDZ 173); CDZ173; CDZ-173; 1-[(3S)-3-[[6-[6-methoxy-5-(trifluoromethyl)pyridin-3-yl]-7,8-dihydro-5H-pyrido[4,3-d]pyrimidin-4-yl]amino]pyrrolidin-1-yl]propan-1-one; Leniolisib [INN]; Leniolisib (CDZ173); Leniolisib (USAN/INN); CDZ173-NX; SCHEMBL323054; GTPL9424; CHEMBL3643413; BDBM118299; EX-A2854; MFCD30470232; s8752; ZB1510; CS-7524; DC22326; SB18839; Example 67 [WO2012004299]; 1-{(S)-3-[6-(6-methoxy-5-trifluoromethyl-pyridin-3-yl)-5,6,7,8-tetrahydro-pyrido[4,3-d]pyrimidin-4-y; 1-{(S)-3-[6-(6-methoxy-5-trifluoromethyl-pyridin-3-yl)-5,6,7,8-tetrahydro-pyrido[4,3-d]pyrimidin-4-ylamino]-pyrrolidin-1-yl}-propan-1-one; AS-56217; HY-17635; A16796; D11158; US8653092, 67; Q27282602; 1-Propanone, 1-((3S)-3-((5,6,7,8-tetrahydro-6-(6-methoxy-5-(trifluoromethyl)-3-pyridinyl)pyrido(4,3-d)pyrimidin-4-yl)amino)-1-pyrrolidinyl)-; 9NQ
Click to Show/Hide
|
|||
| External Link | ||||
| Atezolizumab | Approved | [5] | ||
| External Link | ||||
| Bavencio | Approved | [5] | ||
| External Link | ||||
| Tebentafusp | Approved | [19] | ||
| External Link | ||||
| Merimepodib | Approved | [20] | ||
| Synonyms |
Merimebodib; Merimepodib [USAN:INN]; Tyverb/Tykerb; MMPD; 198821-22-6; 2ZL2BA06FU; C23H24N4O6; CHEMBL304087; MERIMEPODIB, VI-21497, VX-497; UNII-2ZL2BA06FU; VI-21497; VX-497; VX497; Vx 497; carbamic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Taxol | Approved | [21] | ||
| Synonyms |
C47H51NO14; weekly paclitaxel; Micellar Paclitaxel; Paclitaxel [USAN:INN:BAN]; SCHEMBL15000506; Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, (2aR-(2aalpha,4beta,4abeta,6beta,9alpha(alphaR*,betaS*),11alpha,12alpha,12aalpha,12balpha))-
Click to Show/Hide
|
|||
| External Link | ||||
| Ramucirumab | Approved | [22] | ||
| Synonyms |
LY3009806
Click to Show/Hide
|
|||
| External Link | ||||
| Tucatinib | Approved | [23] | ||
| Synonyms |
Irbinitinib; 937263-43-9; ONT-380; UNII-234248D0HH; 234248D0HH; N6-(4,4-Dimethyl-4,5-dihydrooxazol-2-yl)-N4-(3-methyl-4-((1,2,4)triazolo(1,5-a)pyridin-7-yloxy)phenyl)quinazoline-4,6-diamine; 4,6-Quinazolinediamine, N6-(4,5-dihydro-4,4-dimethyl-2-oxazolyl)-N4-(3-methyl-4-((1,2,4)triazolo(1,5-a)pyridin-7-yloxy)phenyl)-; ONT 380; 4,6-QuinazolinediaMine, N6-(4,5-dihydro-4,4-diMethyl-2-oxazolyl)-N4-[3-Methyl-4-([1,2,4]triazolo[1,5-a]pyridin-7-yloxy)phenyl]-; Tucatinib [USAN:INN]; 6-DIAMINE
Click to Show/Hide
|
|||
| External Link | ||||
| Antacids | Approved | [24] | ||
| External Link | ||||
| Trastuzumab | Approved | [5] | ||
| Synonyms |
Herceptin; Herceptin (TN); Trastuzumab (INN); Trastuzumab (genetical recombination); Trastuzumab (genetical recombination) (JAN); Trastuzumab (ERBB2 mAb inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| Carbamazepine | Phase 3 | [25] | ||
| Synonyms |
Carbamazepine (iv, epilepsy); Carbamazepine (iv, epilepsy), Lundbeck; Carbamazepine (iv, epilepsy), Ovation Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| Margetuximab | Approved | [5] | ||
| External Link | ||||
| Nivolumab | Approved | [5] | ||
| External Link | ||||
| GRANITE | Phase 3 | [26] | ||
| Synonyms |
Penoxsulam; 219714-96-2; 2-(2,2-difluoroethoxy)-N-(5,8-dimethoxy-[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; UNII-784ELC1SCZ; 784ELC1SCZ; CHEBI:81776; 2-(2,2-difluoroethoxy)-n-(5,8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; Penoxsulam [ISO]; PXD; DSSTox_CID_14803; DSSTox_RID_79204; DSSTox_GSID_34803; SCHEMBL116968; CHEMBL1895913; DTXSID0034803; HSDB 7887; AMY12535; BCP18718; EBD18529; Tox21_301010; MFCD07363876; ZINC13827750; AKOS025401685; NCGC00163715-01; NCGC00163715-02; NCGC00163715-03; NCGC00254912-01; AC-24494; Penoxsulam 100 microg/mL in Acetonitrile; CAS-219714-96-2; FT-0696708; Penoxsulam, PESTANAL(R), analytical standard; C18481; Q22808507; 2-(2,2-Difluoroethoxy)-6-trifluoromethyl-N-(5, 8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)benzenesulfonamide; 2-(2,2-Difluoroethoxy)-N-(5,8-dimethoxy[1,2,4]-triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; 2-(2,2-difluoroethoxy)-N-{5,8-dimethoxy-[1,2,4]triazolo[1,5-c]pyrimidin-2-yl}-6-(trifluoromethyl)benzene-1-sulfonamide; 2-(2,2-difluoroethyl)-N-(5,8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; Benzenesulfonamide, 2-(2,2-difluoroethoxy)-N-(5,8-dimethoxy(1,2,4)triazolo(1,5-c)pyrimidin-2-yl)-6-(trifluoromethyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| Zolbetuximab | Phase 3 | [27] | ||
| Synonyms |
IMAB362
Click to Show/Hide
|
|||
| External Link | ||||
| Tusamitamab ravtansine | Phase 3 | [28] | ||
| Synonyms |
SAR408701
Click to Show/Hide
|
|||
| External Link | ||||
| Andecaliximab | Phase 3 | [29] | ||
| External Link | ||||
| ABP 980 | Phase 3 | [30] | ||
| External Link | ||||
| GS-5745 | Phase 3 | [21] | ||
| External Link | ||||
| S-1 | Phase 3 | [31] | ||
| Synonyms |
Ciprofibrate-coa; Ciprofibrate-coenzyme A; Coenzyme A, ciprofibrate-; AC1L4TRG; AC1Q3T4H; 111900-25-5; s-{1-[(2r,3s,4r,5r)-5-(6-amino-9h-purin-9-yl)-4-hydroxy-3-(phosphonooxy)tetrahydrofuran-2-yl]-3,5,9-trihydroxy-8,8-dimethyl-3,5-dioxido-10,14-dioxo-2,4,6-trioxa-11,15-diaza-3; E5,5; E5-diphosphaheptadecan-17-yl} 2-[4-(2,2-dichlorocyclopropyl)phenoxy]-2-methylpropanethioate(non-preferred name); Coenzyme A, S-(2-(4-(2,2-dichlorocyclopropyl)phenoxy)-2-methylpropanoate)
Click to Show/Hide
|
|||
| External Link | ||||
| Lonsurf | Phase 3 | [5] | ||
| External Link | ||||
| GDC-0068 | Phase 3 | [21] | ||
| Synonyms |
RG7440
Click to Show/Hide
|
|||
| External Link | ||||
| Edotecarin | Phase 3 | [32] | ||
| Synonyms |
ED-749; Edotecarin < Prop INN; J-107088; PF-804950; 12-(beta-D-Glucopyranosyl)-2,10-dihydroxy-6-[2-hydroxy-1-(hydroxymethyl)ethylamino]-6,7,12,13-tetrahydro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-5,7-dione
Click to Show/Hide
|
|||
| External Link | ||||
| RG3638 | Phase 3 | [33] | ||
| Synonyms |
Onartuzumab
Click to Show/Hide
|
|||
| External Link | ||||
| G17DT | Phase 3 | [34] | ||
| Synonyms |
Gastrimmune; Insegia
Click to Show/Hide
|
|||
| External Link | ||||
| DE-766 | Phase 3 | [35] | ||
| External Link | ||||
| Tesetaxel | Phase 2 | [36] | ||
| Synonyms |
DJ-927; 333754-36-2; UNII-UG97LO5M8Y; UG97LO5M8Y; Tesetaxel [INN]; DJ927; DJ 927; CHEMBL2107787; SCHEMBL12060837; DB12019; Z-3104; (2AS,2BR,3S,4S,6S,8AR,10R,11AS,11BR,13AR)-2A-ACETOXY-6-(((2R,3S)-3-((TERT-BUTOXYCARBONYL)AMINO)-3-(3-FLUOROPYRIDIN-2-YL)-2-HYDROXYPROPANOYL)OXY)-10-((DIMETHYLAMINO)METHYL)-4-HYDROXY-7,11B,14,14-TETRAMETHYL-2A,2B,3,4,5,6,8A,11A,11B,12,13,13A-DODECAHYDRO-2H-4,8-METHANOOXETO[3'',2'':3',4']BENZO[1',2':3,4]CYCLODECA[1,2-D][1,3]DIOXOL-3-YL BENZOATE
Click to Show/Hide
|
|||
| External Link | ||||
| Nelipepimut S | Phase 3 | [37] | ||
| Synonyms |
E75
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-986205 | Phase 3 | [5] | ||
| Synonyms |
KRTIYQIPSAGSBP-KLAILNCOSA-N; 1923833-60-6; BMS986205; UNII-0A7729F42K; 0A7729F42K; GTPL9707; SCHEMBL18826792; SCHEMBL17740982; SCHEMBL19105151; EX-A2606; AKOS032954040; HY-101560; CS-0021719; Q29213697; (R)-N-(4-chlorophenyl)-2-((1s,4S)-4-(6-fluoroquinolin-4-yl)cyclohexyl)propanamide; (2R)-N-(4-chlorophenyl)-2-[4-(6-fluoroquinolin-4-yl)cyclohexyl]propanamide; (2R)-N-(4-Chlorophenyl)-2-(4-(6-fluoro-4-quinolyl)cyclohexyl)propanamide, cis; Cyclohexaneacetamide, N-(4-chlorophenyl)-4-(6-fluoro-4-quinolinyl)-alpha-methyl-, cis-(alphaR)-
Click to Show/Hide
|
|||
| External Link | ||||
| Rivoceranib | Phase 3 | [5] | ||
| External Link | ||||
| Claudiximab | Phase 3 | [5] | ||
| Synonyms |
IMAB-362; Anti-GC182 mAbs (cancer), Ganymed; Anti-GC182 monoclonal antibodies (cancer), Ganymed; Anti-CLDN18-2 mAbs (cancer), Ganymed; Anti-CLDN18-2 monoclonal antibodies (cancer), Ganymed
Click to Show/Hide
|
|||
| External Link | ||||
| OS-440 | Phase 3 | [38] | ||
| Synonyms |
CNS modulator (spasticity), Osmotica
Click to Show/Hide
|
|||
| External Link | ||||
| Oraxol | Phase 3 | [5] | ||
| External Link | ||||
| ICI 118,551 | Phase 3 | [21] | ||
| Synonyms |
Ici 118551; (2R,3S)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-3-(propan-2-ylamino)butan-2-ol; CHEMBL198059; CHEBI:73289; ICI-118551; ICI118551; erythro-DL-1-(7-Methylindan-4-yloxy)-3-isopropylaminobutan-2-ol; (2R,3S)-3-(isopropylamino)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]butan-2-ol; 2-Butanol, 1-((2,3-dihydro-7-methyl-1H-inden-4-yl)oxy)-3-((1-methylethyl)amino)-, (2R,3S)-rel-; 2-Butanol, 1-((2,3-dihydro-7-methyl-1H-inden-4-yl)oxy)-3-((1-methylethyl)amino)-, (R*,S*)-(+-)-; ICI-118,551; Ici 111,581; AC1NUNSO
Click to Show/Hide
|
|||
| External Link | ||||
| Evorpacept | Phase 2/3 | [39] | ||
| Synonyms |
ALX148
Click to Show/Hide
|
|||
| External Link | ||||
| BNT141 | Phase 2 | [40] | ||
| External Link | ||||
| Anti-LAG3 | Phase 2 | [30] | ||
| External Link | ||||
| GSK1292263 | Phase 2 | [41] | ||
| External Link | ||||
| MM-111 | Phase 2 | [42] | ||
| External Link | ||||
| Plevitrexed | Phase 2 | [43] | ||
| Synonyms |
ZD 9331; ZD9331; 153537-73-6; Plevitrexed [INN]; ZD-9331; NSC 696259; UNII-L9P2881C3H; CHEMBL126648; (2s)-2-[(2-fluoro-4-{[(4-hydroxy-2,7-dimethylquinazolin-6-yl)methyl](prop-2-yn-1-yl)amino}benzoyl)amino]-4-(2h-tetrazol-5-yl)butanoic acid; L9P2881C3H; Plevitrexed (INN); 172521-94-7; (2S)-2-[[4-[(2,7-dimethyl-4-oxo-1H-quinazolin-6-yl)methyl-prop-2-ynylamino]-2-fluorobenzoyl]amino]-4-(2H-tetrazol-5-yl)butanoic acid; 1H-Tetrazole-5-butanoic acid,
Click to Show/Hide
|
|||
| External Link | ||||
| DS-8201 | Phase 1 | [30] | ||
| Synonyms |
9-Aminofluorene; 9H-Fluoren-9-amine; 525-03-1; FLUOREN-9-AMINE; Fluoren-9-ylamine; UNII-4NHO2K4K5B; CCRIS 7000; BRN 2209545; 4NHO2K4K5B; OUGMRQJTULXVDC-UHFFFAOYSA-N; fluorene-9-ylamine; 9-Amino-fluoren; 9-amino-fluorene; 9H-9-fluorenamine; 9H-fluoren-9-yl-amine; AC1L1VP5; 4-12-00-03390 (Beilstein Handbook Reference); SCHEMBL353865; AC1Q53A2; AC1Q53A1; KS-00000JGC; CTK1H0380; DTXSID90200496; MolPort-001-794-448; HMS1780P20; 9H-fluoren-9-ylamine hydrochloride; ZINC1724407; ALBB-023296; CA-733; SBB005783; AKOS000264388; MCULE-8757055914; DS-
Click to Show/Hide
|
|||
| External Link | ||||
| XL880 | Phase 2 | [44] | ||
| Synonyms |
GSK 089; GSK 1363089; GSK1363089; XL 880; GSK1363089, GSK089, foretinib, EXEL-2880, XL880; 88Z; MET inhibitors
Click to Show/Hide
|
|||
| External Link | ||||
| Matuzumab | Phase 2 | [45] | ||
| Synonyms |
EMD-62000; EMD-72000; Anti-EGF receptor humanized antibody (iv, cancer), Merck KGaA/ Takeda Pharmaceuticals; Anti-EGFR humanized mAb (iv, cancer), Merck KGaA/ Takeda Pharmaceuticals; Anti-epidermal growth factor receptor humanized antibody (iv, cancer), Merck KGaA/ Takeda Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| BAY-57-9352 | Phase 2 | [21] | ||
| Synonyms |
Telatinib; Bay 57-9352
Click to Show/Hide
|
|||
| External Link | ||||
| Bemarituzumab | Phase 2 | [46] | ||
| External Link | ||||
| PEGPH20 | Phase 2 | [5] | ||
| External Link | ||||
| Plevitrexed (R)-isomer | Phase 2 | [47] | ||
| Synonyms |
YW3548
Click to Show/Hide
|
|||
| External Link | ||||
| APR-246 | Phase 2 | [48] | ||
| Synonyms |
Eprenetapopt
Click to Show/Hide
|
|||
| External Link | ||||
| CRS-207 | Phase 2 | [37] | ||
| External Link | ||||
| Opdivo + Yervoy | Phase 3 | [5] | ||
| External Link | ||||
| CT-041 | Phase 1/2 | [49] | ||
| External Link | ||||
| BPX-601 | Phase 1/2 | [50] | ||
| External Link | ||||
| Anti-MUC1 CAR-T cells | Phase 1/2 | [51] | ||
| External Link | ||||
| Anti-Mesothelin CAR-T cells | Phase 1/2 | [52] | ||
| External Link | ||||
| Anti-HER2 CAR-T | Phase 1/2 | [53] | ||
| External Link | ||||
| CAR-T Cells targeting EpCAM | Phase 1/2 | [54] | ||
| External Link | ||||
| PAT-SC1 | Phase 1/2 | [55] | ||
| Synonyms |
SC-1; Adjuvant therapy (gastric cancer), University of Wurzburg; SC-1 (gastric cancer), CAT; SC-1 (gastric cancer), Debiopharm; SC-1 (gastric cancer), Patrys; SC-1 (stomach cancer), OncoMab
Click to Show/Hide
|
|||
| External Link | ||||
| ASP2138 | Phase 1 | [56] | ||
| External Link | ||||
| SAR443216 | Phase 1 | [57] | ||
| External Link | ||||
| AMG 199 | Phase 1 | [58] | ||
| External Link | ||||
| AMG 910 | Phase 1 | [59] | ||
| External Link | ||||
| Alofanib | Phase 1 | [60] | ||
| Synonyms |
1612888-66-0; 3-(N-(4-methyl-2-nitro-5-(pyridin-3-yl)phenyl)sulfamoyl)benzoic acid; RPT-835(alofanib); UNII-LQX7RFK8MZ; RPT-835; RPT835; LQX7RFK8MZ; ES000835; Alofanib [INN]; Alofanib(RPT835); Syn007154; CHEMBL4594436; SCHEMBL18660613; AMY16650; BCP31905; EX-A2731; MFCD30533418; NSC790182; s8754; Benzoic acid, 3-(((4-methyl-2-nitro-5-(3-pyridinyl)phenyl)amino)sulfonyl)-; NSC-790182; SB19665; AC-31695; AK668992; AS-56846; HY-17601; CS-0014684; RPT 835; Q27283135; 3-{[4-methyl-2-nitro-5-(pyridin-3-yl)phenyl]sulfamoyl}benzoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| HER2-specific CAR T cell | Phase 1 | [61] | ||
| External Link | ||||
| Anti-CEA-CAR T | Phase 1 | [62] | ||
| External Link | ||||
| XR-5944 | Phase 1 | [63] | ||
| Synonyms |
MLN-944; XR-11576 analogs; XR-5000 analogs; XR-5942
Click to Show/Hide
|
|||
| External Link | ||||
| A168 | Phase 1 | [64] | ||
| External Link | ||||
| EGFR806-specific CAR T cell | Phase 1 | [65] | ||
| External Link | ||||
| AbGn-107 | Phase 1 | [5] | ||
| External Link | ||||
| FPA144 | Phase 1 | [30] | ||
| External Link | ||||
| Minnelide 001 | Phase 1 | [21] | ||
| External Link | ||||
| CAR-T cells targeting EpCAM | Phase 1 | [66] | ||
| External Link | ||||
| Anti-CEA CAR-T cells | Phase 1 | [67] | ||
| External Link | ||||
| EPCAM-targeted CAR-T cells | Clinical trial | [68] | ||
| External Link | ||||
| PMID28460551-Compound-1 | Patented | [69] | ||
| External Link | ||||
| Conjugated 3-(indolyl)-and 3-(azaindolyl)-4-arylmaleimide compound 1 | Patented | [70] | ||
| Synonyms |
PMID28621580-Compound-WO2012084683c62
Click to Show/Hide
|
|||
| External Link | ||||
| TOPIXANTRONE HYDROCHLORIDE | Discontinued in Phase 2 | [71] | ||
| Synonyms |
SCHEMBL1418986; Topixantrone hydrochloride < Prop INNM; BBR-3409 (dimaleate); 5-[2-(Dimethylamino)ethylamino]-2-[2-(2-hydroxyethylamino)ethyl]indazolo[4,3-gh]isoquinolin-6(2H)-one dihydrochloride
Click to Show/Hide
|
|||
| External Link | ||||
| MDL 101,731 | Discontinued in Phase 2 | [72] | ||
| Synonyms |
Tezacitabine; Fmdc cpd; 130306-02-4; UNII-7607Y95N9S; Mdl 101731; (E)-2'-Deoxy-2'-(fluoromethylene) cytidine; MDL-101731; 2'-Deoxy-2'-(fluoromethylene)cytidine; 7607Y95N9S; Cytidine, 2'-deoxy-2'-(fluoromethylene)-, (2E)-; (E)-2'-Deoxy-2'-(fluoromethylene)cytidine; Tezacitabine [INN]; tezaciabine; Tezacitabine, anhydrous; AC1O5KIG; SCHEMBL18724; SCHEMBL18725; Tezacitabine, anhydrous [INN]; CHEMBL2105467; C10H12FN3O4; DTXSID10156446; GFFXZLZWLOBBLO-ASKVSEFXSA-N; ZINC3777826; KW-2331
Click to Show/Hide
|
|||
| External Link | ||||
| BBR-3438 | Discontinued in Phase 2 | [73] | ||
| Synonyms |
Nortopixantrone; UNII-PH2639TAB4; PH2639TAB4; Nortopixantrone [INN:BAN]; AC1MI4ZO; CHEMBL150303; SCHEMBL7804438
Click to Show/Hide
|
|||
| External Link | ||||
| IPI-493 | Discontinued in Phase 1 | [74] | ||
| Synonyms |
[(3R,5R,6S,7R,8E,10R,11R,12Z,14E)-21-amino-6-hydroxy-5,11-dimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-17-azabicyclo[16.3.1]docosa-1(21),8,12,14,18-pentaen-10-yl] carbamate; AC1NS08X; SCHEMBL16226496; SCHEMBL16225851
Click to Show/Hide
|
|||
| External Link | ||||
| Kanjinti | Application submitted | [5] | ||
| External Link | ||||
| Anti-CD9 mab | Investigative | [75] | ||
| Synonyms |
ALB-6; Anti-CD9 mAb (gastric cancer); Anti-CD9 mAb (gastric cancer), Osaka University
Click to Show/Hide
|
|||
| External Link | ||||
References