m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03500
|
[1], [2] | |||
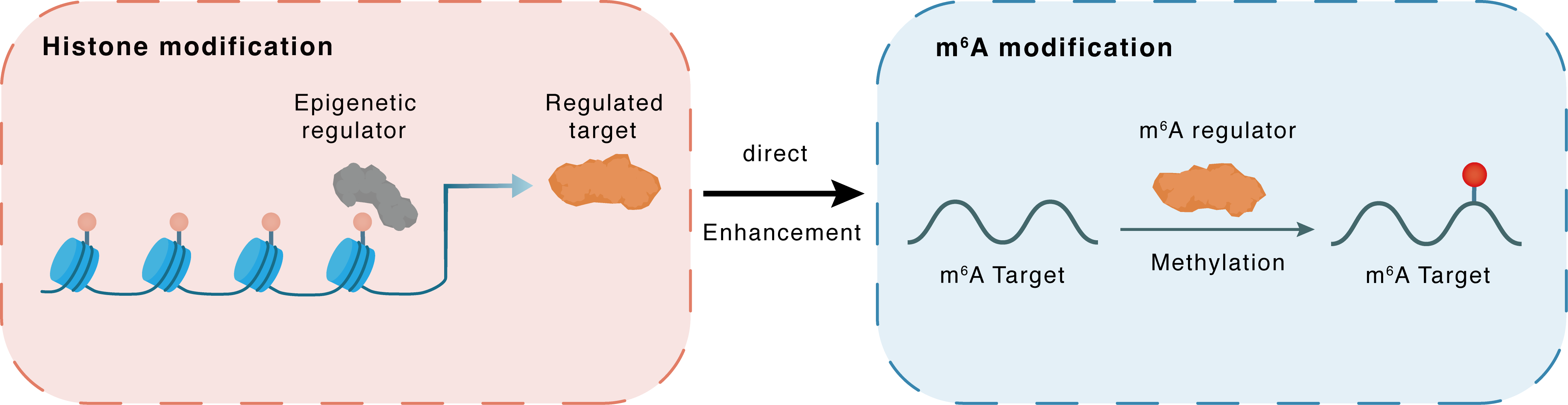 Histone modification
H3K18la
Epigenetic Regulator
METTL14
Direct
Enhancement
m6A modification
MTOR
MTOR
METTL14
Methylation
Histone modification
H3K18la
Epigenetic Regulator
METTL14
Direct
Enhancement
m6A modification
MTOR
MTOR
METTL14
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 14 (METTL14) | WRITER | |||
| m6A Target | Serine/threonine-protein kinase mTOR (MTOR) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Regulated Target | Histone H3 lysine 18 lactylation (H3K18la) | View Details | |||
| Downstream Gene | METTL14 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | Histone modification directly impacts m6A modification through modulating the level of m6A regulator | ||||
| Crosstalk Summary | Histone H3 lysine 18 lactylation (H3K18la) was found to upregulate METTL14 expression. In conclusion, METTL14 knockdown promotes stemness in GC by mediating m6A modification of ATF5 mRNA, which activates the WDR74/beta-catenin axis, making METTL14 a potential therapeutic target for gastric cancer treatment. The m6A modification level was decreased in GC and METTL14 was a key regulator resulting in m6A disorder in GC. METTL14 overexpression suppressed GC cell proliferation and aggression by deactivating the PI3K/AKT/Serine/threonine-protein kinase mTOR (MTOR) pathway and the EMT pathway, respectively. | ||||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | |||
In-vitro Model |
GES-1 | Normal | Homo sapiens | CVCL_EQ22 | |
| SGC-7901 | Gastric carcinoma | Homo sapiens | CVCL_0520 | ||
| MGC-803 | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Serine/threonine-protein kinase mTOR (MTOR) | 72 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Novolimus | Approved | [3] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Temsirolimus | Approved | [4] | ||
| Synonyms |
Torisel
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1760 nM | |||
| External Link | ||||
| Everolimus | Approved | [5] | ||
| Synonyms |
Afinitor; Afinitor (TN); CERTICAN(R); Certican; Certican (TN); Everolimus (JAN/USAN/INN); Everolimus [USAN]; MTOR kinase inhibitors; NVP-RAD-001; RAD 001; RAD-001; RAD-001C; RAD001; RAD001, SDZ-RAD, Certican, Zortress, Afinitor, Everolimus; SDZ-RAD; Zortress
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Zotarolimus | Approved | [6] | ||
| Synonyms |
Abt-578; Zotarolimus (TN)
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 3.3 nM | |||
| External Link | ||||
| Sirolimus | Approved | [7] | ||
| Synonyms |
53123-88-9; Rapamune; Rapamycin (Sirolimus); AY-22989; Rapammune; sirolimusum; WY-090217; RAPA; Antibiotic AY 22989; AY 22989; UNII-W36ZG6FT64; CCRIS 9024; CHEBI:9168; SILA 9268A; W36ZG6FT64; HSDB 7284; C51H79NO13; NSC 226080; DE-109; NCGC00021305-05; DSSTox_CID_3582; DSSTox_RID_77091; DSSTox_GSID_23582; Cypher; Supralimus; Wy 090217; Perceiva; RAP; RPM; Rapamycin from Streptomyces hygroscopicus; SIIA 9268A; LCP-Siro; MS-R001; Rapamune (TN); Rapamycin (TN); Sirolimus (RAPAMUNE); Rapamycin C-7, analog 4; Sirolimus (USAN/INN); Sirolimus [USAN:BAN:INN]; Sirolimus, Rapamune,Rapamycin; Heptadecahydro-9,27-dihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-hydroxy; 23,27-Epoxy-3H-pyrido(2,1-c)(1,4)oxaazacyclohentriacontine; 23,27-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine; 23,27-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine-1,5,11,28,29; 3H-pyrido(2,1-c)(1,4)oxaazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone; Sirolimus (MTOR inhibitor)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PF-04449913 | Approved | [8] | ||
| Synonyms |
Glasdegib; 1095173-27-5; PF 04449913; UNII-K673DMO5H9; K673DMO5H9; CHEMBL2043437; Glasdegib (PF-04449913); Glasdegib [USAN:INN]; Glasdegib (USAN/INN); PF-04449913;Glasdegib; GTPL8201; Glasdegib(PF-04449913); EX-A858; MolPort-035-789-706; SFNSLLSYNZWZQG-VQIMIIECSA-N; ZINC68251434; PF-913; BDBM50385635; 2640AH; AKOS027324121; CS-2
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Ridaforolimus | Phase 3 | [9] | ||
| Synonyms |
Deforolimus; AP 23573; MK 8669; AP-23573; MK-8669; AP23573, MK-8669, Ridaforolimus, Deforolimus
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| INK128 | Phase 2 | [10] | ||
| Synonyms |
1224844-38-5; Sapanisertib; INK-128; INK 128; INK 128 (MLN0128); TAK-228; UNII-JGH0DF1U03; JGH0DF1U03; 5-(4-amino-1-isopropyl-1H-pyrazolo[3,4-d]pyrimidin-3-yl)benzo[d]oxazol-2-amine; INK-0128; 3-(2-Amino-5-benzoxazolyl)-1-(1-methylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine; C15H15N7O; 5-(4-amino-1-propan-2-ylpyrazolo[3,4-d]pyrimidin-3-yl)-1,3-benzoxazol-2-amine; 5-(4-Amino-1-isopropyl-1H-pyrazolo[3,4-d]-pyrimidin-3-yl)benzo[d]oxazol-2-amine; Sapanisertib (USAN/INN)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 1 nM | |||
| External Link | ||||
| OSI-027 | Phase 2 | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| ABI-009 | Phase 2 | [12] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Salirasib | Discontinued in Phase 1/2 | [3] | ||
| Synonyms |
162520-00-5; Farnesylthiosalicylic acid; S-Farnesylthiosalicylic acid; UNII-MZH0OM550M; MZH0OM550M; CHEMBL23293; AK186909; Farnesyl Thiosalicylic Acid; 2-[[(2E,6E)-3,7,11-Trimethyl-2,6,10-dodecatrien-1-yl]thio]benzoic Acid; 2-[(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]sulfanylbenzoic acid; 2-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienylthio)benzoic acid; 2-(((2E,6E)-3,7,11-Trimethyl-2,6,10-dodecatrienyl)sulfanyl)benzoic acid; Benzoic acid, 2-(((2E,6E)-3,7,11-trimethyl-2,6,10-dodecatrienyl)thio)-; FTS; Farnesylthiosalicyclic acid; FTS, Thyreos; Ras antagonists, Thyreos; S-trans; Th-101; Trans-farnesylthiosalicylicacid; FTS (oral, cancer), Concordia; Farnesylthiosalicyclic acid (oral, cancer), Concordia; Ras-inhibitors (cancer), Concordia; FTS (oral, cancer), Concordia/Ono; KD032
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| SAR245409 | Phase 2 | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 157 nM | |||
| External Link | ||||
| SF1126 | Phase 2 | [12] | ||
| Synonyms |
CC-1126; SF-1126; L-Serine, N2-(1,4-dioxo-4-((4-(4-oxo-8-phenyl-4H-1-benzopyran-2-yl)morpholinium-4-yl)methoxy)butyl)-L-arginylglycyl-L-alpha-aspartyl-, inner salt
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PF-05212384 | Phase 2 | [13] | ||
| Synonyms |
PKI-587; 1197160-78-3; Gedatolisib; PKI587; PKI 587; 1-(4-(4-(Dimethylamino)piperidine-1-carbonyl)phenyl)-3-(4-(4,6-dimorpholino-1,3,5-triazin-2-yl)phenyl)urea; PF 05212384; UNII-96265TNH2R; PF-05212384 (PKI-587); CHEMBL592445; 96265TNH2R; N-[4-[[4-(Dimethylamino)-1-piperidinyl]carbonyl]phenyl]-N'-[4-[4,6-di(4-morpholinyl)-1,3,5-triazin-2-yl]phenyl]urea; Gedatolisib (PF-05212384, PKI-587); Urea, N-(4-((4-(dimethylamino)-1-piperidinyl)carbonyl)phenyl)-N'-(4-(4,6-di-4-morpholinyl-1,3,5-triazin-2-yl)phenyl)-
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 0.4 nM | |||
| External Link | ||||
| LY3023414 | Phase 2 | [14] | ||
| MOA | Modulator | |||
| External Link | ||||
| PF-04691502 | Phase 2 | [15] | ||
| Synonyms |
1013101-36-4; PF 04691502; UNII-4W39NS61KI; 4W39NS61KI; 2-amino-8-((1r,4r)-4-(2-hydroxyethoxy)cyclohexyl)-6-(6-methoxypyridin-3-yl)-4-methylpyrido[2,3-d]pyrimidin-7(8H)-one; CHEMBL1234354; PF04691502; 2-Amino-8-[trans-4-(2-Hydroxyethoxy)cyclohexyl]-6-(6-Methoxypyridin-3-Yl)-4-Methylpyrido[2,3-D]pyrimidin-7(8h)-One; 2-Amino-8-[4-(2-hydroxyethoxy)cyclohexyl]-6-(6-methoxypyridin-3-yl)-4-methylpyrido[2,3-d]pyrimidin-7-one
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 7.9 nM | |||
| External Link | ||||
| GDC-0980/RG7422 | Phase 2 | [3] | ||
| Synonyms |
Apitolisib; 1032754-93-0; GDC-0980; GDC0980; RG7422; (S)-1-(4-((2-(2-aminopyrimidin-5-yl)-7-methyl-4-morpholinothieno[3,2-d]pyrimidin-6-yl)methyl)piperazin-1-yl)-2-hydroxypropan-1-one; UNII-1C854K1MIJ; GDC-0980 (RG7422); Apitolisib (GDC-0980, RG7422); 1C854K1MIJ; CHEMBL1922094; RG-7422; (2s)-1-(4-{[2-(2-Aminopyrimidin-5-Yl)-7-Methyl-4-(Morpholin-4-Yl)thieno[3,2-D]pyrimidin-6-Yl]methyl}piperazin-1-Yl)-2-Hydroxypropan-1-One; J-502360; C23H30N8O3S
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | Ki = 17 nM | |||
| External Link | ||||
| BEZ235 | Phase 2 | [16] | ||
| Synonyms |
BEZ-235; S14-0511; NVP-BEZ-235; NVP-BEZ235, BEZ235; 2-(4-(2,3-dihydro-3-methyl-2-oxo-8-(quinolin-3-yl)imidazo[4,5-c]quinolin-1-yl)phenyl)-2-methylpropanenitrile
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| MM-141 | Phase 2 | [12] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PQR309 | Phase 2 | [3] | ||
| Synonyms |
Bimiralisib; 1225037-39-7; PI3K-IN-2; PQR-309; UNII-6Z3QHB00LB; 6Z3QHB00LB; 5-(4,6-dimorpholino-1,3,5-triazin-2-yl)-4-(trifluoromethyl)pyridin-2-amine; 5-[bis(morpholin-4-yl)-1,3,5-triazin-2-yl]-4-(trifluoromethyl)pyridin-2-amine; 5-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)-4-(trifluoromethyl)pyridin-2-amine; Bimiralisib [INN]; Bimiralisib [USAN]; Bimiralisib [WHO-DD]; NCB5; SCHEMBL1309049; GTPL8383; Bimiralisib free base; ADGGYDAFIHSYFI-UHFFFAOYSA-N; EX-A2018; BCP15887; PQR-309(PI3K-IN-2)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 62 nM | |||
| External Link | ||||
| AZD2014 | Phase 2 | [17] | ||
| Synonyms |
1009298-59-2; Vistusertib; AZD-2014; AZD 2014; UNII-0BSC3P4H5X; 0BSC3P4H5X; cc-551; 3-[2,4-Bis((3S)-3-methylmorpholin-4-yl)pyrido[5,6-e]pyrimidin-7-yl]-N-methylbenzamide; CHEMBL2336325; 3-[2,4-Bis((3S)-3-methyLmorpholin-4-yl)pyrido-[5,6-e]pyrimidin-7-yl]-N-methylbenzamide; C25H30N6O3; 3-(2,4-bis((S)-3-methylmorpholino)pyrido[2,3-d]pyrimidin-7-yl)-N-methylbenzamide; 3-[2,4-bis[(3S)-3-methylmorpholin-4-yl]pyrido[2,3-d]pyrimidin-7-yl]-N-methylbenzamide; Vistusertib [INN]; Vistusertib [USAN]; Vistusertib (JAN/INN)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2.8 nM | |||
| External Link | ||||
| CC-223 | Phase 1/2 | [18] | ||
| Synonyms |
GW 791343 HYDROCHLORIDE; GW791343 trihydrochloride; 309712-55-8; 1019779-04-4; GW791343 HCl; GW791343 (trihydrochloride); GW791343; GW-791343; 2-[(3,4-Difluorophenyl)amino]-N-[2-methyl-5-(1-piperazinylmethyl)phenyl]-acetamide trihydrochloride; GW791343 (HCL); GW-791343 hydrochloride; C20H27Cl3F2N4O; GW 791343 Trihydrochloride; C20H24F2N4O.3ClH; CTK8F0044; EX-A438; GW 791343 HCl; WSBRAHWNJBXXJM-UHFFFAOYSA-N; MolPort-023-219-209; BCP23425; AKOS024457596; CS-1030; BCP9000749; API0008007; HY-15470; BCP0726000290; RT-017402; KB-272661
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| BGT226 | Phase 1/2 | [19] | ||
| Synonyms |
BGT-226 free base; 915020-55-2; UNII-ZXE7F2GMJJ; BGT226 free base; ZXE7F2GMJJ; BGT-226; 8-(6-Methoxypyridin-3-yl)-3-methyl-1-[4-(piperazin-1-yl)-3-trifluoromethylphenyl]-1,3-dihydroimidazo[4,5-c]quinolin-2-one; CHEBI:71967; BGT 226; NVPBGT226; 8-(6-methoxypyridin-3-yl)-3-methyl-1-[4-(piperazin-1-yl)-3-(trifluoromethyl)phenyl]-1,3-dihydro-2H-imidazo[4,5-c]quinolin-2-one; 8-(6-Methoxy-pyridin-3-yl)-3-methyl-1-(4-piperazin-1-yl-3-trifluoromethyl-phenyl)-1,3-dihydro-imidazo[4,5-c]quinolin-2-one; NPV-BGT226; SCHEMBL146939
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| ME-344 | Phase 1/2 | [20] | ||
| Synonyms |
NV-128; NV-344; MTOR inhibitor (cancer), Novogen; MTOR inhibitors (cancer), Marshall Edwards
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BI 860585 | Phase 1 | [21] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| LAM-001 | Phase 1 | [22] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| DS-3078 | Phase 1 | [23] | ||
| MOA | Modulator | |||
| External Link | ||||
| GDC-0349 | Phase 1 | [24] | ||
| Synonyms |
MTORC1/2 inhibitors
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 3.8 nM | |||
| External Link | ||||
| CERC 006 | Phase 1 | [25] | ||
| Synonyms |
(+/-)-Cyclohexanecarboxylic acid, 4-(4-amino-5-(7-methoxy-1H-indol-2-yl)imidazo(5,1-f)(1,2,4)triazin-7-yl)-, trans-; (1r,4r)-4-(4-Amino-5-(7-methoxy-1H-indol-2-yl)imidazo[5,1-f][1,2,4]triazin-7-yl)cyclohexane-1-carboxylic acid; (1r,4r)-4-(4-amino-5-(7-methoxy-1H-indol-2-yl)imidazo[5,1-f][1,2,4]triazin-7-yl)cyclohexanecarboxylic acid; (1r,4r)-4-(4-Amino-5-(7-methoxy-1H-indol-2-yl)imidazo-[5,1-f][1,2,4]triazin-7-yl)cyclohexanecarboxylic acid; 1187559-66-5; 25MKH1SZ0M; 4-(4-Amino-5-(7-methoxy-1H-indol-2-yl)imidazo[5,1-f] [1,2,4]Triazin-7-yl)cyclohexanecarboxylic Acid; 4-[(5Z)-4-amino-5-(7-methoxyindol-2-ylidene)-1H-imidazo[5,1-f][1,2,4]triazin-7-yl]cyclohexane-1-carboxylic acid; 4-[4-amino-5-(7-methoxy-1H-indol-2-yl)imidazo[5,1-f][1,2,4]triazin-7-yl]cyclohexane-1-carboxylic acid; 4-[4-amino-5-(7-methoxy-2-indolylidene)-1H-imidazo[5,1-f][1,2,4]triazin-7-yl]-1-cyclohexanecarboxylic acid; 936890-98-1; 936890-98-1 (free acid); A-1065; AC-31517; AEVI-006; AKOS030238938; AKOS037643584; AM81260; AS-17003; ASP 7486; ASP4786; ASP7486; ASP-7486; BCP02613; BCP9001034; BDBM185151; BRD-K94294671-003-01-3; CCG-268721; CERC 006; CERC006; CERC-006; CHEBI:91363; CHEMBL2132692; CHEMBL3120215; CS-0257; Cyclohexanecarboxylic acid, 4-(4-amino-5-(7-methoxy-1H-indol-2-yl)imidazo(5,1-f)(1,2,4)triazin-7-yl)-, trans-; Cyclohexanecarboxylic acid, 4-[4-amino-5-(7-methoxy-1h-indol-2-yl)imidazo[5,1-f][1,2,4]triazin-7-yl]-, trans-; DB12387; DTXSID901025951; EX-A143; F17371; HMS3656H05; HMS3748I11; HY-10423; J-523839; JROFGZPOBKIAEW-HAQNSBGRSA-N; LS-14875; MLS006011006; NCGC00250395-01; NCGC00386179-01; NCGC00386179-04; NCGC00387858-03; NSC800810; NSC-800810; OSI 027; OSI027; OSI-027; Q27163231; Q27253978; s2624; SB19259; SCHEMBL20482333; SCHEMBL22594988; SCHEMBL22787096; SCHEMBL976795; SCHEMBL976796; SMR004702804; SW220246-1; trans-4-(4-Amino-5-(7-methoxy-1H-indol-2-yl)imidazo[5,1-f][1,2,4]triazin-7-yl)cyclohexanecarboxylic acid; TRANS-4-[4-AMINO-5-(7-METHOXY-1H-INDOL-2-YL)IMIDAZO[5,1-F][1,2,4]TRIAZIN-7-YL]CYCLOHEXANECARBOXYLIC ACID; trans-4-[4-amino-5-(7-methoxy-1H-indol-2-yl)imidazo[5,1-f][1,2,4]triazin-7-yl]-cyclohexanecarboxylic acid; trans-4-[4-amino-5-(7-methoxy-1H-indol-2-yl)-imidazo[5,1-f][1,2,4]triazin-7-yl]-cyclohexanecarboxylic acid; UNII-25MKH1SZ0M
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| DS-7423 | Phase 1 | [26] | ||
| MOA | Modulator | |||
| External Link | ||||
| PWT-33597 | Phase 1 | [27] | ||
| Synonyms |
PI3 kinase alpha/mTOR dual inhibitor (cancer), Pathway Therapeutics
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| VS-5584 | Phase 1 | [3] | ||
| Synonyms |
5-(9-Isopropyl-8-methyl-2-morpholino-9H-purin-6-yl)pyrimidin-2-amine; 1246560-33-7; VS-5584 (SB2343); UNII-W71J4X250V; SB-2343; SB2343; CHEMBL3393066; W71J4X250V; 5-(8-methyl-2-morpholin-4-yl-9-propan-2-ylpurin-6-yl)pyrimidin-2-amine; C17H22N8O; QYBGBLQCOOISAR-UHFFFAOYSA-N; SCHEMBL539098; GTPL8382; EX-A288; DTXSID10677328; MolPort-035-757-944; HMS3652B16; BCP08247; 2797AH; ZINC95644685; s7016; VS5584; BDBM50059635; AKOS024465057; 5-(9-isopropyl-8-methyl-2-morpholin-4-yl-9H-purin-6-yl)-pyrimidin-2-ylamine; SB16877
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| PMID25726713-Compound-49 | Patented | [28] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1 nM | |||
| External Link | ||||
| PMID25726713-Compound-51 | Patented | [28] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1 nM | |||
| External Link | ||||
| PMID25726713-Compound-48 | Patented | [28] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1 nM | |||
| External Link | ||||
| PMID25726713-Compound-47 | Patented | [28] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1 nM | |||
| External Link | ||||
| PMID25726713-Compound-50 | Patented | [28] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1 nM | |||
| External Link | ||||
| AZD8055 | Discontinued in Phase 1/2 | [29] | ||
| Synonyms |
1009298-09-2; AZD-8055; AZD 8055; [5-[2,4-Bis((3S)-3-methylmorpholin-4-yl)pyrido[2,3-d]pyrimidin-7-yl]-2-methoxyphenyl]methanol; (5-(2,4-bis((S)-3-methylmorpholino)pyrido[2,3-d]pyrimidin-7-yl)-2-methoxyphenyl)methanol; UNII-970JJ37FPW; 970JJ37FPW; CHEMBL1801204; AK109550; (5-(2,4-Bis((S)-3-methylmorpholino)pyrido-[2,3-d]pyrimidin-7-yl)-2-methoxyphenyl)methanol; (5-(2,4-bis((3S)-3-methylmorpholin-4-yl)pyrido(2,3-d)pyrimidin-7-yl)-2-methoxyphenyl)methanol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4 nM | |||
| External Link | ||||
| TAFA-93 | Discontinued in Phase 1 | [30] | ||
| Synonyms |
MTOR inhibitor, Isotechnika; Rapamycin prodrug, Isotechnika; Transplant rejection therapy, Isotechnika
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SCR-44001 | Terminated | [31] | ||
| Synonyms |
MTOR pathway inhibitors (cancer); PI3K modulators, BioImage; SCR-0044001; SCR-0334654; SCR-0335319; TOP-216; MTOR pathway inhibitors (cancer), TopoTarget; MTOR pathway inhibitors (cancer), BioImage/TopoTarget
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (4-(6-morpholino-9H-purin-2-yl)phenyl)methanol | Investigative | [32] | ||
| Synonyms |
CHEMBL594669; SCHEMBL4442909
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4500 nM | |||
| External Link | ||||
| Rapamycin complexed with immunophilin FKBP12 | Investigative | [33] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| C-16-(S)-3-methylindolerapamycin | Investigative | [34] | ||
| Synonyms |
CHEMBL503885
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| PF-05094037 | Investigative | [3] | ||
| Synonyms |
PF-05171310; PF-05181059; MTOR inhibitors (cancer), Pfizer
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SX-MTR1 | Investigative | [3] | ||
| Synonyms |
MTOR modulators (small peptide mimetics, bladder cancer), Serometrix
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| 2-(6-morpholino-9H-purin-2-yl)phenol | Investigative | [32] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2500 nM | |||
| External Link | ||||
| P-2281 | Investigative | [3] | ||
| Synonyms |
MTOR inhibitor (ulcerative colitis), Piramal
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| X-387 | Investigative | [3] | ||
| Synonyms |
MTOR inhibitors (cancer), Shanghai Institute of Materia Medica
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-(6-morpholino-9H-purin-2-yl)phenol | Investigative | [32] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 450 nM | |||
| External Link | ||||
| EM-101 | Investigative | [3] | ||
| Synonyms |
EM-100 series; LY-3; LY-303511; MTOR pathway inhibitors (cancer), Emiliem; MTOR pathway inhibitors (cancer), NIH
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| EC-0845 | Investigative | [3] | ||
| Synonyms |
MTOR modulator (inflammatory disease), Endocyte
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| 4-(2-(thiophen-2-yl)-9H-purin-6-yl)morpholine | Investigative | [32] | ||
| Synonyms |
CHEMBL604876; SCHEMBL4439490
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2800 nM | |||
| External Link | ||||
| SB-2280 | Investigative | [3] | ||
| Synonyms |
SB-2602; Selective mTOR inhibitors (cancer); Selective mTOR inhibitors (cancer), S*BIO
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-(2-(thiophen-3-yl)-9H-purin-6-yl)morpholine | Investigative | [32] | ||
| Synonyms |
CHEMBL608095; SCHEMBL4438208
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3900 nM | |||
| External Link | ||||
| torin 1 | Investigative | [35] | ||
| Synonyms |
Torin-1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4.32 nM | |||
| External Link | ||||
| EC-0565 | Investigative | [3] | ||
| Synonyms |
Folate-everolimus conjugate (inflammation), Endocyte
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| OXA-01 | Investigative | [3] | ||
| Synonyms |
MTORC1/mTORC2 inhibitor (cancer) OSI Pharmaceuticals
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| AR-mTOR-26 | Investigative | [3] | ||
| Synonyms |
AR-mTOR-1; MTORC1/2 inhibitors (cancer); MTORC1/2 inhibitors (cancer), Array BioPharma
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-chloro-N-(6-cyanopyridin-3-yl)propanamide | Investigative | [36] | ||
| Synonyms |
1112994-35-0; SCHEMBL1483919; CHEMBL446834; VFOLQYOVUCHHET-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-(6-morpholino-9H-purin-2-yl)phenol | Investigative | [32] | ||
| Synonyms |
CHEMBL593515; SCHEMBL4443377; FUDQNOGEMXSUSQ-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1650 nM | |||
| External Link | ||||
| AP-21967 | Investigative | [34] | ||
| Synonyms |
CHEMBL525042; SCHEMBL18176922; C-16-(S)-7-methylindolerapamycin
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| 4-(2-(1H-indol-6-yl)-9H-purin-6-yl)morpholine | Investigative | [32] | ||
| Synonyms |
CHEMBL611630
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 150 nM | |||
| External Link | ||||
| Torin2 | Investigative | [37] | ||
| Synonyms |
Torin 2; 1223001-51-1; Torin-2; 9-(6-aminopyridin-3-yl)-1-[3-(trifluoromethyl)phenyl]benzo[h][1,6]naphthyridin-2-one; 9-(6-AMINO-3-PYRIDINYL)-1-[3-(TRIFLUOROMETHYL)PHENYL]-BENZO[H]-1,6-NAPHTHYRIDIN-2(1H)-ONE; CHEMBL1765602; C24H15F3N4O; CHEBI:90682; 9-(6-Aminopyridin-3-Yl)-1-[3-(Trifluoromethyl)phenyl]benzo[h][1,6]naphthyridin-2(1h)-One; 9-(6-AMINOPYRIDIN-3-YL)-1-(3-(TRIFLUOROMETHYL)PHENYL)BENZO[H][1,6]NAPHTHYRIDIN-2(1H)-ONE; 9-(6-Aminopyridin-3-yl)-1-(3-(trifluoromethyl)-phenyl)benzo[h][1,6]naphthyridin-2(1H)-one; BENZO[H]-1,6-NAPHTHYRIDIN-2(1H)-ONE, 9-(6-AMINO-3-PYRIDINYL)-1-[3-(TRIFLUOROMETHYL)PHENYL]-; 9-(6-Amino-3-pyridinyl)-1-[3-(trifluoromethyl)phenyl]benzo[h]-1,6-naphthyridin-2(1H)-one; cc-275; MLS006011167; GTPL8839; SCHEMBL6876328; AOB3537; DTXSID00679917; EX-A431; HMS3265O05; HMS3265O06; HMS3265P05; HMS3265P06; HMS3651N13; BCP02612; ABP000908; BDBM50341209; MFCD18782652; NSC775727; s2817; ZINC71318831; AKOS024458055; CCG-265003; CS-0236; NSC-775727; PB34957; NCGC00263216-01; NCGC00263216-02; NCGC00263216-09; NCGC00263216-13; 9-(6-AMINO-PYRIDIN-3-YL)-1-(3-TRIFLUOROMETHYL-PHENYL)-1H-BENZO[H][1,6]NAPHTHYRIDIN-2-ONE; AC-31520; AK171126; AS-74405; HY-13002; SMR004702936; AB0035864; DB-084736; FT-0700124; SW218309-2; Y0293; Q-4148; J-519481; BRD-K68174511-001-01-7; Q27089008; 9-(6-amino-3-pyridyl)-1-[3-(trifluoromethyl)phenyl]benzo[h][1,6]naphthyridin-2-one; 17G; 9-(6-Amino-3-pyridinyl)-1-[3-(trifl uoromethyl)phenyl]-benzo[h]-1,6-naphthyridin-2(1H) -one; 9-(6-AMINOPYRIDIN-3-YL)-1-[3-(TRIFLUOROMETHYL)PHENYL]-1H,2H-BENZO[H]1,6-NAPHTHYRIDIN-2-ONE
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| HM-5016699 | Investigative | [3] | ||
| Synonyms |
Dual PI3K/mTOR inhibitor (cancer); Dual PI3K/mTOR inhibitor (cancer), Hutchison
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PF-03772304 | Investigative | [38] | ||
| Synonyms |
MTOR inhibitors, Biotica; MTOR inhibitors, Wyeth; PF-04979064; PF-05017255; PF-05168899; WYE-125132; WYE-132; WYE-178; WYE-354; WYE-600; WYE-687; Imidazolo and pyrazolopyrimidine derivatives (cancer), Pfizer; Imidazolopyrimidine derivatives (cancer), Pfizer; Imidazolopyrimidine derivatives (cancer), Wyeth; Non-rapamycin mTOR/PI3K inhibitors (cancer); PI3K/mTOR signalling inhibitors (cancer), Wyeth; Non-rapamycin mTOR/PI3K inhibitors (cancer), Pfizer; 5H-pyrrolo[3,2-d]pyridimine analogs (cancer), Wyeth
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PP-242 | Investigative | [39] | ||
| Synonyms |
PP242; TORKinib
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 8 nM | |||
| External Link | ||||
| CU-906 | Investigative | [3] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| P-6915 | Investigative | [3] | ||
| Synonyms |
PI3K/mTOR inhibitors (cancer), Piramal
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-Morpholin-4-yl-pyrimido[2,1-a]isoquinolin-4-one | Investigative | [40] | ||
| Synonyms |
Compound 401; 168425-64-7; 2-morpholino-4H-pyrimido[2,1-a]isoquinolin-4-one; 2-(morpholin-4-yl)pyrimido[2,1-a]isoquinolin-4-one; CHEMBL179242; 2-(4-MORPHOLINYL)-4H-PYRIMIDO[2,1-A]ISOQUINOLIN-4-ONE; Compound401; SCHEMBL10092321; KS-00001DEG; CTK4D2994; DTXSID20434626; MolPort-023-276-726; HMS3229D15; EX-A1016; BCP04303; BDBM50159620; ZINC13608047; AKOS016369524; CS-5624; NCGC00378805-02; HY-19341; KB-224235; M2537; B7337; S-7713; J-010456
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5300 nM | |||
| External Link | ||||
| 2-(2-Methyl-morpholin-4-yl)-benzo[h]chromen-4-one | Investigative | [40] | ||
| Synonyms |
CHEMBL435507; SCHEMBL3545107
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4800 nM | |||
| External Link | ||||
| Ethyl 1-[(1H-benzimidazol-2(3H)one-5-yl)sulfonyl]-1H-pyrrole-2-carboxylate | Investigative | [40] | ||
| Synonyms |
2-(Morpholin-4-yl)-benzo[h]chromen-4-one; 154447-35-5; NU7026; NU 7026; DNA-PK Inhibitor II; NU-7026; 2-morpholino-4H-benzo[h]chromen-4-one; LY293646; LY-293646; 2-(4-Morpholinyl)-4H-naphthol[1,2-b]pyran-4-one; 2-(4-morpholinyl)-4H-naphtho[1,2-b]pyran-4-one; CHEMBL104468; AK186905; DNA-Dependent Protein Kinase Inhibitor II; 2-morpholin-4-ylbenzo[h]chromen-4-one; SCHEMBL610237; ZINC9230; GTPL5959; KS-00000XHI; CTK0E7833; CHEBI:92165; DTXSID10432010; AOB2835; MolPort-009-019-548; HMS3229C11; EX-A1100; BCP04736; IN1364; s2893
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 6400 nM | |||
| External Link | ||||
| PP121 | Investigative | [39] | ||
| Synonyms |
PP-121; PP 121
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| 2B72: Gastric cancer | 81 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Leniolisib | Approved | [41] | ||
| Synonyms |
1354690-24-6; Leniolisib free base; UNII-L22772Z9CP; (S)-1-(3-((6-(6-methoxy-5-(trifluoromethyl)pyridin-3-yl)-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-4-yl)amino)pyrrolidin-1-yl)propan-1-one; L22772Z9CP; 1354690-24-6 (free base); leniolisib(CDZ 173); CDZ173; CDZ-173; 1-[(3S)-3-[[6-[6-methoxy-5-(trifluoromethyl)pyridin-3-yl]-7,8-dihydro-5H-pyrido[4,3-d]pyrimidin-4-yl]amino]pyrrolidin-1-yl]propan-1-one; Leniolisib [INN]; Leniolisib (CDZ173); Leniolisib (USAN/INN); CDZ173-NX; SCHEMBL323054; GTPL9424; CHEMBL3643413; BDBM118299; EX-A2854; MFCD30470232; s8752; ZB1510; CS-7524; DC22326; SB18839; Example 67 [WO2012004299]; 1-{(S)-3-[6-(6-methoxy-5-trifluoromethyl-pyridin-3-yl)-5,6,7,8-tetrahydro-pyrido[4,3-d]pyrimidin-4-y; 1-{(S)-3-[6-(6-methoxy-5-trifluoromethyl-pyridin-3-yl)-5,6,7,8-tetrahydro-pyrido[4,3-d]pyrimidin-4-ylamino]-pyrrolidin-1-yl}-propan-1-one; AS-56217; HY-17635; A16796; D11158; US8653092, 67; Q27282602; 1-Propanone, 1-((3S)-3-((5,6,7,8-tetrahydro-6-(6-methoxy-5-(trifluoromethyl)-3-pyridinyl)pyrido(4,3-d)pyrimidin-4-yl)amino)-1-pyrrolidinyl)-; 9NQ
Click to Show/Hide
|
|||
| External Link | ||||
| Atezolizumab | Approved | [12] | ||
| External Link | ||||
| Bavencio | Approved | [12] | ||
| External Link | ||||
| Tebentafusp | Approved | [42] | ||
| External Link | ||||
| Merimepodib | Approved | [43] | ||
| Synonyms |
Merimebodib; Merimepodib [USAN:INN]; Tyverb/Tykerb; MMPD; 198821-22-6; 2ZL2BA06FU; C23H24N4O6; CHEMBL304087; MERIMEPODIB, VI-21497, VX-497; UNII-2ZL2BA06FU; VI-21497; VX-497; VX497; Vx 497; carbamic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Taxol | Approved | [22] | ||
| Synonyms |
C47H51NO14; weekly paclitaxel; Micellar Paclitaxel; Paclitaxel [USAN:INN:BAN]; SCHEMBL15000506; Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, (2aR-(2aalpha,4beta,4abeta,6beta,9alpha(alphaR*,betaS*),11alpha,12alpha,12aalpha,12balpha))-
Click to Show/Hide
|
|||
| External Link | ||||
| Ramucirumab | Approved | [44] | ||
| Synonyms |
LY3009806
Click to Show/Hide
|
|||
| External Link | ||||
| Tucatinib | Approved | [45] | ||
| Synonyms |
Irbinitinib; 937263-43-9; ONT-380; UNII-234248D0HH; 234248D0HH; N6-(4,4-Dimethyl-4,5-dihydrooxazol-2-yl)-N4-(3-methyl-4-((1,2,4)triazolo(1,5-a)pyridin-7-yloxy)phenyl)quinazoline-4,6-diamine; 4,6-Quinazolinediamine, N6-(4,5-dihydro-4,4-dimethyl-2-oxazolyl)-N4-(3-methyl-4-((1,2,4)triazolo(1,5-a)pyridin-7-yloxy)phenyl)-; ONT 380; 4,6-QuinazolinediaMine, N6-(4,5-dihydro-4,4-diMethyl-2-oxazolyl)-N4-[3-Methyl-4-([1,2,4]triazolo[1,5-a]pyridin-7-yloxy)phenyl]-; Tucatinib [USAN:INN]; 6-DIAMINE
Click to Show/Hide
|
|||
| External Link | ||||
| Antacids | Approved | [46] | ||
| External Link | ||||
| Trastuzumab | Approved | [12] | ||
| Synonyms |
Herceptin; Herceptin (TN); Trastuzumab (INN); Trastuzumab (genetical recombination); Trastuzumab (genetical recombination) (JAN); Trastuzumab (ERBB2 mAb inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| Carbamazepine | Phase 3 | [47] | ||
| Synonyms |
Carbamazepine (iv, epilepsy); Carbamazepine (iv, epilepsy), Lundbeck; Carbamazepine (iv, epilepsy), Ovation Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| Margetuximab | Approved | [12] | ||
| External Link | ||||
| Nivolumab | Approved | [12] | ||
| External Link | ||||
| GRANITE | Phase 3 | [48] | ||
| Synonyms |
Penoxsulam; 219714-96-2; 2-(2,2-difluoroethoxy)-N-(5,8-dimethoxy-[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; UNII-784ELC1SCZ; 784ELC1SCZ; CHEBI:81776; 2-(2,2-difluoroethoxy)-n-(5,8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; Penoxsulam [ISO]; PXD; DSSTox_CID_14803; DSSTox_RID_79204; DSSTox_GSID_34803; SCHEMBL116968; CHEMBL1895913; DTXSID0034803; HSDB 7887; AMY12535; BCP18718; EBD18529; Tox21_301010; MFCD07363876; ZINC13827750; AKOS025401685; NCGC00163715-01; NCGC00163715-02; NCGC00163715-03; NCGC00254912-01; AC-24494; Penoxsulam 100 microg/mL in Acetonitrile; CAS-219714-96-2; FT-0696708; Penoxsulam, PESTANAL(R), analytical standard; C18481; Q22808507; 2-(2,2-Difluoroethoxy)-6-trifluoromethyl-N-(5, 8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)benzenesulfonamide; 2-(2,2-Difluoroethoxy)-N-(5,8-dimethoxy[1,2,4]-triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; 2-(2,2-difluoroethoxy)-N-{5,8-dimethoxy-[1,2,4]triazolo[1,5-c]pyrimidin-2-yl}-6-(trifluoromethyl)benzene-1-sulfonamide; 2-(2,2-difluoroethyl)-N-(5,8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide; Benzenesulfonamide, 2-(2,2-difluoroethoxy)-N-(5,8-dimethoxy(1,2,4)triazolo(1,5-c)pyrimidin-2-yl)-6-(trifluoromethyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| Zolbetuximab | Phase 3 | [49] | ||
| Synonyms |
IMAB362
Click to Show/Hide
|
|||
| External Link | ||||
| Tusamitamab ravtansine | Phase 3 | [50] | ||
| Synonyms |
SAR408701
Click to Show/Hide
|
|||
| External Link | ||||
| Andecaliximab | Phase 3 | [51] | ||
| External Link | ||||
| ABP 980 | Phase 3 | [52] | ||
| External Link | ||||
| GS-5745 | Phase 3 | [22] | ||
| External Link | ||||
| S-1 | Phase 3 | [53] | ||
| Synonyms |
Ciprofibrate-coa; Ciprofibrate-coenzyme A; Coenzyme A, ciprofibrate-; AC1L4TRG; AC1Q3T4H; 111900-25-5; s-{1-[(2r,3s,4r,5r)-5-(6-amino-9h-purin-9-yl)-4-hydroxy-3-(phosphonooxy)tetrahydrofuran-2-yl]-3,5,9-trihydroxy-8,8-dimethyl-3,5-dioxido-10,14-dioxo-2,4,6-trioxa-11,15-diaza-3; E5,5; E5-diphosphaheptadecan-17-yl} 2-[4-(2,2-dichlorocyclopropyl)phenoxy]-2-methylpropanethioate(non-preferred name); Coenzyme A, S-(2-(4-(2,2-dichlorocyclopropyl)phenoxy)-2-methylpropanoate)
Click to Show/Hide
|
|||
| External Link | ||||
| Lonsurf | Phase 3 | [12] | ||
| External Link | ||||
| GDC-0068 | Phase 3 | [22] | ||
| Synonyms |
RG7440
Click to Show/Hide
|
|||
| External Link | ||||
| Edotecarin | Phase 3 | [54] | ||
| Synonyms |
ED-749; Edotecarin < Prop INN; J-107088; PF-804950; 12-(beta-D-Glucopyranosyl)-2,10-dihydroxy-6-[2-hydroxy-1-(hydroxymethyl)ethylamino]-6,7,12,13-tetrahydro-5H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-5,7-dione
Click to Show/Hide
|
|||
| External Link | ||||
| RG3638 | Phase 3 | [55] | ||
| Synonyms |
Onartuzumab
Click to Show/Hide
|
|||
| External Link | ||||
| G17DT | Phase 3 | [56] | ||
| Synonyms |
Gastrimmune; Insegia
Click to Show/Hide
|
|||
| External Link | ||||
| DE-766 | Phase 3 | [57] | ||
| External Link | ||||
| Tesetaxel | Phase 2 | [58] | ||
| Synonyms |
DJ-927; 333754-36-2; UNII-UG97LO5M8Y; UG97LO5M8Y; Tesetaxel [INN]; DJ927; DJ 927; CHEMBL2107787; SCHEMBL12060837; DB12019; Z-3104; (2AS,2BR,3S,4S,6S,8AR,10R,11AS,11BR,13AR)-2A-ACETOXY-6-(((2R,3S)-3-((TERT-BUTOXYCARBONYL)AMINO)-3-(3-FLUOROPYRIDIN-2-YL)-2-HYDROXYPROPANOYL)OXY)-10-((DIMETHYLAMINO)METHYL)-4-HYDROXY-7,11B,14,14-TETRAMETHYL-2A,2B,3,4,5,6,8A,11A,11B,12,13,13A-DODECAHYDRO-2H-4,8-METHANOOXETO[3'',2'':3',4']BENZO[1',2':3,4]CYCLODECA[1,2-D][1,3]DIOXOL-3-YL BENZOATE
Click to Show/Hide
|
|||
| External Link | ||||
| Nelipepimut S | Phase 3 | [59] | ||
| Synonyms |
E75
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-986205 | Phase 3 | [12] | ||
| Synonyms |
KRTIYQIPSAGSBP-KLAILNCOSA-N; 1923833-60-6; BMS986205; UNII-0A7729F42K; 0A7729F42K; GTPL9707; SCHEMBL18826792; SCHEMBL17740982; SCHEMBL19105151; EX-A2606; AKOS032954040; HY-101560; CS-0021719; Q29213697; (R)-N-(4-chlorophenyl)-2-((1s,4S)-4-(6-fluoroquinolin-4-yl)cyclohexyl)propanamide; (2R)-N-(4-chlorophenyl)-2-[4-(6-fluoroquinolin-4-yl)cyclohexyl]propanamide; (2R)-N-(4-Chlorophenyl)-2-(4-(6-fluoro-4-quinolyl)cyclohexyl)propanamide, cis; Cyclohexaneacetamide, N-(4-chlorophenyl)-4-(6-fluoro-4-quinolinyl)-alpha-methyl-, cis-(alphaR)-
Click to Show/Hide
|
|||
| External Link | ||||
| Rivoceranib | Phase 3 | [12] | ||
| External Link | ||||
| Claudiximab | Phase 3 | [12] | ||
| Synonyms |
IMAB-362; Anti-GC182 mAbs (cancer), Ganymed; Anti-GC182 monoclonal antibodies (cancer), Ganymed; Anti-CLDN18-2 mAbs (cancer), Ganymed; Anti-CLDN18-2 monoclonal antibodies (cancer), Ganymed
Click to Show/Hide
|
|||
| External Link | ||||
| OS-440 | Phase 3 | [60] | ||
| Synonyms |
CNS modulator (spasticity), Osmotica
Click to Show/Hide
|
|||
| External Link | ||||
| Oraxol | Phase 3 | [12] | ||
| External Link | ||||
| ICI 118,551 | Phase 3 | [22] | ||
| Synonyms |
Ici 118551; (2R,3S)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-3-(propan-2-ylamino)butan-2-ol; CHEMBL198059; CHEBI:73289; ICI-118551; ICI118551; erythro-DL-1-(7-Methylindan-4-yloxy)-3-isopropylaminobutan-2-ol; (2R,3S)-3-(isopropylamino)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]butan-2-ol; 2-Butanol, 1-((2,3-dihydro-7-methyl-1H-inden-4-yl)oxy)-3-((1-methylethyl)amino)-, (2R,3S)-rel-; 2-Butanol, 1-((2,3-dihydro-7-methyl-1H-inden-4-yl)oxy)-3-((1-methylethyl)amino)-, (R*,S*)-(+-)-; ICI-118,551; Ici 111,581; AC1NUNSO
Click to Show/Hide
|
|||
| External Link | ||||
| Evorpacept | Phase 2/3 | [61] | ||
| Synonyms |
ALX148
Click to Show/Hide
|
|||
| External Link | ||||
| BNT141 | Phase 2 | [62] | ||
| External Link | ||||
| Anti-LAG3 | Phase 2 | [52] | ||
| External Link | ||||
| GSK1292263 | Phase 2 | [63] | ||
| External Link | ||||
| MM-111 | Phase 2 | [64] | ||
| External Link | ||||
| Plevitrexed | Phase 2 | [65] | ||
| Synonyms |
ZD 9331; ZD9331; 153537-73-6; Plevitrexed [INN]; ZD-9331; NSC 696259; UNII-L9P2881C3H; CHEMBL126648; (2s)-2-[(2-fluoro-4-{[(4-hydroxy-2,7-dimethylquinazolin-6-yl)methyl](prop-2-yn-1-yl)amino}benzoyl)amino]-4-(2h-tetrazol-5-yl)butanoic acid; L9P2881C3H; Plevitrexed (INN); 172521-94-7; (2S)-2-[[4-[(2,7-dimethyl-4-oxo-1H-quinazolin-6-yl)methyl-prop-2-ynylamino]-2-fluorobenzoyl]amino]-4-(2H-tetrazol-5-yl)butanoic acid; 1H-Tetrazole-5-butanoic acid,
Click to Show/Hide
|
|||
| External Link | ||||
| DS-8201 | Phase 1 | [52] | ||
| Synonyms |
9-Aminofluorene; 9H-Fluoren-9-amine; 525-03-1; FLUOREN-9-AMINE; Fluoren-9-ylamine; UNII-4NHO2K4K5B; CCRIS 7000; BRN 2209545; 4NHO2K4K5B; OUGMRQJTULXVDC-UHFFFAOYSA-N; fluorene-9-ylamine; 9-Amino-fluoren; 9-amino-fluorene; 9H-9-fluorenamine; 9H-fluoren-9-yl-amine; AC1L1VP5; 4-12-00-03390 (Beilstein Handbook Reference); SCHEMBL353865; AC1Q53A2; AC1Q53A1; KS-00000JGC; CTK1H0380; DTXSID90200496; MolPort-001-794-448; HMS1780P20; 9H-fluoren-9-ylamine hydrochloride; ZINC1724407; ALBB-023296; CA-733; SBB005783; AKOS000264388; MCULE-8757055914; DS-
Click to Show/Hide
|
|||
| External Link | ||||
| XL880 | Phase 2 | [66] | ||
| Synonyms |
GSK 089; GSK 1363089; GSK1363089; XL 880; GSK1363089, GSK089, foretinib, EXEL-2880, XL880; 88Z; MET inhibitors
Click to Show/Hide
|
|||
| External Link | ||||
| Matuzumab | Phase 2 | [67] | ||
| Synonyms |
EMD-62000; EMD-72000; Anti-EGF receptor humanized antibody (iv, cancer), Merck KGaA/ Takeda Pharmaceuticals; Anti-EGFR humanized mAb (iv, cancer), Merck KGaA/ Takeda Pharmaceuticals; Anti-epidermal growth factor receptor humanized antibody (iv, cancer), Merck KGaA/ Takeda Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| BAY-57-9352 | Phase 2 | [22] | ||
| Synonyms |
Telatinib; Bay 57-9352
Click to Show/Hide
|
|||
| External Link | ||||
| Bemarituzumab | Phase 2 | [68] | ||
| External Link | ||||
| PEGPH20 | Phase 2 | [12] | ||
| External Link | ||||
| Plevitrexed (R)-isomer | Phase 2 | [69] | ||
| Synonyms |
YW3548
Click to Show/Hide
|
|||
| External Link | ||||
| APR-246 | Phase 2 | [70] | ||
| Synonyms |
Eprenetapopt
Click to Show/Hide
|
|||
| External Link | ||||
| CRS-207 | Phase 2 | [59] | ||
| External Link | ||||
| Opdivo + Yervoy | Phase 3 | [12] | ||
| External Link | ||||
| CT-041 | Phase 1/2 | [71] | ||
| External Link | ||||
| BPX-601 | Phase 1/2 | [72] | ||
| External Link | ||||
| Anti-MUC1 CAR-T cells | Phase 1/2 | [73] | ||
| External Link | ||||
| Anti-Mesothelin CAR-T cells | Phase 1/2 | [74] | ||
| External Link | ||||
| Anti-HER2 CAR-T | Phase 1/2 | [75] | ||
| External Link | ||||
| CAR-T Cells targeting EpCAM | Phase 1/2 | [76] | ||
| External Link | ||||
| PAT-SC1 | Phase 1/2 | [77] | ||
| Synonyms |
SC-1; Adjuvant therapy (gastric cancer), University of Wurzburg; SC-1 (gastric cancer), CAT; SC-1 (gastric cancer), Debiopharm; SC-1 (gastric cancer), Patrys; SC-1 (stomach cancer), OncoMab
Click to Show/Hide
|
|||
| External Link | ||||
| ASP2138 | Phase 1 | [78] | ||
| External Link | ||||
| SAR443216 | Phase 1 | [79] | ||
| External Link | ||||
| AMG 199 | Phase 1 | [80] | ||
| External Link | ||||
| AMG 910 | Phase 1 | [81] | ||
| External Link | ||||
| Alofanib | Phase 1 | [82] | ||
| Synonyms |
1612888-66-0; 3-(N-(4-methyl-2-nitro-5-(pyridin-3-yl)phenyl)sulfamoyl)benzoic acid; RPT-835(alofanib); UNII-LQX7RFK8MZ; RPT-835; RPT835; LQX7RFK8MZ; ES000835; Alofanib [INN]; Alofanib(RPT835); Syn007154; CHEMBL4594436; SCHEMBL18660613; AMY16650; BCP31905; EX-A2731; MFCD30533418; NSC790182; s8754; Benzoic acid, 3-(((4-methyl-2-nitro-5-(3-pyridinyl)phenyl)amino)sulfonyl)-; NSC-790182; SB19665; AC-31695; AK668992; AS-56846; HY-17601; CS-0014684; RPT 835; Q27283135; 3-{[4-methyl-2-nitro-5-(pyridin-3-yl)phenyl]sulfamoyl}benzoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| HER2-specific CAR T cell | Phase 1 | [83] | ||
| External Link | ||||
| Anti-CEA-CAR T | Phase 1 | [84] | ||
| External Link | ||||
| XR-5944 | Phase 1 | [85] | ||
| Synonyms |
MLN-944; XR-11576 analogs; XR-5000 analogs; XR-5942
Click to Show/Hide
|
|||
| External Link | ||||
| A168 | Phase 1 | [86] | ||
| External Link | ||||
| EGFR806-specific CAR T cell | Phase 1 | [87] | ||
| External Link | ||||
| AbGn-107 | Phase 1 | [12] | ||
| External Link | ||||
| FPA144 | Phase 1 | [52] | ||
| External Link | ||||
| Minnelide 001 | Phase 1 | [22] | ||
| External Link | ||||
| CAR-T cells targeting EpCAM | Phase 1 | [88] | ||
| External Link | ||||
| Anti-CEA CAR-T cells | Phase 1 | [89] | ||
| External Link | ||||
| EPCAM-targeted CAR-T cells | Clinical trial | [90] | ||
| External Link | ||||
| PMID28460551-Compound-1 | Patented | [91] | ||
| External Link | ||||
| Conjugated 3-(indolyl)-and 3-(azaindolyl)-4-arylmaleimide compound 1 | Patented | [92] | ||
| Synonyms |
PMID28621580-Compound-WO2012084683c62
Click to Show/Hide
|
|||
| External Link | ||||
| TOPIXANTRONE HYDROCHLORIDE | Discontinued in Phase 2 | [93] | ||
| Synonyms |
SCHEMBL1418986; Topixantrone hydrochloride < Prop INNM; BBR-3409 (dimaleate); 5-[2-(Dimethylamino)ethylamino]-2-[2-(2-hydroxyethylamino)ethyl]indazolo[4,3-gh]isoquinolin-6(2H)-one dihydrochloride
Click to Show/Hide
|
|||
| External Link | ||||
| MDL 101,731 | Discontinued in Phase 2 | [94] | ||
| Synonyms |
Tezacitabine; Fmdc cpd; 130306-02-4; UNII-7607Y95N9S; Mdl 101731; (E)-2'-Deoxy-2'-(fluoromethylene) cytidine; MDL-101731; 2'-Deoxy-2'-(fluoromethylene)cytidine; 7607Y95N9S; Cytidine, 2'-deoxy-2'-(fluoromethylene)-, (2E)-; (E)-2'-Deoxy-2'-(fluoromethylene)cytidine; Tezacitabine [INN]; tezaciabine; Tezacitabine, anhydrous; AC1O5KIG; SCHEMBL18724; SCHEMBL18725; Tezacitabine, anhydrous [INN]; CHEMBL2105467; C10H12FN3O4; DTXSID10156446; GFFXZLZWLOBBLO-ASKVSEFXSA-N; ZINC3777826; KW-2331
Click to Show/Hide
|
|||
| External Link | ||||
| BBR-3438 | Discontinued in Phase 2 | [95] | ||
| Synonyms |
Nortopixantrone; UNII-PH2639TAB4; PH2639TAB4; Nortopixantrone [INN:BAN]; AC1MI4ZO; CHEMBL150303; SCHEMBL7804438
Click to Show/Hide
|
|||
| External Link | ||||
| IPI-493 | Discontinued in Phase 1 | [96] | ||
| Synonyms |
[(3R,5R,6S,7R,8E,10R,11R,12Z,14E)-21-amino-6-hydroxy-5,11-dimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-17-azabicyclo[16.3.1]docosa-1(21),8,12,14,18-pentaen-10-yl] carbamate; AC1NS08X; SCHEMBL16226496; SCHEMBL16225851
Click to Show/Hide
|
|||
| External Link | ||||
| Kanjinti | Application submitted | [12] | ||
| External Link | ||||
| Anti-CD9 mab | Investigative | [97] | ||
| Synonyms |
ALB-6; Anti-CD9 mAb (gastric cancer); Anti-CD9 mAb (gastric cancer), Osaka University
Click to Show/Hide
|
|||
| External Link | ||||
References