m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03541
|
[1] | |||
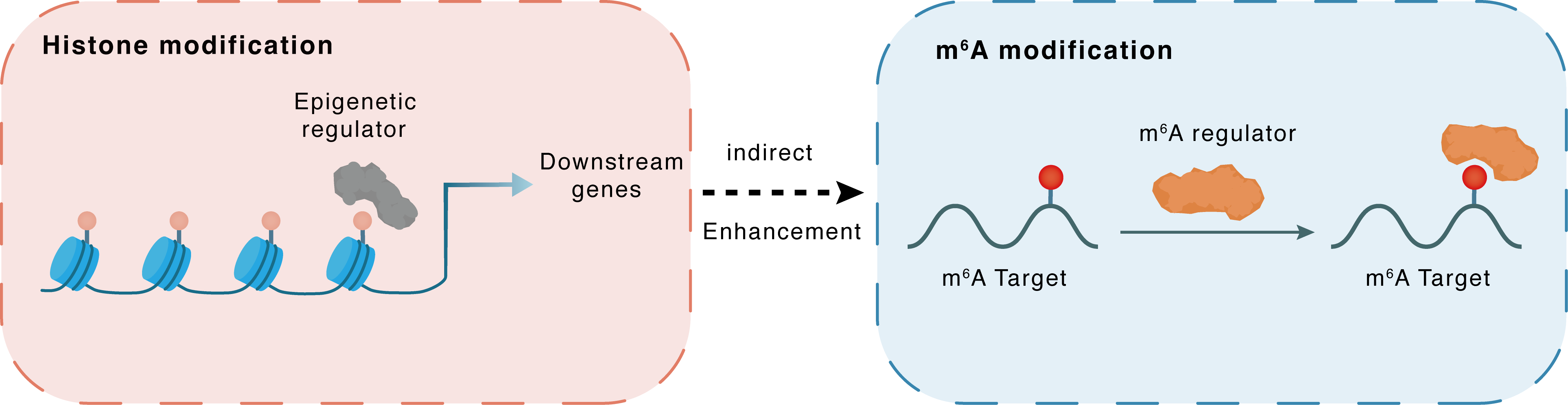 Histone modification
H3K27me3
EZH2
miR-454-3p
Indirect
Enhancement
m6A modification
NFKB1
NFKB1
YTHDF2
Histone modification
H3K27me3
EZH2
miR-454-3p
Indirect
Enhancement
m6A modification
NFKB1
NFKB1
YTHDF2
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | YTH domain-containing family protein 2 (YTHDF2) | READER | |||
| m6A Target | Nuclear factor NF-kappa-B p105 subunit (NF-Kappa-B/NFKB1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone-lysine N-methyltransferase EZH2 (EZH2) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 27 trimethylation (H3K27me3) | View Details | |||
| Downstream Gene | miR-454-3p | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | Histone methyltransferase EZH2 inhibited hsa-miR-454-3p through Histone H3 lysine 27 trimethylation (H3K27me3) and promoted m6A modification of PTEN/MYC/UBXN1/Nuclear factor NF-kappa-B p105 subunit (NF-Kappa-B/NFKB1) to induce glioma M2 macrophage polarization. miR-454-3p promoted PTEN expression by inhibiting m6A modification through binding to the enzyme YTHDF2 | ||||
| Responsed Disease | Brain cancer | ICD-11: 2A00 | |||
In-vitro Model |
THP-1 | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | |
| In-vivo Model | Luciferase-labeled A172 cells (1,000,000 cells per mouse) were mixed with polarized macrophages (200,000 cells per mouse), and the mixture was injected into the axilla of nude mice. In detail, two groups of nude mice were injected with pLenti-EZH2-GFP-treated A172 cells and polarized macrophages (n = 8) or pLenti-HK-GFP-treated A172 cells and polarized macrophages. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Histone-lysine N-methyltransferase EZH2 (EZH2) | 74 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Tazemetostat | Approved | [2] | ||
| Synonyms |
EPZ-6438; 1403254-99-8; EPZ6438; UNII-Q40W93WPE1; N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(morpholinomethyl)-[1,1'-biphenyl]-3-carboxamide; Q40W93WPE1; EPZ 6438; E7438; (1,1'-Biphenyl)-3-carboxamide, N-((1,2-dihydro-4,6-dimethyl-2-oxo-3-pyridinyl)methyl)-5-(ethyl(tetrahydro-2H-pyran-4-yl)amino)-4-methyl-4'-(4-morpholinylmethyl)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 2.5 nM | |||
| External Link | ||||
| DS-3201b | Phase 2 | [3] | ||
| Synonyms |
Valemetostat; 1809336-39-7; UNII-60RD0234VE; 60RD0234VE; 1809336-39-7 (free base); DS-3201; (2R)-7-chloro-2-[trans-4-(dimethylamino)cyclohexyl]-N-[(4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl]-2,4-dimethyl-1,3-benzodioxole-5-carboxamide; Valemetostat 2HCl; Valemetostat [INN]; Valemetostat (DS-3201); CHEMBL4597193; EZH1/2 inhibitor DS-3201; SCHEMBL18393626; SCHEMBL18393627; SCHEMBL18639210; EX-A3423; DS3201; NSC813381; s8926; NSC-813381; HY-109108; CS-0039740; D11551
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CPI-1205 | Phase 1/2 | [4] | ||
| Synonyms |
HPODOLXTMDHLLC-QGZVFWFLSA-N; 1621862-70-1; UNII-455J2479FY; CPI1205; CPI 1205; 455J2479FY; (R)-N-((4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-methyl-1-(1-(1-(2,2,2-trifluoroethyl)piperidin-4-yl)ethyl)-1H-indole-3-carboxamide; GTPL9115; SCHEMBL17329268; MolPort-044-560-382; KS-000006BA; EX-A1068; s8353; AKOS030628484; ZINC220982768; CS-7648; compound 13 [PMID: 27739677]; HY-100021; J3.556.402K; N-[(4-methoxy-6-methyl-2-oxo-1H-pyridin-3-yl)methyl]-2-methyl-1-[(1R)-1-[1-(2,2,2-trifluoroethyl)piperidin-4-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SHR2554 | Phase 1/2 | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| CPI-0209 | Phase 1/2 | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| GSK2816126 | Phase 1 | [7] | ||
| Synonyms |
GSK 126; GSK-126
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | Ki = 0.5 nM | |||
| External Link | ||||
| PF-06821497 | Phase 1 | [8] | ||
| Synonyms |
UNII-S4L4MM20B6; S4L4MM20B6; CHEMBL4080228; PF06821497; 1844849-10-0; 5,8-dichloro-2-[(4-methoxy-6-methyl-2-oxo-1,2-dihydropyridin-3-yl)methyl]-7-[(R)-methoxy(oxetan-3-yl)methyl]-3,4-dihydroisoquinolin-1(2H)-one; SCHEMBL17330426; GTPL10516; BDBM50246967; NSC800019; DB14799; HY-101571A; NSC-800019; compound 23a [PMID: 29211475]; CS-0092626; Q29209799; 1(2H)-Isoquinolinone, 5,8-dichloro-2-((1,2-dihydro-4-methoxy-6-methyl-2-oxo-3-pyridinyl)methyl)-3,4-dihydro-7-((R)-methoxy-3-oxetanylmethyl)-; 5,8-dichloro-2-[(4-methoxy-6-methyl-2-oxo-1H-pyridin-3-yl)methyl]-7-[(R)-methoxy(oxetan-3-yl)methyl]-3,4-dihydroisoquinolin-1-one; CJD
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| DS-3201 | Phase 1 | [4] | ||
| Synonyms |
QTGYNKYZRZATJB-UHFFFAOYSA-N; 701899-63-0; 2-(2-(2-Methyl-1H-imidazol-5-yl)ethyl)isoindoline-1,3-dione; 2-(2-(2-Methyl-1H-imidazol-5-yl)-ethyl)isoindoline-1,3-dione; SCHEMBL7743461; MolPort-035-945-474; MolPort-035-690-353; ZINC98086069; AKOS024459002; AKOS024262663; MCULE-2708350770; FCH4077443; AK158834; AX8292467; ST2403812; 2-[2-(2-methyl-1H-imidazol-5-yl)ethyl]-2,3-dihydro-1H-isoindole-1,3-dione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| HH2853 | Phase 1 | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-33 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID28394193-Compound-Figure3bI | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-35 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| PMID28394193-Compound-54 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-24 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-27 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-25 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-50 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-47 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2 nM; Ki < 1 nM | |||
| External Link | ||||
| PMID28394193-Compound-21 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-41 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-53 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 80 nM | |||
| External Link | ||||
| PMID28394193-Compound-Figure5aVIII | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-38 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-51 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-31 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 20 nM | |||
| External Link | ||||
| PMID28394193-Compound-42 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-15 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-52 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-32 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| PMID28394193-Compound-23 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-29 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID28394193-Compound-30 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 16 nM | |||
| External Link | ||||
| PMID28394193-Compound-39 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-49 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1 nM; IC50 < 2 nM; Ki > 0.01 nM; Ki < 0.04 nM | |||
| External Link | ||||
| PMID28394193-Compound-43 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-40 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-Figure3bIII | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-36 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 316 nM | |||
| External Link | ||||
| PMID28394193-Compound-28 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 32 nM | |||
| External Link | ||||
| PMID28394193-Compound-22 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-18 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-16 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-44 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-20 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-19 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-37 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-Figure3bII | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-26 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID28394193-Compound-17 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-34 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| PMID28394193-Compound-56 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.93 nM | |||
| External Link | ||||
| PMID28394193-Compound-46 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| PMID28394193-Compound-57 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6.45 nM | |||
| External Link | ||||
| PMID28394193-Compound-14 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 21900 nM | |||
| External Link | ||||
| PMID28394193-Compound-12 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 10 nM | |||
| External Link | ||||
| PMID26882240-Compound-1 | Patented | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28394193-Compound-55 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 8.13 nM | |||
| External Link | ||||
| PMID28394193-Compound-45 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 168000 nM | |||
| External Link | ||||
| PMID28394193-Compound-13 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1470 nM | |||
| External Link | ||||
| EPZ005687 | Investigative | [12] | ||
| Synonyms |
EPZ-005687; EPZ 005687
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| EI1 | Investigative | [13] | ||
| Synonyms |
KB-145943
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| UNC1999 | Investigative | [14] | ||
| Synonyms |
UNC 1999; UNC-1999
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MS1943 | Investigative | [5] | ||
| Synonyms |
2225938-17-8; SCHEMBL21271666; EX-A3962; s8918; HY-133129; CS-0112146; 6-(6-(4-(2-(2-((3r,5r,7r)-adamantan-1-yl)acetamido)ethyl)piperazin-1-yl)pyridin-3-yl)-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-1-isopropyl-1H-indazole-4-carboxamide; 6-(6-(4-(2-(2-(Adamantan-1-yl)acetamido)ethyl)piperazin-1-yl)pyridin-3-yl)-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-1-isopropyl-1H-indazole-4-carboxamide
Click to Show/Hide
|
|||
| MOA | Degrader | |||
| External Link | ||||
| GSK343 | Investigative | [15] | ||
| Synonyms |
compound 6 [PMID 24900432]; GSK 343
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 174 nM | |||
| External Link | ||||
| PMID28394193-Compound-11 | Patented | [16] | ||
| External Link | ||||
| PMID28394193-Compound-10 | Patented | [16] | ||
| External Link | ||||
| PMID28394193-Compound-14 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 21900 nM | |||
| External Link | ||||
| PMID28394193-Compound-12 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 10 nM | |||
| External Link | ||||
| PMID28394193-Compound-13 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1470 nM | |||
| External Link | ||||
| PMID28394193-Compound-56 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.93 nM | |||
| External Link | ||||
| PMID28394193-Compound-46 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| PMID28394193-Compound-57 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6.45 nM | |||
| External Link | ||||
| PMID28394193-Compound-55 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 8.13 nM | |||
| External Link | ||||
| PMID28394193-Compound-45 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 168000 nM | |||
| External Link | ||||
| Nuclear factor NF-kappa-B p105 subunit (NF-Kappa-B/NFKB1) | 2 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| P54 | Phase 2 | [17] | ||
| MOA | Modulator | |||
| External Link | ||||
| CAT 1004 | Phase 2 | [18] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 2A00: Brain cancer | 53 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Zinostatin stimalamer | Approved | [19] | ||
| Synonyms |
Smancs (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Motexafin gadolinium | Approved | [20] | ||
| Synonyms |
Xcytrin; Gadolinium texaphyrin; GdT2B2; GD-Tex; Motexafin gadolinium (USAN); PCI-0120; Xcytrin (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Lomustine | Approved | [21] | ||
| Synonyms |
Belustine; CCNU; CINU; Cecenu; CeeNU; Chloroethylcyclohexylnitrosourea; Lomustina; Lomustinum; Bristol Myers Squibb Brand of Lomustine; CCNU [Chloroethyl nitrosoureas]; Cyclohexyl chloroethyl nitrosourea; Lomustine medac Brand; Medac Brand of Lomustine; Rhone Poulenc Rorer Brand of Lomustine; OR5087; RB 1509; SRI 2200; Bristol-Myers Squibb Brand of Lomustine; CeeNU (TN); Lomustina [INN-Spanish]; Lomustinum [INN-Latin]; NPFAPI-06; Rhone-Poulenc Rorer Brand of Lomustine; CeeNU, CCNU, Lomustine; Lomustine (USAN/INN); Lomustine [USAN:BAN:INN]; N-(2-Chloroethyl)-N'-cyclohexyl-N-nitrosourea; (Chloro-2-ethyl)-1-cyclohexyl-3-nitrosourea; (Cloro-2-etil)-1-cicloesil-3-nitrosourea; (Cloro-2-etil)-1-cicloesil-3-nitrosourea [Italian];1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea; 1-(2-Chloroethyl)-3-cyclohexyl-1-nitrosourea [Chloroethyl nitrosoureas]; 1-(2-Chloroethyl)-3-cyclohexylnitrosourea
Click to Show/Hide
|
|||
| External Link | ||||
| Borocaptate sodium B 10 | Approved | [19] | ||
| External Link | ||||
| DTI-015 | Approved | [22] | ||
| Synonyms |
Carmustine; 154-93-8; 1,3-Bis(2-chloroethyl)-1-nitrosourea; BCNU; Carmustin; Nitrumon; Carmubris; Gliadel; BiCNU; Bi CNU; Carmustinum; Bischlorethylnitrosurea; Bischlorethylnitrosourea; Carmustina; Becenun; Becenum; Bischloroethyl nitrosourea; N,N'-BIS(2-CHLOROETHYL)-N-NITROSOUREA; Bis(2-chloroethyl)nitrosourea; Urea, N,N'-bis(2-chloroethyl)-N-nitroso-; Gliadel Wafer; FDA 0345; Bischloroethylnitrosourea; SRI 1720; 1,3-Bis(2-chloroethyl)nitrosourea; BiCNU (TN); Carmustinum [INN-Latin]; Carmustina [INN-Spanish]; DTI 015; NCI-C04773; SK; Injectable carmustine, Direct Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| Prinomastat | Approved | [23] | ||
| Synonyms |
AG-3354; AG-3362; Prinomastat (USAN/INN); (3S)-N-hydroxy-2,2-dimethyl-4-(4-pyridin-4-yloxyphenyl)sulfonylthiomorpholine-3-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| INO-1001 | Phase 3 | [24] | ||
| Synonyms |
Hypoxanthine arabinoside; LT00454797; 9-[(3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-3H-purin-6-one
Click to Show/Hide
|
|||
| External Link | ||||
| GliAtak | Phase 3 | [25] | ||
| Synonyms |
GliAtak (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| SOT-107 | Phase 3 | [26] | ||
| Synonyms |
TransMID
Click to Show/Hide
|
|||
| External Link | ||||
| ICT-107 | Phase 3 | [27] | ||
| External Link | ||||
| Cintredekin besudotox | Phase 3 | [28] | ||
| External Link | ||||
| Rindopepimut | Phase 3 | [29] | ||
| External Link | ||||
| DCVax-Ovarian | Phase 3 | [30] | ||
| Synonyms |
DCVax-L; Dendritic cell-based immunotherapy (ovarian cancer), Northwest Biotherapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| DCVax-Brain | Phase 3 | [31] | ||
| Synonyms |
Dendritic cell-based immunotherapy (brain cancer), Northwest Biotherapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| TVI-Brain-1 | Phase 2/3 | [32] | ||
| External Link | ||||
| NLG8189 | Phase 2/3 | [4] | ||
| Synonyms |
1-Methyl-D-tryptophan; Indoximod; 110117-83-4; D-Tryptophan, 1-methyl-; D-1MT; Indoximod (NLG-8189); D-1-methyltryptophan; UNII-TX5CYN1KMZ; D-(+)-1-Methyltryptophan; TX5CYN1KMZ; (R)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic acid; (2R)-2-amino-3-(1-methylindol-3-yl)propanoic acid; NSC-721782; (2R)-2-amino-3-(1-methyl-3-indolyl)propanoic acid; 1-MT; (2R)-2-azanyl-3-(1-methylindol-3-yl)propanoic acid; (2R)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic acid; D-l-Methyltryptophan; Indoximod [USAN:INN]; NLG-8189; NLG 8189
Click to Show/Hide
|
|||
| External Link | ||||
| Synthetic survivin peptide vaccine | Phase 2 | [33] | ||
| External Link | ||||
| Fresolimumab | Phase 2 | [34] | ||
| Synonyms |
GC-1008
Click to Show/Hide
|
|||
| External Link | ||||
| DNX-2401 | Phase 2 | [35] | ||
| Synonyms |
Tasadenoturev
Click to Show/Hide
|
|||
| External Link | ||||
| TVI-Brain-1 cancer vaccine | Phase 2 | [36] | ||
| External Link | ||||
| PDT with Photofrin | Phase 2 | [4] | ||
| External Link | ||||
| F18-ML-10 | Phase 2 | [37] | ||
| External Link | ||||
| PT2385 | Phase 2 | [38] | ||
| Synonyms |
ONBSHRSJOPSEGS-INIZCTEOSA-N; PT-2385; UNII-6O16716DXP; 1672665-49-4; 6O16716DXP; SCHEMBL16555810; ZINC230453533; AKOS030526641; HY-12867; PT2385,1672665-49-4, PT 2385,PT-2385; Benzonitrile, 3-(((1S)-2,2-difluoro-2,3-dihydro-1-hydroxy-7-(methylsulfonyl)-1H-inden-4-yl)oxy)-5-fluoro-; 3-{[(1s)-2,2-Difluoro-1-Hydroxy-7-(Methylsulfonyl)-2,3-Dihydro-1h-Inden-4-Yl]oxy}-5-Fluorobenzonitrile; 3-(((1S)-2,2-Difluoro-1-hydroxy-7-methanesulfonyl-2,3-dihydro-1hinden-4-yl)oxy)-5-fluorobenzonitrile; 79A
Click to Show/Hide
|
|||
| External Link | ||||
| ABT-414 | Phase 2 | [39] | ||
| External Link | ||||
| CLR1404-I-124 | Phase 1/2 | [40] | ||
| External Link | ||||
| DM-CHOC-PEN | Phase 2 | [41] | ||
| External Link | ||||
| L-alanosine | Phase 2 | [42] | ||
| Synonyms |
SDX-102
Click to Show/Hide
|
|||
| External Link | ||||
| APX005M | Phase 2 | [4] | ||
| External Link | ||||
| WP-1066 | Phase 1/2 | [36] | ||
| Synonyms |
WP1066; 857064-38-1; (S,E)-3-(6-Bromopyridin-2-yl)-2-cyano-N-(1-phenylethyl)acrylamide; WP 1066; UNII-63V8AIE65T; 63V8AIE65T; AK-99218; C17H14BrN3O; (E)-3-(6-bromopyridin-2-yl)-2-cyano-N-[(1S)-1-phenylethyl]prop-2-enamide; MLS006010178; SCHEMBL1315826; QCR-16; SCHEMBL1315831; GTPL7972; CHEMBL1923234; EX-A760; AOB1497; DTXSID50235007; MolPort-044-723-708; MolPort-023-219-149; ZINC13983221; AKOS016007983; WP1066/WP-1066; CS-2736; DB12679; 2-Propenamide, 3-(6-bromo-2-pyridinyl)-2-cyano-N-((1S)-1-phenylethyl)-, (2E)-; HY-15312
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-EGFRvIII CAR transduced PBL | Phase 1/2 | [43] | ||
| External Link | ||||
| RG6156 | Phase 1 | [44] | ||
| External Link | ||||
| rhenium-186 | Phase 1 | [45] | ||
| Synonyms |
(~186~Re)Rhenium; 14998-63-1; 186Re; DTXSID60933825; Q18882928; RHENIUM (186-RE); Rhenium Re-186; Rhenium, isotope of mass 186; Rhenium-186; RHENIUM-186 [WHO-DD]; UNII-ZU7F1ET6TM; ZU7F1ET6TM
Click to Show/Hide
|
|||
| External Link | ||||
| IGV-001 | Phase 1 | [46] | ||
| External Link | ||||
| DA-3607 | Phase 1 | [47] | ||
| Synonyms |
Ad-stTRAIL; TRAIL adenoviral gene therapy (cancer), Dong-A
Click to Show/Hide
|
|||
| External Link | ||||
| DC/I540/KLH vaccine | Phase 1 | [48] | ||
| Synonyms |
HTERT:540-548; Telomerase: 540-548 peptide vaccine; DC/I540/KLH vaccine (cancer); Dendritic cell/hTERT peptide I540/keyhole limpet hemocyanin vaccine, Dana-Farber; DC/I540/KLH vaccine (cancer), Dana-Farber
Click to Show/Hide
|
|||
| External Link | ||||
| KX2-361 | Phase 1 | [49] | ||
| Synonyms |
KX-02
Click to Show/Hide
|
|||
| External Link | ||||
| MR1-1 | Phase 1 | [50] | ||
| Synonyms |
MR1-1KDEL; EGFR-specific immunotoxin, IVAX; Anticancer immunotoxin (EGFR-specific), IVAX
Click to Show/Hide
|
|||
| External Link | ||||
| INdoximod + temozolomide | Phase 1 | [36] | ||
| External Link | ||||
| Anti-CD133-CAR vector-transduced T cells | Phase 1 | [51] | ||
| External Link | ||||
| 8H9 | Phase 1 | [52] | ||
| External Link | ||||
| CC-8490 | Phase 1 | [53] | ||
| External Link | ||||
| Sitimagene ceradenovec | Discontinued in Phase 3 | [54] | ||
| Synonyms |
Cerepro; EG-009; HSV thymidine kinase gene therapy, Ark
Click to Show/Hide
|
|||
| External Link | ||||
| Ranagengliotucel-T | Discontinued in Phase 3 | [55] | ||
| Synonyms |
Glionix; Brain tumor vaccine, NovaRx; Antisense (TGFbeta) brain tumor vaccine, NovaRx
Click to Show/Hide
|
|||
| External Link | ||||
| Oncolysin S | Discontinued in Phase 2 | [56] | ||
| Synonyms |
N901-bR
Click to Show/Hide
|
|||
| External Link | ||||
| Labradimil | Discontinued in Phase 2 | [57] | ||
| Synonyms |
Cereport; Lobradimil; Receptor mediated permeabilizer; RMP 7; DRG-0182; RMP-7; N2-((S)-2-(L-Arginyl-L-prolyl-trans-4-hydroxy-L-prolylglycyl-3-(2-thienyl)-L-alanyl-L-seryl-L-prolinamido)-3-(p-methoxyphenyl)propyl)-L-arginine; (S-(R*,R*))-L-Arginyl-L-prolyl-trans-4-hydroxy-L-prolyl-3-(2-thienyl)-L-alanylglycyl-L-seryl-N-(2-((4-((aminoiminomethyl)amino)-1-carboxybutyl)amino)-1-((4-methoxyphenyl)methyl)ethyl)-L-prolinamide
Click to Show/Hide
|
|||
| External Link | ||||
| Brain tumor vaccine | Discontinued in Phase 1 | [58] | ||
| Synonyms |
Brain cancer vaccine, IRC; Brain tumor vaccine, IRC; IR-850; Established cancer cell line therapy (brain cancer), IRC; GM-CSF vaccine (brain cancer), IRC
Click to Show/Hide
|
|||
| External Link | ||||
| PCNU | Terminated | [59] | ||
| Synonyms |
NSC-95466
Click to Show/Hide
|
|||
| External Link | ||||
| 131I-81C6 | Terminated | [60] | ||
| Synonyms |
Neuradiab; MAb-81C6; Iodine-131-81C6; Astatine-211-MAb-81C6; Iodine-131-MAb-81C6; Iodine-131-ch-81C6; Iodine-131-ch-81C6-F(ab)2; 211At-MAb-81C6
Click to Show/Hide
|
|||
| External Link | ||||
| NSD-551 | Terminated | [61] | ||
| Synonyms |
BK channel activator (cancer), NeuroSearch/TopoTarget
Click to Show/Hide
|
|||
| External Link | ||||
| AGT-2000 | Investigative | [62] | ||
| Synonyms |
Gene therapy (intravenous, brain cancer), ArmaGen
Click to Show/Hide
|
|||
| External Link | ||||
| NV.XOD.09 | Investigative | [63] | ||
| External Link | ||||
| EDP-19 | Investigative | [63] | ||
| Synonyms |
SiRNA (convection-enhanced delivery, brain tumor), Sheba Medical Center/BioLineRx
Click to Show/Hide
|
|||
| External Link | ||||
| MIQ-004 | Investigative | [63] | ||
| Synonyms |
M-IQ-004
Click to Show/Hide
|
|||
| External Link | ||||
References